Abstract
Objective
To assess if the effect of a single treatment episode with nicotine replacement therapy (NRT) enhances smoking cessation over many years.
Data sources
Meta‐analysis of all randomised controlled trials of NRT with final follow‐up more than one year after the start of treatment. Twelve eligible trials were identified, all placebo‐controlled, having final follow‐ups ranging from 2–8 years. All had earlier follow‐ups at 12 months. They comprised 2408 active and 2384 placebo treatment participants.
Data synthesis
The odds ratio (OR) in favour of NRT at final follow‐up was 1.99 (95% confidence interval (CI) 1.50 to 2.64). There was no evidence that the effect varied according to length of final follow‐up (β = 0.92, p = 0.28) or duration of initial NRT treatment (β = 0.99, p > 0.5). The overall relapse rate between the 12 months and final follow‐up was 30.0% (95% CI 23.5% to 37.5%). This rate did not differ between NRT and control groups (OR 1.11, 95% CI 0.78 to 1.59), or length of initial NRT treatment. There was also no evidence that it varied according to length of final follow up. Due to relapse, the overall efficacy of NRT treatment in terms of additional ex‐smokers declined from 10.7% over and above placebo (6.6% to 14.8%) after one year to 7.2% (3.8% to 11.3%) at an average of 4.3 years follow up.
Conclusions
The relative efficacy of a single course of NRT remains constant over many years. The majority of relapse after 12 months occurs within the first or second year and is not detectable thereafter, suggesting that NRT has a permanent effect on smoking cessation. However, initial relapse after one year has the effect of diminishing the number of ex‐smokers that can be ultimately attributed to NRT. Results after only 6–12 months of follow‐up, as used in existing reviews and treatment guidelines, will overestimate the lifetime benefit and cost‐efficacy of NRT by about 30%. Because the long‐term benefit of NRT is modest, tobacco dependence treatment might be better viewed as a chronic disorder, requiring repeated episodes of treatment.
Keywords: tobacco dependence, nicotine replacement therapy, long‐term follow‐up, meta‐analysis
Estimates from 50 years' observation of the natural history of smoking and mortality indicate that smokers compared to never smokers die on average 10 years younger if they continue to smoke throughout their lives.1 However, permanent cessation at ages 40, 50 and 60 gains approximately 9, 6 and 3 years of life, respectively. These epidemiological findings have recently received support from the first randomised cessation trial where the long‐term health benefits were also assessed.2
Nicotine replacement therapy (NRT) is the most widely used pharmacological adjunct to advice and behavioural support when treating tobacco dependence. Because it is safe for virtually all smokers and is the only medicine easily available over the counter and on general sale, it is likely to remain the most popular treatment for the foreseeable future. NRT partially replaces the nicotine from cigarettes over the initial 8–12 week period of stopping smoking. It reduces tobacco withdrawal symptoms during the period when they are most severe, enabling the smoker time to adjust to life without cigarettes. Evidence for NRT effectiveness comes from over 100 placebo‐controlled trials with final follow‐up 6–12 months after the start of treatment. Meta‐analyses of these trials give odds ratios in favour of active treatment ranging from 1.7 to 2.3 according to NRT product.3,4
The conventional goal of even brief and inexpensive treatment is lifetime smoking cessation, or at least a sufficient period of cessation to give a measurable health benefit. Therefore, trials and meta‐analyses that only measure cessation during the first year fall short of providing evidence for the efficacy of NRT according to the treatment goal. It is well‐known that relapse to smoking continues beyond 12 months, and that this could have a substantial impact on the true long‐term effectiveness of NRT.5,6 Even if relapse after 12 months is similar in NRT and control groups, giving a stable odds ratio over time, the net NRT benefit, as measured by the difference in cessation rates, might be substantially reduced. Therefore, the essential question for clinicians, policymakers and health economists—as to whether NRT is effective in promoting enduring, permanent smoking cessation—remains unanswered.
To assess if the efficacy of a single treatment episode with NRT is maintained long‐term, we conducted a meta‐analysis of results from all controlled trials having follow‐up results beyond 12 months.
METHODS
Identification of trials
The authors independently searched the Cochrane Central Register of Controlled Trials, Medline, PubMed, Embase, and Psychmed for randomised trials of NRT with an end point beyond 12 months after the start of treatment. Subsequently, we wrote to all members of the Society for Research on Nicotine and Tobacco and Globalink Tobacco Control, enquiring about knowledge of additional long‐term studies.
Inclusion criteria
Eligible studies were randomised trials that had a treatment aim of permanent smoking cessation after a defined “quit day”. We also required that treatment and control conditions differed only by the inclusion of an active NRT product in the treatment arm, and that the proportion of those randomised who succeeded in maintaining smoking cessation was available for follow up times beyond 12 months of starting treatment.
Data extraction and coding
We independently abstracted data from the eligible trials using a standardised spreadsheet. We abstracted data on the number of participants randomised to treatment and control arms, the number of participants who were abstinent from smoking at 12 months from the start of treatment and at end points beyond 12 months, type of NRT product, dose, duration of treatment, and type of primary outcome measure. Only study arms of standard recommended doses of NRT were included, except where lower doses had specifically been given to light smokers.
Outcome measures
The proportion of those originally randomised in each study arm who had maintained smoking cessation at the time of follow up (“the success rate”) was taken as the measure of outcome. To assess the specific effect of a single treatment episode, we chose the more stringent prolonged abstinence criteria (continuous complete abstinence, or continuous abstinence allowing limited lapses) in preference to point prevalence (abstinent during the week before follow up) when both measures were reported. In this way, we hoped to reduce contamination bias by excluding abstinence attributable to subsequent cessation attempts, rather than the tested treatment. Similarly, we used results that included biochemically verified abstinence in preference to self‐report only abstinence, whenever both criteria were reported. Where results from more than one follow up beyond 12 months were given, we only included in the analysis the results from the last follow up.
Statistical methods
We used the odds ratio (NRT: control) to estimate the effect of NRT additional to the effect of any advice or behavioural counselling that was also given. Initially, we also considered a direct analysis of the absolute difference between success rates in NRT and control conditions but, as has previously been observed in meta‐analyses of the short term effect of NRT, this measure exhibited a high degree of heterogeneity and consequent loss of statistical precision.3 Instead, we estimated the absolute benefit of NRT in terms of the percentage of additional smokers stopping by applying the pooled odds ratio to the pooled control condition success rate.7
We used a random effects model to give a pooled estimate of the overall odds ratio and standard error on the basis that genuine heterogeneity is highly plausible across the varied clinical settings and populations covered by the included trials, and we wished to properly allow for this. We calculated random effects estimates using DerSimonian and Laird's re‐weighting method, which also gave a measure of heterogeneity via a Q‐statistic.8 We used the regression method to test the symmetry of the trial funnel plot which might indicate publication bias due to small studies with low or null effects not reaching publication.9 The funnel plot is a graph plotting the effect size of each trial (odds ratio/log odds ratio) against the precision (related to sample size) of each trial. Where no bias exists the graph will be symmetrical about the overall mean effect with an equal number of small trials either side of the mean. Where asymmetry was evident we used the Klein method to estimate how many “missing” null trials would be required before the overall pooled effect was also null (odds ratio = 1), and also the somewhat over‐corrective “trim and fill” method to impute results for “missing” small trials and re‐calculate the overall pooled effect on the basis of a symmetric funnel.9,10,11 We also conducted meta‐regression analyses to examine if the trial effect sizes were related to the length of final follow up, length of time that subjects used NRT, and type of NRT used.12
RESULTS
The trials
Twelve controlled trials reporting cessation results 2–8 years after treatment (weighted mean 4.3 years), involving 4792 participants (2408 NRT, 2384 control), were identified and included in the analysis (table 1).6,13,14,15,16,17,18,19,20,21,22,23 All were placebo‐controlled and all also provided results at 12 months of follow‐up, either in the same or in previous publications. They comprised five trials of the nicotine patch, four of nicotine gum and three of nicotine nasal spray, studied across nine countries in four continents. Most allowed participants to use NRT for about three months, but four allowed longer use (overall weighted mean 21.9 weeks). All gave NRT in addition to supportive advice or counselling. In one trial of the nicotine nasal spray, all participants were also given active nicotine patches for a shorter duration.15
Table 1 Nicotine replacement therapy (NRT) trials with final end points more than one year after treatment.
| Study | Time of final follow‐up | NRT product* | Dose | Control condition† | Maximum period of NRT use | Abstinence criterion‡ | Biochemical verification§ |
|---|---|---|---|---|---|---|---|
| Blondal 1989 | 2 years | Gum | 4 mg | Placebo | 12 weeks | Continuous | None |
| Blondal 1997 | 2 years | NNS | 0.5 mg | Placebo | 52 weeks | Continuous | CO <10 ppm |
| Blondal 1999 | 6 years | NNS | 0.5 mg | Placebo | 52 weeks | Sustained | CO <10 ppm |
| Clavel 1997 | 4 years | Gum | 2 mg | Placebo | 26 weeks | Sustained | CO <10 ppm |
| Daughton 1999 | 4.6 years | Patch | 21 mg/24 h | Placebo | 12 weeks | Sustained | CO <10 ppm¶ |
| Glavas 2003 | 5 years | Patch | 21 mg/24 h | Placebo | 3 weeks | PP | CO <10 ppm |
| Herrera 1995 | 2 years | Gum | 2 mg | Placebo | 12 weeks | Sustained | CO <10 ppm |
| Mikkelsen 1994 | 3 years | Patch | 15 mg/16 h | Placebo | 16 weeks | Sustained | CO <10 ppm |
| Richmond 1997 | 3 years | Patch | 21 mg/24 h | Placebo | 10 weeks | Continuous | CO <10 ppm |
| Stapleton 1998 | 3.3 years | NNS | 0.5 mg | Placebo | 52 weeks | Sustained | CO <10 ppm |
| Tonnesen 1988 | 2 years | Gum | 2 mg | Placebo | 16 weeks | Continuous | CO<10ppm |
| Yudkin 2003 | 8 years | Patch | 21 mg/24 h | Placebo | 12 weeks | Continuous | Cot <15 ng/ml |
*Gum, nicotine chewing gum; NNS, nicotine nasal spray; Patch, nicotine transdermal patch.
†In addition to placebo, control group received the same counselling and support as NRT group. This varied in type and intensity across studies.
‡Continuous = no smoking after start of outcome observation period. Sustained = continuous abstinence but allowing some very limited smoking lapses throughout the follow‐up period. PP (point prevalence) = abstinence from smoking in the week before follow up.
§CO, expired‐air carbon monoxide; Cot, saliva cotinine.
¶CO verification not conducted in all clinical centres in study.
The trials spanned the full spectrum of clinical settings where NRT is frequently used, from specialist cessation clinics where more intensive support was given, to primary care where participants received only brief advice. However, it was not possible to reliably code for each trial a measure of the intensity of support, or the expertise with which it was given. Sample sizes appeared to reflect the fact that adding NRT is generally thought to improve the success of support alone in an approximately multiplicative fashion, with more successes attributable to NRT when more support is given.3 Therefore, smaller trials tended to be conducted in specialist clinics and larger trials in settings where the adjunctive support was minimal. All the included trials were conducted in clinical settings, rather than in “self‐help” settings, such as when NRT is purchased over the counter in pharmacies or supermarkets or given without support in community programmes.24
All but one study excluded smokers of less than 10 or 15 cigarettes per day and gave standard NRT doses delivering about 1 mg of nicotine per hour. In the one study that included lighter smokers, these received appropriately lower NRT doses.18 Another study randomised smokers of 15 or more cigarettes per day to either placebo or to full strength (21 mg) or lower dose (14 mg or 7 mg) patches from the outset.17 Only the full strength dose arm was included in our analysis because the two lower doses are generally recommended only for weaning down the nicotine dose, following several weeks using full strength patches.
Exclusions
Two trials with follow up beyond 12 months were excluded because of inadequate control conditions. Most notable was the large Lung Health Study that reported highly significant results five years after the start of treatment but compared extensive behavioural support and NRT with a “usual care” intervention.25 Similarly, the trial by Tonnesen (1988) compared NRT and group counselling to brief advice only.26
Relative effect of NRT in sustaining long‐term smoking cessation
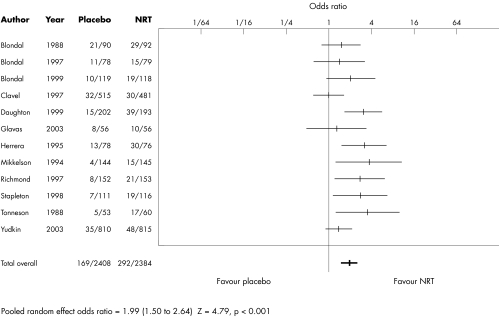
Of the 12 included trials, six provided statistical evidence of an NRT effect at final follow up and six gave null results (table 2). Combining the data from all trials provided good evidence for the efficacy of NRT in sustaining smoking cessation beyond 12 months (fig 1). A random effects model gave odds ratios in favour of NRT of 1.99 (95% confidence interval (CI) 1.50 to 2.64, z = 4.79, p < 0.001). The heterogeneity statistic was 18.7 (df = 11, p = 0.08), suggesting that a fixed‐effect model would have given similar results. This estimate is close to the odds ratio for the effect of NRT after only 12 months of follow‐up in these 12 trials (OR 2.13, 95% CI 1.68 to 2.69).
Table 2 Effect of NRT on long‐term smoking cessation.
| Study | NRT | Placebo | Weight (%) | OR (95% CI) |
|---|---|---|---|---|
| Blondal 1989 | 29/92* (30)† | 21/90 (22) | 9.9 | 1.51 (0.78 to 2.92) |
| Blondal 1997 | 15/79 (20) | 11/78 (13) | 7.3 | 1.43 (0.61 to 3.34) |
| Blondal 1999 | 19/118 (32) | 10/119 (13) | 7.7 | 2.09 (0.93 to 4.72) |
| Clavel 1997 | 30/481 (53) | 32/515 (43) | 12.5 | 1.00 (0.60 to 1.68) |
| Daughton 1999 | 39/193 (50) | 15/202 (19) | 10.3 | 3.16 (1.68 to 5.94) |
| Glavas 2003 | 10/56 (13) | 8/56 (9) | 5.7 | 1.30 (0.47 to 3.60) |
| Herrera 1995 | 30/76 (37) | 13/78 (17) | 8.5 | 3.26 (1.54 to 6.92) |
| Mikkelsen 1994 | 15/145 (24) | 4/144 (6) | 4.8 | 4.04 (1.31 to 12.5) |
| Richmond 1997 | 21/153 (29) | 8/152 (14) | 7.3 | 2.86 (1.23 to 6.69) |
| Stapleton 1998 | 19/116 (33) | 7/111 (14) | 6.7 | 2.91 (1.17 to 7.23) |
| Tonnesen 1988 | 17/60 (23) | 5/53 (12) | 5.2 | 3.80 (1.29 to 11.2) |
| Yudkin 2003 | 48/815 (91) | 35/810 (62) | 14.0 | 1.39 (0.89 to 2.17) |
| Total | 292/2384 (435) | 169/2408 (244) | 100 | 1.99 (1.50 to 2.64) |
Random effects test of overall effect: Z = 4.79, p<0.001.
*Number of participants who successfully maintained smoking cessation/number entering trial.
†Figures in brackets give number successful at earlier 12 month follow up.
CI, confidence interval; OR, odds ratio.
Figure 1 Effect of nicotine replacement therapy (NRT) on longer term smoking cessation.
There was evidence that the funnel plot was asymmetrical (p = 0.02), suggesting some publication bias with small, null studies not having reached publication. However, the Klein method indicated that an additional 93 such trials would be required to reverse the conclusion that NRT had a positive effect. The “trim and fill” adjustment method for possible bias imputed four studies and gave a slightly lower odds ratio in favour of NRT (OR 1.57, 95% CI 1.15 to 2.13).
We examined the effect of NRT product type, the length of final follow up (2–8 years) and the length of time patients were allowed to use NRT (3–52 weeks) in a meta‐regression analysis. There was no evidence of a difference between the products (χ2 = 0.32, df = 2, p > 0.5). The odds ratios for gum, nasal spray, and patch were 1.80, 2.03 and 2.16, respectively. There was no evidence that the effect of NRT changed over time according to length of follow‐up (linear χ2 = 1.20, df = 1, p = 0.28). For the eight trials with final follow up within four years, the odds ratio was 2.07, and for the four trials with final follow up beyond four years, it was 1.88. There was also no evidence that the duration of NRT treatment influenced long‐term effectiveness (linear χ2 = 0.06, df = 1, p > 0.5).
Relapse to smoking after one year of cessation
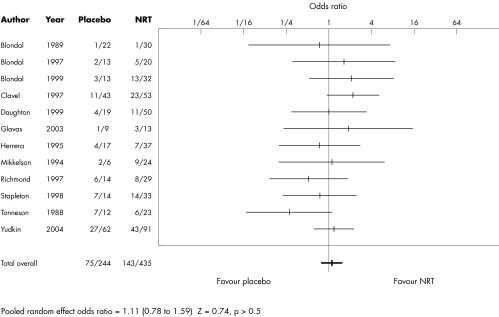
Although statistical power was limited to examine subsequent relapse among those surviving to 12 months, we found no suggestion of a difference between NRT and control groups (OR 1.11, 95% CI 0.78 to 1.59) (table 3, fig 2). There was also no suggestion in a meta‐regression analysis that this overall lack of effect had been masked by a difference according to NRT product (χ2 = 0.18, df = 2, p > 0.5), length of follow up (χ2 = 1.1, df = 1, p > 0.5), or length of NRT use (χ2 = 0.75, df = 1, p > 0.5).
Table 3 Effect of NRT treatment for less than 12 months on relapse after 12 months of smoking cessation.
| Study | NRT | Placebo | Weight (%) | OR (95% CI) |
|---|---|---|---|---|
| Blondal 1989 | 1/30* | 1/22 | 1.6 | 0.72 (0.04 to12.2) |
| Blondal 1997 | 5/20 | 2/13 | 3.8 | 1.83 (0.30 to 11.3) |
| Blondal 1999 | 13/32 | 3/13 | 5.9 | 2.28 (0.52 to 9.92) |
| Clavel 1997 | 23/53 | 11/43 | 16.7 | 2.23 (0.93 to 5.35) |
| Daughton 1999 | 11/50 | 4/19 | 7.7 | 1.06 (0.29 to 3.84) |
| Glavas 2003 | 3/13 | 1/9 | 2.1 | 2.40 (0.21 to 27.7) |
| Herrera 1995 | 7/37 | 4/17 | 6.6 | 0.76 (0.19 to 3.05) |
| Mikkelsen 1994 | 9/24 | 2/6 | 3.6 | 1.20 (0.18 to 7.93) |
| Richmond 1997 | 8/29 | 6/14 | 7.2 | 0.51 (0.13 to 1.93) |
| Stapleton 1998 | 14/33 | 7/14 | 8.2 | 0.74 (0.21 to 2.56) |
| Tonnesen 1988 | 6/23 | 7/12 | 5.9 | 0.25 (0.06 to 1.11) |
| Yudkin 2003 | 43/91 | 27/62 | 30.5 | 1.16 (0.61 to 2.22) |
| Total | 143/435 | 75/244 | 100 | 1.11 (0.78 to 1.59) |
Random effects test of overall effect: Z = 0.74, p>0.5.
*Numbers abstinent for 12 months who relapsed before final follow up/numbers abstinent for 12 months.
Figure 2 Effect of NRT treatment for less than 12 months on relapse after 12 months of smoking cessation.
Although there was no evidence that NRT relative to placebo affected long‐term relapse, a substantial number of trial participants (218/679) did relapse after 12 months. In all 12 studies the pooled odds of relapse was 0.43 (95% CI 0.31 to 0.60), equivalent to a 30.0% (95% CI 23.5% to 37.5%) relapse rate. In a meta‐regression analysis, there was no evidence that this rate varied according to the NRT product being tested (χ2 = 1.2, df = 2, p > 0.5), or duration of use (χ2 = 0.9, df = 1, p > 0.5). More interestingly, the relapse rate did not differ by time of final follow up (χ2 = 2.46, df = 1, p = 0.13). For the eight trials with final follow up within four years the odds of relapse was 0.41 and for the four trials with final follow up beyond four years it was 0.46.
Estimating the long‐term net benefit of NRT treatment
To estimate from the pooled odds ratio the long‐term benefit of NRT in terms of the percentage increase in ex‐smokers, we also needed to estimate the pooled long‐term success rate in the control arms of the trials. The random effects method gave an overall rate of 8.6% (95% CI 5.9% to 12.3%). However, there was clear evidence of heterogeneity (X2 = 63, df = 11, p < 0.001), indicating that this overall rate cannot be considered applicable to all situations. Consequently, if in a particular setting the non‐NRT control rate differs from the overall figure estimated here (8.6%), due possibly to differing intensity and expertise of the counselling support, or to the characteristics of the smokers being treated, then the net benefit of NRT will also differ. For example, if the non‐NRT control rate is expected to be about 5%, then by applying the common odds ratio (1.99) the net long‐term benefit of adding NRT is estimated to be 4.6% (95% CI 2.4% to 7.6%). If, however, the success rate without NRT is expected to be about 12.5%, then the benefit from adding NRT is estimated to be 9.6% (95% CI 5.2% to 14.9%). For the estimated overall control rate for these trials (8.6%) the improvement gained by adding NRT is 7.2% (95% CI 3.8% to 11.3%).
Applying the same method to the overall control rate (12.3%) and odds ratio (2.13) estimated after only 12 months of follow up gives a net NRT benefit of 10.7% (95% CI 6.6% to 14.8%). The reduction in the estimated absolute benefit of NRT between the one year and long‐term follow up (32.7%) is therefore almost entirely due to the 30% relapse rate between these times, and only 2.7% is due to the slight reduction in the odds ratio.
DISCUSSION
This is the first systematic review of the long‐term effect of NRT in the treatment of tobacco dependence. Therefore, it provides the most reliable evidence to date that NRT aids in achieving the treatment goal of permanent smoking cessation. The odds ratio in favour of NRT at long‐term follow up was similar to that found after only 12 months, suggesting that the relative effect of NRT remains stable over time. The addition of NRT to the brief advice or behavioural support offered in the included studies gave an odds ratio of 2 and represents a 70–90% increase in the cessation rate achieved without NRT. However, since the long‐term success rate without NRT is extremely modest, these figures disguise the true overall impact of NRT which is similarly modest and represents success for only about 7% of all those treated in these trials.
The results show that an estimated 30% of those regarded as quitters after 12 months will subsequently relapse. Although this rate was similar for NRT and control arms, leaving the odds ratio essentially unchanged, it had the effect of reducing the benefit attributable to NRT in terms of the percentage of additional ex‐smokers. This attrition of continuing abstainers “closed the difference” between NRT and control rates simply by lowering both in equal proportions. Importantly, we could detect no greater relapse among trials with longer rather than shorter follow up, suggesting that most of the relapse after 12 months takes place in the following year or two. Further, it suggests that the effect of NRT estimated here is likely to be permanent and that studies with longer follow ups are unlikely to reveal further relapse. Although initially surprising, our results are supported by those modelled from the large cross‐sectional NHANES‐I population survey (National Health and Nutrition Examination Survey) of self‐help cessation attempts.25 This study observed an asymptote in relapse at five years, with only a very small, non‐detectable, change in survival rates between three and five years. The estimated 35% relapse rate after 12 months was similar to the rate in the current study. It should be noted, however, that late relapse is not specific to NRT but occurs as well for untreated smokers.27
All the trials included in our review are also included in the major meta‐analyses of NRT efficacy during the initial 6–12 months after the start of treatment.3,4 The odds ratio for NRT in the more recent Cochrane review is 1.77 (95% CI 1.66 to 1.88). The estimate for the 12 month results in the 12 long‐term trials (OR 1.99, 95% CI 1.50 to 2.64) is slightly larger and lies outside this confidence interval. However, confidence intervals for the two estimates have considerable overlap and there is no evidence of a difference between the two (Z = 0.83, p = 0.2). Although this cannot be regarded as evidence that the long‐term trials are a representative subset of all trials, it does suggest that the long‐term results, in essentially clinical settings, might also hold for the larger group of more diverse Cochrane trials where no long‐term follow‐ups are available, including those in community and over‐the‐counter (OTC) settings.
What this paper adds
There are now sufficient trials with follow‐ups beyond one year to give a good estimate of the long‐term benefit of nicotine replacement therapy (NRT). This study shows that results after only 6–12 months of follow‐up, as used in existing reviews and treatment guidelines, will overestimate the lifetime benefit and cost‐efficacy of NRT by about 30%. Because the long‐term benefit of NRT is modest, tobacco dependence treatment might be better viewed as a chronic, relapsing disorder requiring repeated episodes of treatment.
We found that small trials tended to have larger NRT effects leading to an asymmetric funnel plot. Using the “trim and fill” method, which attempts to impute the results of the “missing”, less effective, small trials marginally reduced the overall estimate. However, in this context bias detection and adjustment methods based on the assumption that the effect size will be similar in small and large trials may not be appropriate.9 Trials with smaller sample sizes tend to be conducted in specialist centres, where more intensive behavioural support is given and the adjunctive effect of NRT is consequently expected to be larger. Compared to larger trials in generalist settings, such as primary care, these trials may also exert more control over trial procedures, producing higher compliance with treatment and perhaps most importantly, successfully following up a higher proportion of participants over many years.23 Such factors may have contributed to a possibly false impression that some small trials with results similar to those in larger trials have not reached publication, when in fact they do not exist.
This review focused on the long‐term impact of the current “one‐shot” therapeutic approach to treatment with NRT and found significant but modest effects. Although such treatment is still likely to be highly cost‐effective in terms of life‐years gained,28 the substantial amount of relapse observed even after a year of abstinence, and the fact that more than 90% of those treated do not succeed, questions whether this therapeutic approach is the most appropriate. Our results support the notion that nicotine addiction, like others, should be viewed as a chronic recurring disease of the brain,29 and that its treatment should probably be closer to the long‐term treatment of other chronic diseases, such as hypertension, than that used for acute diseases like infections. For many smokers at least, a chronic, prolonged treatment is probably necessary and should include the encouragement to make repeated quit attempts accompanied with multiple treatment episodes over many years. To date, only one study has thoroughly investigated the effect of prolonged treatment on health outcomes. The results in terms of reducing smoking and morbidity have been encouraging.2
Footnotes
Financial support: none.
Competing interest statement: JFE has been reimbursed by manufacturers of nicotine replacement products for attending international conferences and to give lectures on smoking cessation. The Institute of Social and Preventive Medicine of the University of Geneva has received grants from Novartis and Pfizer to develop computer‐tailored smoking cessation counselling programs, led by JFE. JS has acted as an adviser to several organisations with an interest in smoking cessation, including manufacturers of nicotine replacement products, for which he has received remuneration. He has also conducted a number of Medial Research Council funded clinical trials that also received support from the manufacturers of nicotine replacement products.
References
- 1.Doll R, Peto R, Boreham J.et al Mortality in relation to smoking: 50 years' observation on male British doctors. BMJ 20043281519–1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anthonisen N, Skeins A, Wise R.et al The effects of a smoking cessation intervention on 14. 5‐year mortality: a randomized clinical trial, Ann Intern Med 2005142233–239. [DOI] [PubMed] [Google Scholar]
- 3.Silagy C, Lancaster T, Stead L.et alNicotine replacement therapy for smoking cessation. The Cochrane Library, Issue 1, 2005. Chichester, UK: John Wiley & Sons, Ltd,
- 4.Fiore M C, Baily W C, Cohen S J.et alTreating tobacco use and dependence. Clinical Practice Guideline. Rockville, Maryland: US Department of Health and Human Services, Public Health Service. June 2000
- 5.US Department of Health and Human Services The health benefits of smoking cessation. A report of the Surgeon General, 1990. Rockville, Maryland: Public Health Service, Centers for Disease Control, Office on Smoking and Health, 1990, (DHHS Publication No (CDC) 90‐8416. )
- 6.Stapleton J A, Sutherland G, Russell M A. How much does relapse after one year erode effectiveness of smoking cessation treatments? Long‐term follow up of randomised trial of nicotine nasal spray. BMJ 1998316830–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sackett D L, Haynes R B, Guyatt G H.et alClinical epidemiology: a basic science for clinical medicine. 2nd ed. Little, Brown and Company 1991
- 8.Fleiss J. The statistical basis of meta‐analysis. Statistical Methods in Medical Research 19932121–145. [DOI] [PubMed] [Google Scholar]
- 9.Sterne J A C, Egger M, Davey Smith G. Investigating and dealing with publication and other biases in meta‐analysis. BMJ 2001323101–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leandro G.Meta‐analysis in medical research. The handbook for the understanding and practice of meta‐analysis. Oxford: Blackwell BMJ Books, 2005
- 11.Duval S, Tweedie R. Trim and fill: a simple funnel‐plot‐based method of testing and adjusting for publication bias in meta‐analysis. Biometrics 200056455–463. [DOI] [PubMed] [Google Scholar]
- 12.Smith G D, Egger M. Going beyond the grand mean: subgroup analysis in meta‐analysis of randomized trials. In: Egger M, Smith GD, Altman DG. Systematic reviews in health care: meta‐analysis in context. London: BMJ Publishing Group, 2001
- 13.Blöndal T. Controlled trial of nicotine polacrilex gum with supportive measures. Arch Intern Med 19891491818–1821. [DOI] [PubMed] [Google Scholar]
- 14.Blöndal T, Franzon M, Westin A. A double‐blind randomized trial of nicotine nasal spray as an aid in smoking cessation. Eur Respir J 1997101585–1590. [DOI] [PubMed] [Google Scholar]
- 15.Blöndal T, Gudmundsson L J, Olafsdottir I.et al Nicotine nasal spray with nicotine patch for smoking cessation: randomised trial with six year follow up. BMJ 1999318285–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clavel‐Chapelon F, Paoletti C, Benhamou S. Smoking cessation rates 4 years after treatment by nicotine gum and acupuncture. Prev Med 19972625–28. [DOI] [PubMed] [Google Scholar]
- 17.Daughton D M, Fortmann S P, Glover E D.et al The smoking cessation efficacy of varying doses of nicotine patch delivery systems 4 to 5 years post‐quit day. Prev Med 199928113–118. [DOI] [PubMed] [Google Scholar]
- 18.Glavas D, Rumboldt M, Rumboldt Z. Smoking cessation with nicotine replacement therapy among health care workers: randomized double‐blind study. Croat Med J 200344219–224. [PubMed] [Google Scholar]
- 19.Herrera N, Franco R, Herrera L.et al Nicotine gum, 2 and 4 mg, for nicotine dependence. A double‐blind placebo‐controlled trial within a behavior modification support program. Chest 1995108447–451. [DOI] [PubMed] [Google Scholar]
- 20.Mikkelsen K L, Tønnesen P. Norregaard J. Three‐year outcome of two‐ and three‐year sustained abstainers from a smoking cessation study with nicotine patches. J Smoking‐Related Dis 1994595–100. [Google Scholar]
- 21.Richmond R L, Kehoe L, de Almeida Neto A C. Three year continuous abstinence in a smoking cessation study using the nicotine transdermal patch. Heart 199778617–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tønnesen P, Fryd V, Hansen M.et al Effect of nicotine chewing gum in combination with group counseling on the cessation of smoking. N Engl J Med 198831815–18. [DOI] [PubMed] [Google Scholar]
- 23.Yudkin P, Hey K, Roberts S.et al Abstinence from smoking eight years after participation in randomised controlled trial of nicotine patch. BMJ 200332728–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hughes J R, Shiffman S.et al A meta‐analysis of the efficacy of over‐the‐counter nicotine replacement. Tob Control 20031221–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Anthonisen N R, Connett J E.et al Effects of smoking intervention and the use of an inhaled anticholinergic bronchodilator on the rate of decline of FEV1. JAMA 19942721497–1505. [PubMed] [Google Scholar]
- 26.Tonneson P, Fryd V.et al Two and four mg nicotine chewing gum and group counselling in smoking cessation: an open, randomized controlled trial with 22 month follow‐up. Addictive Behaviours 19881317–27. [DOI] [PubMed] [Google Scholar]
- 27.Hughes J R, Keely J, Naud S. Shape of the relapse curve and long‐term abstinence among untreated smokers. Addiction 20049929–38. [DOI] [PubMed] [Google Scholar]
- 28.Stapleton J A, Lowin A, Russell M A H. Prescription of transdermal nicotine patches for smoking cessation in general practice: evaluation of cost‐effectiveness. Lancet 1999354210–215. [DOI] [PubMed] [Google Scholar]
- 29.Leshner A I. Addiction is a brain disease, and it matters. Science 199727845–47. [DOI] [PubMed] [Google Scholar]