Abstract
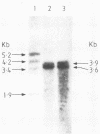
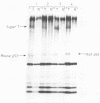
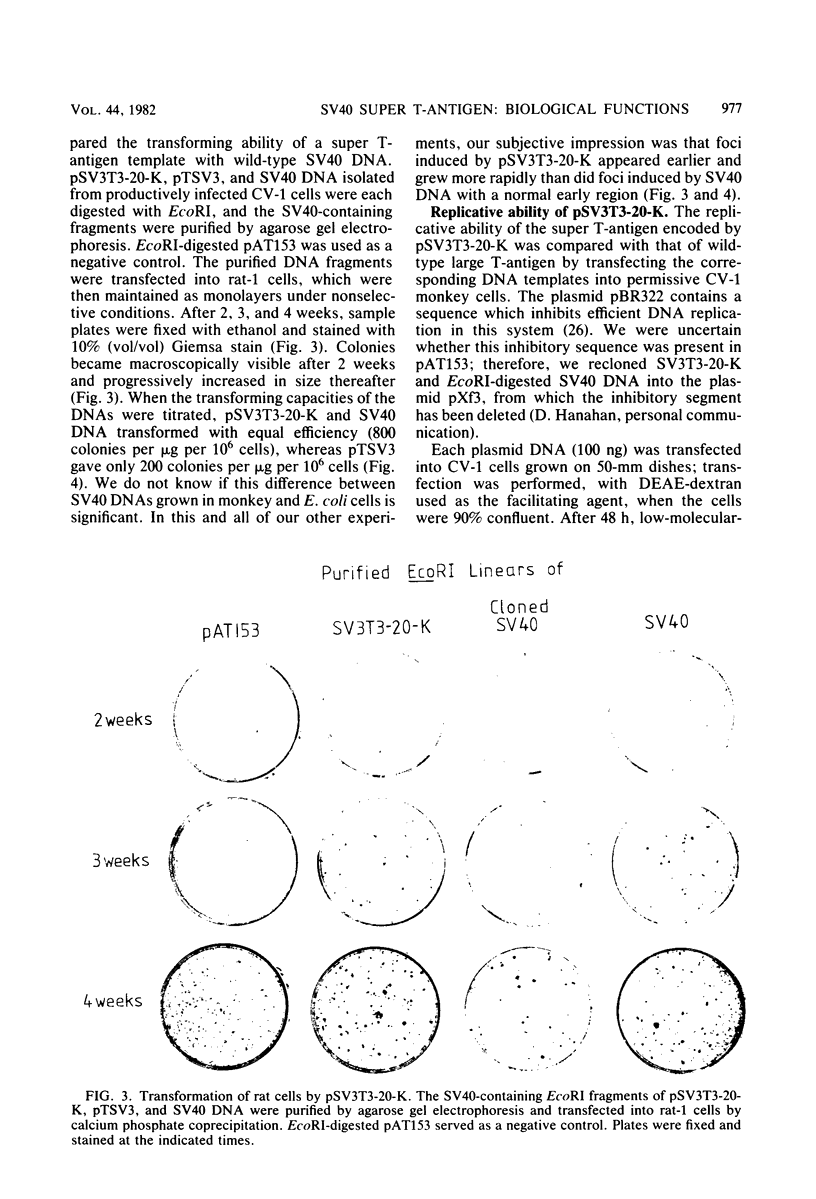
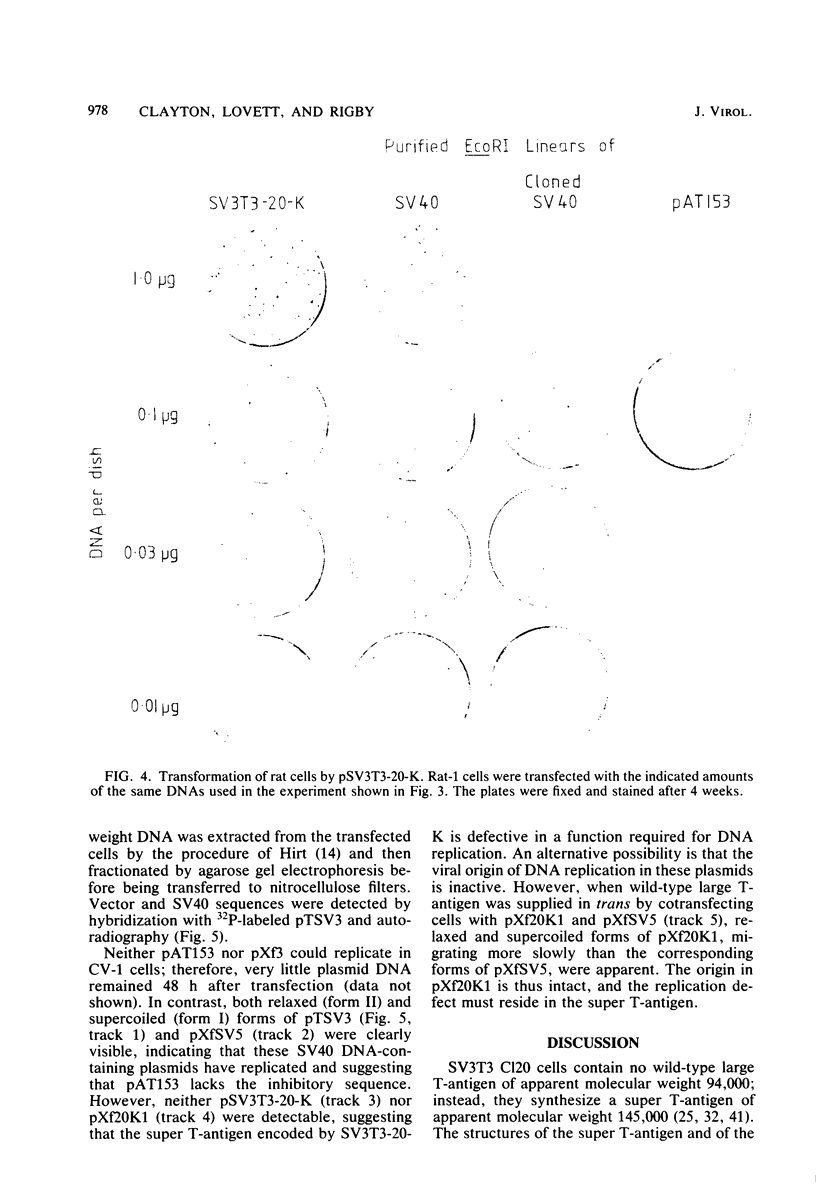
The SV3T3 C120 line of simian virus 40-transformed mouse cells synthesizes no large T-antigen of molecular weight 94,000 but instead a super T-antigen of molecular weight 145,000. In the accompanying paper (Lovett et al., J. Virol. 44:963-973, 1982), we showed that the integrated viral DNA segment SV3T3-20-K contains a perfect, in-phase, tandem duplication of 1.212 kilobases within the large T-antigen coding sequences. Our data suggested that this integrated template encodes mRNAs of 3.9 and 3.6 kilobases, the smaller of which directs the synthesis of the super T-antigen of molecular weight 145,000. We transfected the DNA segment SV3T3-20-K into nonpermissive rat cells and into TK- mouse L cells and analyzed the T-antigens and viral mRNAs in the transfectants; these data prove directly the coding assignments suggested previously. The super T-antigen retained the ability to induce morphological transformation, and may even transform better than the wild-type protein. It also retained the ability to bind to the cell-coded p53 protein. Transfection into permissive CV-1 cells showed that the super T-antigen encoded by SV3T3-20-K was incapable of initiating DNA replication at the viral origin. The duplication in SV3T3-20-K thus defines a mutation which separates the transformation and DNA replication functions of large T-antigen. We discuss why such mutations may be selected in transformed cells.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Basilico C., Gattoni S., Zouzias D., Valle G. D. Loss of integrated viral DNA sequences in polyomatransformed cells is associated with an active viral A function. Cell. 1979 Jul;17(3):645–659. doi: 10.1016/0092-8674(79)90272-1. [DOI] [PubMed] [Google Scholar]
- Bender M. A., Brockman W. W. Rearrangement of integrated viral DNA sequences in mouse cells transformed by simian virus 40. J Virol. 1981 Jun;38(3):872–879. doi: 10.1128/jvi.38.3.872-879.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C., Simmons D. T., Martin M. A., Mora P. T. Identification and partial characterization of new antigens from simian virus 40-transformed mouse cells. J Virol. 1979 Aug;31(2):463–471. doi: 10.1128/jvi.31.2.463-471.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chia W., Rigby P. W. Fate of viral DNA in nonpermissive cells infected with simian virus 40. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6638–6642. doi: 10.1073/pnas.78.11.6638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chia W., Scott M. R., Rigby P. W. The construction of cosmid libraries of eukaryotic DNA using the Homer series of vectors. Nucleic Acids Res. 1982 Apr 24;10(8):2503–2520. doi: 10.1093/nar/10.8.2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark R., Lane D. P., Tjian R. Use of monoclonal antibodies as probes of simian virus 40 T antigen ATPase activity. J Biol Chem. 1981 Nov 25;256(22):11854–11858. [PubMed] [Google Scholar]
- Clayton C. E., Rigby P. W. Cloning and characterization of the integrated viral DNA from three lines of SV40-transformed mouse cells. Cell. 1981 Aug;25(2):547–559. doi: 10.1016/0092-8674(81)90073-8. [DOI] [PubMed] [Google Scholar]
- Colantuoni V., Dailey L., Basilico C. Amplification of integrated viral DNA sequences in polyoma virus-transformed cells. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3850–3854. doi: 10.1073/pnas.77.7.3850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosman D. J., Tevethia M. J. Characterization of a temperature-sensitive, DNA-positive, nontransforming mutant of simian virus 40. Virology. 1981 Jul 30;112(2):605–624. doi: 10.1016/0042-6822(81)90306-8. [DOI] [PubMed] [Google Scholar]
- Della Valle G., Fenton R. G., Basilico C. Polyoma large T antigen regulates the integration of viral DNA sequences into the genome of transformed cells. Cell. 1981 Feb;23(2):347–355. doi: 10.1016/0092-8674(81)90130-6. [DOI] [PubMed] [Google Scholar]
- Harlow E., Crawford L. V., Pim D. C., Williamson N. M. Monoclonal antibodies specific for simian virus 40 tumor antigens. J Virol. 1981 Sep;39(3):861–869. doi: 10.1128/jvi.39.3.861-869.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassell J. A., Lukanidin E., Fey G., Sambrook J. The structure and expression of two defective adenovirus 2/simian virus 40 hybrids. J Mol Biol. 1978 Apr 5;120(2):209–247. doi: 10.1016/0022-2836(78)90065-7. [DOI] [PubMed] [Google Scholar]
- Hassell J. A., Topp W. C., Rifkin D. B., Moreau P. E. Transformation of rat embryo fibroblasts by cloned polyoma virus DNA fragments containing only part of the early region. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3978–3982. doi: 10.1073/pnas.77.7.3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Hiscott J. B., Murphy D., Defendi V. Instability of integrated viral DNA in mouse cells transformed by simian virus 40. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1736–1740. doi: 10.1073/pnas.78.3.1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiscott J., Murphy D., Defendi V. Amplification and rearrangement of integrated SV40 DNA sequences accompany the selection of anchorage-independent transformed mouse cells. Cell. 1980 Nov;22(2 Pt 2):535–543. doi: 10.1016/0092-8674(80)90363-3. [DOI] [PubMed] [Google Scholar]
- Kress M., May E., Cassingena R., May P. Simian virus 40-transformed cells express new species of proteins precipitable by anti-simian virus 40 tumor serum. J Virol. 1979 Aug;31(2):472–483. doi: 10.1128/jvi.31.2.472-483.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai C. J., Nathans D. A map of temperature-sensitive mutants of simian virus 40. Virology. 1975 Jul;66(1):70–81. doi: 10.1016/0042-6822(75)90179-8. [DOI] [PubMed] [Google Scholar]
- Lane D. P., Lane E. B. A rapid antibody assay system for screening hybridoma cultures. J Immunol Methods. 1981;47(3):303–307. doi: 10.1016/0022-1759(81)90286-6. [DOI] [PubMed] [Google Scholar]
- Lania L., Boast S., Fried M. Excision of polyoma virus genomes from chromosomal DNA by homologous recombination. Nature. 1982 Jan 28;295(5847):349–350. doi: 10.1038/295349a0. [DOI] [PubMed] [Google Scholar]
- Lania L., Gandini-Attardi D., Griffiths M., Cooke B., De Cicco D., Fried M. The polyoma virus 100K large T-antigen is not required for the maintenance of transformation. Virology. 1980 Feb;101(1):217–232. doi: 10.1016/0042-6822(80)90497-3. [DOI] [PubMed] [Google Scholar]
- Lania L., Hayday A., Bjursell G., Gandini-Attardi D., Fried M. Organization and expression of integrated polyoma virus DNA sequences in transformed rodent cells. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 1):597–603. doi: 10.1101/sqb.1980.044.01.062. [DOI] [PubMed] [Google Scholar]
- Lania L., Hayday A., Fried M. Loss of functional large T-antigen and free viral genomes from cells transformed in vitro by polyoma virus after passage in vivo as tumor cells. J Virol. 1981 Aug;39(2):422–431. doi: 10.1128/jvi.39.2.422-431.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis A. M., Jr, Martin R. G. Oncogenicity of simian virus 40 deletion mutants that induce altered 17-kilodalton t-proteins. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4299–4302. doi: 10.1073/pnas.76.9.4299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovett M., Clayton C. E., Murphy D., Rigby P. W., Smith A. E., Chaudry F. Structure and synthesis of a simian virus 40 super T-antigen. J Virol. 1982 Dec;44(3):963–973. doi: 10.1128/jvi.44.3.963-973.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lusky M., Botchan M. Inhibition of SV40 replication in simian cells by specific pBR322 DNA sequences. Nature. 1981 Sep 3;293(5827):79–81. doi: 10.1038/293079a0. [DOI] [PubMed] [Google Scholar]
- Martin R. G., Setlow V. P., Edwards C. A., Vembu D. The roles of the simian virus 40 tumor antigens in transformation of Chinese hamster lung cells. Cell. 1979 Jul;17(3):635–643. doi: 10.1016/0092-8674(79)90271-x. [DOI] [PubMed] [Google Scholar]
- Martin R. G. The transformation of cell growth and transmogrification of DNA synthesis by simian virus 40. Adv Cancer Res. 1981;34:1–68. doi: 10.1016/s0065-230x(08)60238-9. [DOI] [PubMed] [Google Scholar]
- May E., Jeltsch J. M., Gannon F. Characterization of a gene encoding a 115 K super T antigen expressed by a SV40-transformed rat cell line. Nucleic Acids Res. 1981 Aug 25;9(16):4111–4128. doi: 10.1093/nar/9.16.4111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May E., Kress M., Daya-Grosjean L., Monier R., May P. Mapping of the viral mRNA encoding a super-T antigen of 115,000 daltons expressed in simian virus 40-transformed rat cell lines. J Virol. 1981 Jan;37(1):24–35. doi: 10.1128/jvi.37.1.24-35.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May P., Kress M., Lange M., May E. New genetic information expressed in SV40-transformed cells: characterization of the 55K proteins and evidence for unusual SV40 mRNAs. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 1):189–200. doi: 10.1101/sqb.1980.044.01.022. [DOI] [PubMed] [Google Scholar]
- McCormick F., Chaudry F., Harvey R., Smith R., Rigby P. W., Paucha E., Smith A. E. T antigens of SV40-transformed cells. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 1):171–178. doi: 10.1101/sqb.1980.044.01.020. [DOI] [PubMed] [Google Scholar]
- McCutchan J. H., Pagano J. S. Enchancement of the infectivity of simian virus 40 deoxyribonucleic acid with diethylaminoethyl-dextran. J Natl Cancer Inst. 1968 Aug;41(2):351–357. [PubMed] [Google Scholar]
- Mertz J. E., Berg P. Defective simian virus 40 genomes: isolation and growth of individual clones. Virology. 1974 Nov;62(1):112–124. doi: 10.1016/0042-6822(74)90307-9. [DOI] [PubMed] [Google Scholar]
- Mishra N. K., Ryan W. L. Effect of 3-methylcholanthrene and dimethylnitrosamine on anchorage dependence of rat fibroblasts chronically infected with Rauscher leukemia virus. Int J Cancer. 1973 Jan 15;11(1):123–130. doi: 10.1002/ijc.2910110114. [DOI] [PubMed] [Google Scholar]
- Moore J. L., Chowdhury K., Martin M. A., Israel M. A. Polyoma large tumor antigen is not required for tumorigenesis mediated by viral DNA. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1336–1340. doi: 10.1073/pnas.77.3.1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison D. A. Transformation in Escherichia coli: cryogenic preservation of competent cells. J Bacteriol. 1977 Oct;132(1):349–351. doi: 10.1128/jb.132.1.349-351.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norgard M. V., Keem K., Monahan J. J. Factors affecting the transformation of Escherichia coli strain chi1776 by pBR322 plasmid DNA. Gene. 1978 Jul;3(4):279–292. doi: 10.1016/0378-1119(78)90038-0. [DOI] [PubMed] [Google Scholar]
- Novak U., Dilworth S. M., Griffin B. E. Coding capacity of a 35 percent fragment of the polyoma virus genome is sufficient to initiate and maintain cellular transformation. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3278–3282. doi: 10.1073/pnas.77.6.3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pintel D., Bouck N., di Mayorca G. Separation of lytic and transforming functions of the simian virus 40 A region: two mutants which are temperature sensitive for lytic functions have opposite effects on transformation. J Virol. 1981 May;38(2):518–528. doi: 10.1128/jvi.38.2.518-528.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Chia W., Clayton C. E., Lovett M. The structure and expression of the integrated viral DNA in mouse cells transformed by simian virus 40. Proc R Soc Lond B Biol Sci. 1980 Nov 19;210(1180):437–450. doi: 10.1098/rspb.1980.0145. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Rigby P. W. SV40 and polyoma viruses: their analysis by deoxyribonucleic acid recombination in vitro and their use as vectors in eukaryotic systems. Biochem Soc Symp. 1979;44:89–102. [PubMed] [Google Scholar]
- Sager R., Anisowicz A., Howell N. Genomic rearrangements in a mouse cell line containing integrated SV40 DNA. Cell. 1981 Jan;23(1):41–50. doi: 10.1016/0092-8674(81)90268-3. [DOI] [PubMed] [Google Scholar]
- Shenk T. E., Carbon J., Berg P. Construction and analysis of viable deletion mutants of simian virus 40. J Virol. 1976 May;18(2):664–671. doi: 10.1128/jvi.18.2.664-671.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A. E., Smith R., Paucha E. Characterization of different tumor antigens present in cells transformed by simian virus 40. Cell. 1979 Oct;18(2):335–346. doi: 10.1016/0092-8674(79)90053-9. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Spangler G. J., Griffin J. D., Rubin H., Livingston D. M. Identification and initial characterization of a new low-molecular-weight virus-encoded T antigen in a line of simian virus 40-transformed cells. J Virol. 1980 Nov;36(2):488–498. doi: 10.1128/jvi.36.2.488-498.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stringer J. R. Mutant of simian virus 40 large T-antigen that is defective for viral DNA synthesis, but competent for transformation of cultured rat cells. J Virol. 1982 Jun;42(3):854–864. doi: 10.1128/jvi.42.3.854-864.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutcliffe J. G. Complete nucleotide sequence of the Escherichia coli plasmid pBR322. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 1):77–90. doi: 10.1101/sqb.1979.043.01.013. [DOI] [PubMed] [Google Scholar]
- Topp W. C., Rifkin D. B., Sleigh M. J. SV40 mutants with an altered small-t protein are tumorigenic in newborn hamsters. Virology. 1981 Jun;111(2):341–350. doi: 10.1016/0042-6822(81)90338-x. [DOI] [PubMed] [Google Scholar]
- Treisman R., Novak U., Favaloro J., Kamen R. Transformation of rat cells by an altered polyoma virus genome expressing only the middle-T protein. Nature. 1981 Aug 13;292(5824):595–600. doi: 10.1038/292595a0. [DOI] [PubMed] [Google Scholar]
- Twigg A. J., Sherratt D. Trans-complementable copy-number mutants of plasmid ColE1. Nature. 1980 Jan 10;283(5743):216–218. doi: 10.1038/283216a0. [DOI] [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigler M., Pellicer A., Silverstein S., Axel R., Urlaub G., Chasin L. DNA-mediated transfer of the adenine phosphoribosyltransferase locus into mammalian cells. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1373–1376. doi: 10.1073/pnas.76.3.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyke J. A., Quade K. Infection of rat cells by avian sarcoma virus: factors affecting transformation and subsequent reversion. Virology. 1980 Oct 30;106(2):217–233. doi: 10.1016/0042-6822(80)90246-9. [DOI] [PubMed] [Google Scholar]