Abstract
Hospital acquired or nosocomial infections continue to be an important cause of morbidity and mortality. The critically ill patient is at particular risk of developing intensive care unit acquired infection, with the lungs being especially vulnerable. Nosocomial bacterial pneumonia occurring after two days of mechanical ventilation is referred to as ventilator associated pneumonia, and is the most common nosocomial infection seen in the intensive care unit. Intubation of the trachea and mechanical ventilation is associated with a 7‐fold to 21‐fold increase in the incidence of pneumonia and up to 28% of patients receiving mechanical ventilation will develop this complication. Its development is associated with an attributable increase in morbidity and mortality. The establishment of an accurate diagnosis of ventilator associated pneumonia remains problematic and as yet there is still no accepted “gold standard” for diagnosis. The responsible pathogens vary according to case mix, local resistance patterns, and methodology of sampling. However, there is general agreement that rapid initiation of appropriate antimicrobial therapy improves outcome.
Keywords: ventilator associated pneumonia, nosocomial infection, critical care
Ventilator associated pneumonia (VAP) is defined as nosocomial pneumonia occurring in a patient after 48 hours of mechanical ventilation via a tracheal or tracheostomy tube. It is commonly classified as either early onset (occurring within 96 hours of start of mechanical ventilation) or late onset (>96 hours after start of mechanical ventilation). It is a common condition, difficult to diagnose accurately, and expensive to treat. Its development prolongs a patient's stay in the intensive care unit (ICU), and is associated with significant morbidity and mortality. Most cases seem to result from aspiration of pathogenic material that commonly colonises the oropharyngeal airways of the critically ill. Simple measures to decrease the incidence of aspiration or reduce the burden of colonisation of the oropharynx may aid in the prevention of ventilator associated pneumonia. A favourable outcome seems to be more likely if appropriate antibiotics are given in a timely manner.
Epidemiology
Accurate information concerning the epidemiology of VAP is lacking, as there is no universally accepted criteria for its diagnosis. Its incidence is also influenced by the case mix studied, and prior exposure to antibiotics. A one day point prevalence study designed to determine the prevalence of ICU acquired infection identified pneumonia as the most common infection with a prevalence of 10%, although this may not be wholly accurate as no strict diagnostic criteria were stipulated.1 However, the clinical course of up to 28% of patients receiving mechanical ventilation will be complicated by an episode of VAP and intubated patients have rates of pneumonia up to 21 times higher than patients without an artificial airway.2,3,4,5,6
The mortality attributable to VAP is difficult to quantify as it is influenced by many different factors including type of infecting organism, underlying comorbidity, severity of host response, and timing of onset. However, VAP does seem to be associated with a significantly higher risk of death. Of seven studies performed using matched controls, five have shown a significant increase in mortality attributable to VAP.2,7,8,9,10,11,12
The development of VAP prolongs the stay in the ICU and is associated with an increase in costs.13 A study by Heyland and colleagues to determine the excess ICU stay attributable to VAP prospectively matched patients with VAP to patients who did not develop clinically suspected pneumonia. They found that the development of VAP was associated with an average of 4.3 days longer in the ICU than control subjects.2 Other studies support these findings.14,15
Pathogenesis
Pneumonia represents the host's inflammatory response to the microbial invasion of the normally sterile lung parenchyma. The magnitude of this response depends on the size and type of the inoculum, the virulence of the organisms involved, and the competence of the host's immune system.
Most cases of VAP are caused by the aspiration of infected secretions from the oropharynx, although a small number of cases may result from direct bloodstream infection.16,17 Critical illness leads to the rapid colonisation of the oropharynx with potentially pathogenic bacteria caused by changes in host defences, previous antibiotic exposure, and changes in either the bacterial adhesins or host surface receptors.18 Aerobic Gram negative bacteria (AGNB) and Staphylococcus aureus rapidly replace normal flora. Other potential sources of infected material include the sinuses and dental plaque. It remains contentious whether the aspiration of infected material from the stomach plays an important part in the development of VAP.19,20,21 However, alkalinisation of the normally acid environment in the stomach leads to overgrowth with AGNB, providing a potential pool of infected material.22
The presence of the cuff on the tracheal tube does not prevent the passage of infected material into the airways.23 Contaminated secretions pool above the high volume low pressure cuff of the tracheal tube commonly used in ICU, and gain access to the trachea along folds in the cuff. These organisms can then gain access to and colonise the biofilm that rapidly coats the inner surface of the tracheal tube.24 This is commonly followed by colonisation of the trachea with pathogenic organisms. The infected material is then propelled into the distal airways by the inspiratory flow provided by the mechanical ventilator. Occasionally, contaminated nebulisers, ventilation circuits or humidifiers may be the source of the infected material.25
A variety of defence mechanisms exist that protect the lung from infection including non‐immune antimicrobial agents in saliva, an intact cough reflex, mucociliary clearance, and cell mediated and humoral immunity. Indeed, healthy adults frequently aspirate oropharyngeal secretions with impunity because of host defence mechanisms.26 However immune dysfunction is well reported in the critically ill and many of these host defence mechanisms are ineffective.27 When infected material reaches the distal airways, the immune mechanisms within the lung attempt to inactivate or kill the offending organism(s). Alveolar macrophages, neutrophils, and elements of the humoral immune system interact to mount an inflammatory response.28,29 If the host's immune system is overwhelmed, then pneumonia develops.
Acute lung injury and acute respiratory distress syndrome are commonplace in the critically ill and are associated with profound changes in the structure and functioning of the alveoli. Martin and colleagues showed that the function of neutrophils obtained from bronchoalveolar lavage fluid of patients with acute respiratory distress syndrome is significantly impaired.30 These changes impair the ability of the lungs defence mechanisms to deal with a bacterial challenge. Pulmonary oedema and alveolar haemorrhage also provide a favourable environment for the proliferation of bacteria.31
Risk factors
Multiple factors have been identified that increase the likelihood of developing VAP (see box).
Intubation is the most important risk factor for developing nosocomial pneumonia.32 Although it is difficult to differentiate between the risk imposed by the mechanical ventilator and its associated tubing and the presence of a tracheal tube, it is known that the incidence of VAP is less when non‐invasive ventilation is used.33,34
Risk factors for the development of ventilator associated pneumonia
Age ⩾60 years
Severity of illness (APACHE II score >16)
Acute or chronic lung disease
Excessive sedation
Enteral nutrition
Severe burns
Supine body position
Glasgow coma scale<9
Use of muscle relaxants
Cigarette smoking
Stress ulcer prophylaxis is routinely used in the critically ill. H2 blockers or the gastroprotective agent sucralfate form the mainstay of treatment. The use of H2 blockers is associated with a change in the acidity of the gastric juices that favours bacterial colonisation with Gram negative bacteria.35 However, the role of gastric pH in the pathogenesis of VAP is controversial. A large, multicentre, randomised, blinded placebo controlled trial involving 1200 mechanically ventilated patients in which sucralfate was compared with the H2 receptor antagonist ranitidine for the prevention of upper gastrointestinal bleeding failed to show any difference in the incidence of VAP.36 However, a number of other studies suggest that lower rates of VAP are seen in patients given a gastroprotective agent rather than agents that block gastric acid secretions.37,38 Despite these discordant results, H2 receptor antagonists are widely used and most clinicians believe that the use of these agents to prevent stress ulcer bleeding outweighs any additional risk of VAP.39
There is an increasing awareness of the importance of body position on the development of VAP.40 Drakulovic and colleagues randomly allocated intubated mechanically ventilated patients to be nursed in either the semi‐recumbent (45°) or the supine body position.41 Microbiologically confirmed pneumonia developed in significantly fewer patients nursed in the semi‐recumbent position (2 of 39 [5%]) than in those nursed supine (11 of 47 [23%], p = 0.018). The exact mechanism by which adoption of the semi‐recumbent position decreases the incidence of nosocomial pneumonia is not completely understood, but is probably related to the reduction in gastro‐oesophageal reflux seen in this position.
Unplanned or failed extubation followed by re‐intubation has been identified as a significant risk factor for the development of VAP.42 It is probable that aspiration of infected upper airway secretions occurs at the time of re‐intubation.
Nasal intubation, by blocking sinus outflow via the nasal ostia, is associated with a higher incidence of nosocomial sinusitis.43 However, it remains inconclusive whether nasal intubation is associated with a higher incidence of VAP. In a trial by Holzapfel and colleagues in which a total of 300 adult mechanically ventilated patients were randomised to either oro‐tracheal or nasal intubation, no statistically significant difference in the occurrence of VAP could be shown between the groups despite more sinusitis in the nasotracheal group.44
Enteral feeding via a nasogastric tube promotes gastro‐oesophageal reflux, the magnitude of which is unchanged by the use of fine bore tubes.45 It is also associated with an increase in gastric pH and colonisation of the stomach with AGNB.46 It is therefore unsurprising that enteral nutrition has been shown to be an independent risk factor for the development of VAP (odds ratio 5.7 (95% CI 1.5 to 22.8), p = 0.013).41 However, as adequate nutrition is essential in the critically ill and the enteral route is generally regarded as superior to parenteral nutrition, most clinicians advocate the commencement of early nasogastric feeding.47
Diagnosis of VAP
The accurate diagnosis of VAP remains problematic. Standard diagnostic features of pneumonia such as fever, tachycardia, leucocytosis, purulent sputum, and consolidation on the chest radiograph are unreliable in the critically ill mechanically ventilated patient. Fever, leucocytosis, and tachycardia are non‐specific findings and are seen in any critically unwell patient with an inflammatory response to an insult, for example, trauma, burns, pancreatitis, etc. Purulent sputum may be caused by tracheobronchitis and does not always signify parenchymal involvement.48 Infiltrates on the chest radiograph can be caused by a number of non‐infective conditions including pulmonary oedema, haemorrhage, and contusions.49 A study by Meduri and colleagues highlighted some of these difficulties. In a prospective study of 50 patients with fever and pulmonary infiltrates, only 42% had a definitive diagnosis of VAP.50 Disappointingly the use of scoring systems, such as the clinical pulmonary infection score, seems to add little to diagnostic accuracy.51
Nevertheless, the diagnosis of VAP is suspected if the patient has a new or progressive infiltrate on the chest radiograph accompanied by clinical findings suggestive of infection such as fever, leucocytosis, and purulent secretions. This is often accompanied by deterioration in gas exchange.
Because clinical suspicion alone is overly sensitive and lacks specificity, further diagnostic tests are required for optimal management. Ideally, microbiological data should be obtained before the start of antibiotic therapy.
Microbiological diagnosis
Despite numerous publications on the subject, controversy still exists on the optimal method of microbiological diagnosis of VAP.52 As the trachea and tracheal tube rapidly become colonised with bacteria in the critically ill patient, cultures of sputum or tracheal aspirates may simply yield colonising organisms. The argument therefore revolves around whether specimens of lower respiratory tract secretions should be collected in an invasive manner or whether quantitative analysis of non‐invasively collected tracheal aspirates is sufficient. Analysing samples using quantitative culture techniques theoretically permits differentiation between oropharyngeal organisms present at low concentrations and the higher concentrations of pathogenic organisms.
Blood cultures have limited value in the diagnosis of VAP and have a very low sensitivity for detecting the pathogenic organism responsible for the pneumonia. However, blood cultures are obviously useful in any patient with signs of sepsis, but the isolation of a micro‐organism in the blood does not confirm that micro‐organism as the pathogen responsible for VAP.53
A recently described immunological method for the diagnosis of VAP holds great promise for the future. The triggering receptor expressed on myeloid cells (TREM‐1) is a member of the immunoglobulin superfamily, and is involved in the acute inflammatory response. Neutrophils express high levels of TREM‐1 on exposure to infected tissues. Gibot and colleagues prospectively studied 148 mechanically ventilated patients with suspected VAP. A rapid immunoblot technique was used to measure soluble TREM‐1 in bronchoalveolar lavage fluid. They showed that the presence of soluble TREM‐1 in the bronchoalveolar lavage (BAL) fluid was a highly accurate method for the diagnosis of fungal or bacterial pneumonia with a sensitivity of 98% and a specificity of 90%.54
Quantitative cultures of tracheal aspirates
Collection of material for microbiological analysis using this technique is quick, simple, and widely available. While there is some evidence to suggest that the use of this method has a high false positive rate in the diagnosis of VAP, other studies suggest that quantitative analysis of tracheal aspirates offers a reliable alternative to invasive techniques.55,56,57 A prospective study by Sanchez‐Nieto and colleagues comparing quantitative analysis on non‐invasively collected tracheal aspirates with invasively collected respiratory samples in 51 patients with suspected VAP showed a high degree of concordance in bacteriological results and no difference in mortality.55 Another study involving 76 patients with suspected VAP, who were randomly allocated to either invasive or a non‐invasive diagnostic strategy, also showed that the invasive strategy had no benefit.58 Both studies used a threshold of 105 colony forming units/ml to distinguish tracheal colonisation from true VAP.
A non‐invasive strategy of diagnosis in those suspected of VAP seems to be associated with higher antibiotic use.59
Invasive techniques of sampling distal airways
Fibre optic bronchoscopy allows direct selective access to the distal airways for sampling. However, there are a number of problems associated with the use of bronchoscopy; the expertise and equipment required is not always available and sampling is often followed by a period of hypoxaemia.60 Accurate results also require rigorous microbiological analysis in laboratories with specialised equipment.
Whether an invasive approach changes outcome remains contentious. Three comparatively small single centre studies comparing mortality in patients with suspected VAP managed on the results obtained by either invasive studies or quantitative analysis of tracheal aspirates failed to find any difference in mortality.55,58,61 A much larger study involving 413 patients suspected of having VAP suggested that an invasive diagnostic strategy was desirable.59 Compared with patients who received clinical management, patients who received invasive management had reduced mortality rate at day 14 (16.2% and 25.8%, p = 0.02) and decreased antibiotic use (mean (SD) number of antibiotic free days, 5.0 (5.1) and 2.2 (3.5), p<0.001). Most recently a meta‐analysis of randomised, controlled trials of invasive diagnostic strategies in suspected VAP reported that an invasive approach did not change mortality (odds ratio 0.89, 95% confidence interval 0.56 to 1.41), but did change antibiotic use (odds ratio for change in antibiotic management after invasive sampling, 2.85, 95% confidence interval 1.45 to 5.59).62
Two techniques are commonly used to obtain distal airway samples with the bronchoscope; BAL or protected specimen brushing (PSB).
Bronchoalveolar lavage
BAL is performed by advancing the bronchoscope into a distal airway and instilling about 130–150 ml of sterile saline.63 Selection of the area of the lung for sampling is guided by the pattern of consolidation on the chest radiograph. As much of the saline a possible is aspirated and sent for quantitative culture. The most frequently used threshold for a positive culture is 104 colony forming units/ml. An examination of 23 studies evaluating the accuracy of BAL methods using fibreoptic bronchoscopy to diagnose VAP reported a mean (SD) sensitivity of 73% (18%) and a mean (SD) specificity of 82% (19%).63
Protected specimen brush
This technique was introduced in 1979 by Wiberley et al in an attempt to reliably obtain lower respiratory tract specimens that are not contaminated with tracheal or tracheal tube organisms.64 This technique entails the advancement of a double lumen catheter system under direct vision into the desired distal airway. Once in position the brush is advanced expelling the carbowax plug at the distal end of the catheter. Brushings are taken and the brush is then retracted into the catheter and removed, thereby protecting the brush from contamination. The brush is suspended in 1 ml of saline and quantitative culture obtained. A threshold of 103 colony forming units/ml is used to signify a positive result. Pooled data from 18 studies show that the diagnostic accuracy of this technique is high, with a sensitivity of 89% (95% confidence interval, 87% to 93%) and a specificity of 94% (95% confidence interval, 92% to 97%).65
Non‐bronchoscopic sampling of distal airways
BAL and PSB can both be performed without the aid of a bronchoscope. Non‐bronchoscopic BAL is performed by blindly advancing a protected suction catheter through the tracheal tube until it becomes wedged in a distal airway. The inner cannula is then advanced past its protective sheath and 100 ml of saline introduced and aspirated. Non‐bronchoscopic PSB is similarly performed by inserting a double lumen catheter through the tracheal tube until resistance is felt and then advancing the brush to obtain a specimen. The good concordance between microbiological data obtained by bronchoscopic and non‐bronchoscopic techniques shows the diffuse nature of parenchymal infection in those with VAP.66
Pathogens
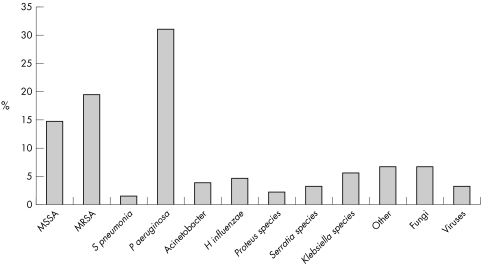
The organisms responsible for VAP vary according to case mix, institution, prior antibiotic exposure, local resistance patterns, and length of mechanical ventilation. Organisms responsible for early onset VAP are largely Staphylococcus aureus, Streptococcus pneumoniae, and Haemophilus influenzae while late onset VAP is often caused by resistant nosocomial pathogens such as Pseudomonas aeruginosa, methicillin resistant Staphylococcus aureus, Klebsiellaspecies, and Acinetobacter baumannii. Figure 1 shows the range of organisms responsible for VAP in an important recent study.67 The isolation of MRSA is more common in elderly patients, and those who have received prior antibiotic therapy.68
Figure 1 Summary of pathogens responsible for VAP in a study of 420 patients. Adapted from Cerra et al.47 MSSA, methicillin sensitive Straphylococcus aureus; MRSA, methicillin resistant Straphylococcus aureus.
Management of suspected VAP
Optimal management of the patient with suspected VAP requires prompt initiation of appropriate antimicrobial therapy and general supportive care. Although microbiological sampling should be performed before start of therapy, this must not delay commencement of antibiotic dosing.39,69 Several studies show that delay in administration of effective therapy is associated with an increase in mortality rate.70,71
Key references
Chastre J, Fagon JY. Ventilator‐associated pneumonia. Am J Respir Crit Care Med 2002;165:867–903.
Kollef MH. The prevention of ventilator‐associated pneumonia. N Engl J Med 1999;340:627–34.
Rello J, Artur Paiva J, Baraibar J, et al. International conference for the development of consensus on the diagnosis and treatment of ventilator‐associated pneumonia. Chest 2001;120:955–70.
Rello J, Diaz E. Pneumonia in the intensive care unit. Crit Care Med 2003;31:2544–51.
Hubmayr RD, Burchardi H, Elliot M, et al. American Thoracic Society Assembly on Critical Care; European Respiratory Society; European Society of Intensive Care Medicine; Societe de Reanimation de Langue Francaise. Statement of the 4th international consensus conference in critical care on ICU‐acquired pneumonia—Chicago, Illinois, May 2002. Intensive Care Med 2002;28:1521–36.
A detailed discussion of appropriate antibiotic regimens is beyond the scope of this article and each ITU should tailor its own regimens to the local microbial epidemiology and susceptibility patterns. This requires close liaison with the hospitals' microbiology service. However, because of the plethora of potential causative organisms a broad spectrum antibiotic should be given initially with activity against enteric Gram negative organisms. In patients with early onset VAP who have not previously received antibiotic therapy, monotherapy with a third generation cephalosporin (for example, cefotaxime) is a reasonable choice. For patients who develop VAP after prolonged mechanical ventilation and prior antibiotic exposure, a combination of antibiotics is required to ensure adequate coverage of potential pathogens. Piperacillin/tazobactam (Tazocin) with a combination of either aminoglycoside or ciprofloxacillin is the preferred choice for empiric therapy.52 A prospective study of 156 patients with clinically suspected VAP showed that those who received initial empiric therapy with antipseudomonal penicillins plus β lactamase inhibitor had lower in‐hospital mortality compared with those who were not treated with these antibiotics. There was also a strong trend towards reduced mortality rates when gentamicin was given as part of the antibiotic regimen.72 Alternatively, an antipseudomonal carbapenem (for example, Meropenem) or an antipseudomonal cephalosporin (for example, Ceftazidime) can be given with either a fluoroquinolone or an aminoglycoside.4 The aminoglycosides should not be used as monotherapy as lung penetration is poor. An antibiotic with activity against MRSA should also form part of the antibiotic regimen if MRSA is a possibility. This is especially probable in those patients who have received prior antibiotic therapy.68 Although the glycopeptide antibiotics teicoplanin and vancomycin form the mainstay of therapy against nosocomial pneumonia attributable to MRSA, there is limited evidence to suggest that the newer agent linezolid is associated with significantly better survival and clinical cure rates than vancomycin in patients with VAP.73
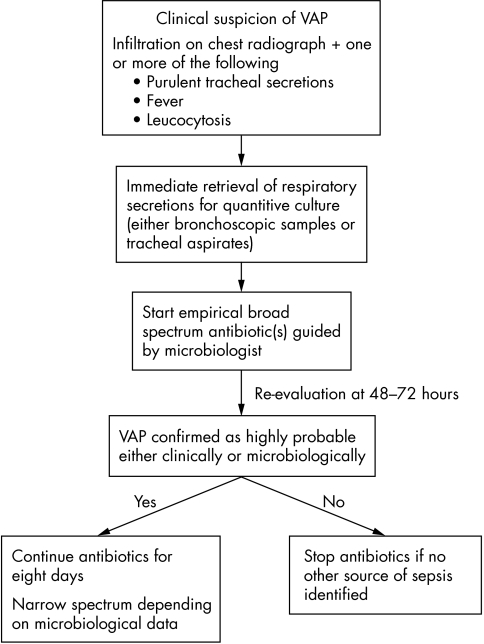
Inappropriate or inadequate therapy is associated with excess mortality, and longer length of stay.74 Subsequent culture data from microbiological samples should be used to tailor antibiotic therapy when it becomes available.75 Figure 2 outlines a straightforward algorithm for the management of suspected VAP.76
Figure 2 A simplified strategy for the management of suspected VAP. Adapted from Torres et al.74
The optimal duration of antimicrobial treatment for VAP is unknown. In a randomised, prospective clinical trial of over 400 patients 8 and 15 days of antibiotic therapy was compared for VAP. This study showed comparable clinical effectiveness with the 8 and 15 day treatment regimens. In particular, there was no excess mortality or increase in microbiologically reported pulmonary infection recurrence in the 8 day group.77
Prevention of VAP
There are several measures that can be taken to reduce the incidence of VAP. Strict hand washing and the use of protective gowns and gloves should be routinely used in the ICU to minimise nosocomial infections.17,78 Patients should be nursed in the semi‐recumbent position (45° angle), gastric distension avoided, and there should not be any unnecessary changes of the ventilator circuit. Nasal intubation should be discouraged and there is increasing evidence to suggest that performance of an early tracheostomy in patients expected to require prolonged mechanical ventilation is beneficial. In a recent study of 120 patients expected to require mechanical ventilation for longer than 14 days, those that were randomised to receive percutaneous dilatational tracheostomy within 48 hours of admission had a significantly lower incidence of VAP than those who received a tracheostomy after 14–16 days (5% compared with 25%, p<0.005).79 Daily interruption of sedative drug infusions has been shown to shorten the duration of mechanical ventilation.80 There is some evidence to suggest that prophylactic parenteral antibiotics may be useful in the prevention of VAP, especially those with a significantly obtunded conscious level.81
Many ICUs are introducing “ventilator bundles”, which are a group of interventions related to patients receiving mechanical ventilation that, when implemented together, result in better outcomes than when implemented individually. Some of the key components of the bundles such a positioning of the patient and avoidance of heavy sedation may reduce the incidence of VAP.
Summary
VAP continues to be an important challenge to the critical care physician and is the most common nosocomial acquired infection among patients with acute respiratory failure. It is difficult to diagnose accurately, and a high index of suspicion is required. If VAP is suspected empirical antibiotics should be given immediately. Although bacteriological sampling is important, it should not significantly delay the start of treatment. As the appropriateness of the initial antibiotic regimen is a vital determinant of outcome, microbiological advice should be sought. There is an increasing prevalence of MRSA and multidrug resistant pathogens in late onset VAP, and antimicrobial therapy should take account of this. Subsequent microbiological findings should be used to tailor antibiotic therapy.
Several, easy to implement strategies have been identified that prevent VAP and these should be used in all ventilated patients. The introduction of “ventilator bundles” may facilitate this.
Multiple choice questions (true (T)/false (F); answers at the end of the references)
-
Which of the following statements is true regarding ventilator associated pneumonia
VAP is the most commonly observed nosocomial infection in the intensive care unit
There are universally accepted diagnostic criteria
The development of VAP has no influence on patient length of stay
In excess of 25% of patients receiving intubation and mechanical ventilation will develop VAP
-
Regarding the pathogenesis of VAP;
VAP is normally caused by haematogenous spread of organisms
A well inflated tracheal tube cuff will reliably prevent aspiration
The aspiration of infected material from the stomach plays an important part in the development of VAP
Acute lung injury does not predispose the patient to the development of VAP
-
The following are confirmed risk factors for the development of VAP
Nursing the patient in a supine position
Enteral feeding
The use of sucralfate as stress ulcer prophylaxis
Obtunded conscious level at the time of intubation
-
Regarding the diagnosis of VAP, which of the following statements are true;
Microbiological specimens should be processed in a non‐quantitative manner
A specimen obtained by bronchoalveolar lavage containing >103 colony forming units/ml signifies invasive infection
Blood cultures have a high sensitivity for detecting the organisms responsible for VAP
A bronchoscope is always required to sample the distal airways
-
The optimal management of VAP always requires;
Identification of the responsible pathogen before the start of antibiotic treatment
An antifungal agent as part of the antibiotic regimen
Close liaison with microbiology service
A microbiological sample obtained from the distal airways
Abbreviations
VAP - ventilator acquired pneumonia
ICU - intensive care unit
AGNB - aerobic Gram negative bacteria
TREM‐1 - triggering receptor expressed on myeloid cells
BAL - bronchoalveolar lavage
PSB - protected specimen brushing
ANSWERS
1. (A) T, (B) F, (C) F, (D) T; 2. (A) F, (B) F, (C) F, (D) F; 3. (A) T, (B) T, (C) F, (D) T; 4. (A) F, (B) F, (C) F, (D) F; 5. (A) F, (B) F, (C) T, (D) F.
Footnotes
Funding: none.
Conflicts of interest: none declared.
References
- 1.Vincent J L, Bihari D J, Suter P M.et al The prevalence of nosocomial infection in intensive care units in Europe. Results of the European prevalence of infection in intensive care (EPIC) study. JAMA 1995274639–644. [PubMed] [Google Scholar]
- 2.Heyland D K, Cook D J, Griffith L.et al The attributable morbidity and mortality of ventilator‐associated pneumonia in the critically ill patient. The Canadian Critical Trials Group. Am J Respir Crit Care Med 19991591249–1256. [DOI] [PubMed] [Google Scholar]
- 3.Celis R, Torres A, Gatell J M.et al Nosocomial pneumonia. A mulitivariate analysis of risk and prognosis. Chest 198893318–324. [DOI] [PubMed] [Google Scholar]
- 4.Chastre J, Fagon J Y. Ventilator‐associated pneumonia. Am J Respir Crit Care Med 2002165867–903. [DOI] [PubMed] [Google Scholar]
- 5.Haley R W, Hooton T M, Culver T H.et al Nosocomial infection in US hospitals 1975–76. Estimated frequency by selected characteristics of patients. Am J Med 198170947–959. [DOI] [PubMed] [Google Scholar]
- 6.Chevret S, Hemmer M, Carlet J.et al Incidence and risk factors of pneumonia acquired in intensive care units. Results from a multi‐centre prospective study on 996 patients. European Cooperative Group on Nosocomial Pneumonia. Intensive Care Med 199319256–264. [DOI] [PubMed] [Google Scholar]
- 7.Fagon J Y, Chastre A J, Hance A J.et al Nosocomial pneumonia in ventilated patients: a cohort study evaluating attributable mortality and hospital stay. Am J Med 199394281–288. [DOI] [PubMed] [Google Scholar]
- 8.Bercault N, Boulain T. Mortality rate attributable to ventilator‐associated nosocomial pneumonia in an adult intensive care unit: a prospective case‐control study. Crit Care Med 2001292303–2309. [DOI] [PubMed] [Google Scholar]
- 9.Craig C P, Connelly S. Effect of intensive care unit nosocomial pneumonia on duration of stay and mortality. Am J Infect Control 198412233–238. [DOI] [PubMed] [Google Scholar]
- 10.Baker A M, Meredith J W, Haponik E F. Pneumonia in intubated trauma patients. Microbiology and outcome. Am J Respir Crit Care Med 1996153343–349. [DOI] [PubMed] [Google Scholar]
- 11.Cunnion K M, Weber D J, Broadhead W E.et al Risk factors for nosocomial pneumonia: comparing adult critical care populations. Am J Respir Crit Care Med 1996153158–162. [DOI] [PubMed] [Google Scholar]
- 12.Papazian L, Bregeon F, Thirion X.et al Effect of ventilator‐associated pneumonia on mortality and morbidity. Am J Respir Crit Care Med 199615491–97. [DOI] [PubMed] [Google Scholar]
- 13.Pinner R W, Haley R W, Blumenstein B A.et al High cost nosocomial infections. Infect Control 19823143–149. [DOI] [PubMed] [Google Scholar]
- 14.Craig C P, Connelly S. Effect of intensive care unit nosocomial pneumonia on duration of stay and mortality. Am J Infect Control 198412233–238. [DOI] [PubMed] [Google Scholar]
- 15.Kappstein I, Schulgen G, Beyer U.et al Prolongation of hospital stay and extra costs due to ventilator‐associated pneumonia in an intensive care unit. Eur J Clin Microbiol Infect Dis 199211504–508. [DOI] [PubMed] [Google Scholar]
- 16.Estes R J, Meduri G U. The pathogenesis of ventilator‐associated pneumonia: I. Mechanisms of bacterial transcolonization and airway inoculation. Intensive Care Med 199521365–383. [DOI] [PubMed] [Google Scholar]
- 17.Kollef M H. The prevention of ventilator‐associated pneumonia. N Engl J Med 1999340627–634. [DOI] [PubMed] [Google Scholar]
- 18.Garrouste‐Orgeas M, Chevret S, Arlet G.et al Oropharyngeal or gastric colonization and nosocomial pneumonia in adult intensive care unit patients. A prospective study based on genomic DNA analysis. Am J Respir Crit Care Med 19971561647–1655. [DOI] [PubMed] [Google Scholar]
- 19.Bonten M J, Gaillard C A, van Tiel F H.et al The stomach is not a source for colonization of the upper respiratory tract and pneumonia in ITU patients. Chest 1994105878–884. [DOI] [PubMed] [Google Scholar]
- 20.Craven D E, Steger K A, Barber T W. Preventing nosocomial pneumonia: state of the art and perspectives for the 1990's. Am J Med 19919144–53. [DOI] [PubMed] [Google Scholar]
- 21.Du Moulin G C, Paterson D G, Hedley‐Whyte J.et al Aspiration of gastric bacteria in antacid‐treated patients: a frequent cause of postoperative colonisation of the airway. Lancet 1982i242–245. [DOI] [PubMed]
- 22.Donowitz L G, Page M C, Mileur B L.et al Alterations of normal gastric flora in critical care patients receiving antacid and ciimetidine therapy. Infect Control 1986723–26. [DOI] [PubMed] [Google Scholar]
- 23.Seegobin R D, van Hasselt G L. Aspiration beyond endotracheal cuffs. Can Anaesth Soc J 198633273–279. [DOI] [PubMed] [Google Scholar]
- 24.Sottile F D, Marrie T J, Prough D S.et al Nosocomial pulmonary infection: possible etiological significance of bacterial adhesion to endotracheal tubes. Crit Care Med 198614265–270. [PubMed] [Google Scholar]
- 25.Craven D E, Lichtenberg D A, Goularte T A.et al Contaminated medication nebulizers in mechanical ventilator circuits. Source of bacterial aerosols. Am J Med 198477834–838. [DOI] [PubMed] [Google Scholar]
- 26.Huxley E J, Viroslav J, Gray W R.et al Pharyngeal aspiration in normal adults and patients with depressed consciousness. Am J Med 197864564–568. [DOI] [PubMed] [Google Scholar]
- 27.Hunter J D. Effects of anaesthesia on the human immune system. Hospital Medicine 199960658–663. [DOI] [PubMed] [Google Scholar]
- 28.Meduri G U, Estes R J. The pathogenesis of ventilator‐associated pneumonia: II. The lower respiratory tract. Intensive Care Med 199521452–461. [DOI] [PubMed] [Google Scholar]
- 29.Young P J, Ridley S A. Ventilator‐associated pneumonia. Anaesthesia 1999541183–1197. [DOI] [PubMed] [Google Scholar]
- 30.Martin T R, Pistorese B P, Hudson L D.et al The function of lung and blood neutrophils in patients with the adult respiratory distress syndrome. Implications for the pathogenesis of lung infections. Am Rev Respir Dis 1991144254–262. [DOI] [PubMed] [Google Scholar]
- 31.LaForce F M, Mullane J F, Boehme R F.et al The effect of pulmonary edema on antibacterial defenses of the lung. J Lab Clin Med 197382634–638. [PubMed] [Google Scholar]
- 32.Rello J, Diaz E. Pneumonia in the intensive care unit. Crit Care Med 2003312544–2551. [DOI] [PubMed] [Google Scholar]
- 33.Guerin C, Girard R, Chemorin C.et al Facial mask noninvasive mechanical ventilation reduces the incidence of nosocomial pneumonia. A prospective epidemiological survey from a single ICU. Intensive Care Med 1997231024–1032. [DOI] [PubMed] [Google Scholar]
- 34.Nourdine K, Combes P, Carton M J.et al Does noninvasive ventilation reduce the ICU nosocomial infection risk? A prospective clinical survey. Intensive Care Med 199925567–573. [DOI] [PubMed] [Google Scholar]
- 35.Hillman K M, Riordan T, O'Farrell S M.et al Colonization of the gastric contents in critically ill patients. Crit Care Med 198210444–447. [DOI] [PubMed] [Google Scholar]
- 36.Cook D, Guyatt G, Marshall J.et al A comparison of sucralfate and ranitidine for the prevention of upper gastrointestinal bleeding in patients requiring mechanical ventilation. Canadian Critical Care Trials Group. N Engl J Med 1998338791–797. [DOI] [PubMed] [Google Scholar]
- 37.Prod'hom G, Leuenberger P, Koerfer J.et al Nosocomial pneumonia in mechanically ventilated patients receiving antacid, ranitidine, or sucralfate as prophylaxis for stress ulcer. A randomized controlled trial. Ann Intern Med 1994120653–662. [DOI] [PubMed] [Google Scholar]
- 38.Driks M R, Craven D E, Celli B R.et al Nosocomial pneumonia in intubated patients given sucralfate as compared with antacids or histamine type 2 blockers. The role of gastric colonization N Engl J Med 19873171376–1382. [DOI] [PubMed] [Google Scholar]
- 39.Hubmayr R D, Burchardi H, Elliot M.et al American Thoracic Society Assembly on Critical Care; European Respiratory Society; European Society of Intensive Care Medicine; Societe de Reanimation de Langue Francaise. Statement of the 4th international consensus conference in critical care on ICU‐acquired pneumonia—Chicago, Illinois, May 2002. Intensive Care Med 2002281521–1536. [DOI] [PubMed] [Google Scholar]
- 40.Webster N R. Importance of position in which patients are nursed in intensive‐care units. Lancet 19993541835–1836. [DOI] [PubMed] [Google Scholar]
- 41.Drakulovic M B, Torres A, Bauer T T.et al Supine body position as a risk factor for nosocomial pneumonia in mechanically ventilated patients: a randomised trial. Lancet 19993541851–1858. [DOI] [PubMed] [Google Scholar]
- 42.Torres A, Gatell J M, Aznar E.et al Re‐intubation increases the risk of nosocomial pneumonia in patients needing mechanical ventilation. Am J Respir Crit Care Med 1995152137–141. [DOI] [PubMed] [Google Scholar]
- 43.Rouby J J, Laurent P, Gosnach M.et al Risk factors and clinical relevance of nosocomial maxillary sinusitis in the critically ill. Am J Respir Crit Care Med 19943776–783. [DOI] [PubMed] [Google Scholar]
- 44.Holzapfel L, Chevret S, Madinier G.et al Influence of long‐term oro‐ or nasotracheal intubation on nosocomial maxillary sinusitis and pneumonia: results of a prospective, randomized, clinical trial. Crit Care Med 1993211132–1138. [DOI] [PubMed] [Google Scholar]
- 45.Ferrer M, Bauer T T, Torres A.et al Effect of nasogastric tube size on gastroesophageal reflux and microaspiration in intubated patients. Ann Intern Med 1999130991–994. [DOI] [PubMed] [Google Scholar]
- 46.Bonten M J, Gaillard C A, van der Hulst R.et al Intermittent enteral feeding: the influence on respiratory and digestive tract colonization in mechanically ventilated intensive‐care‐unit patients. Am J Respir Crit Care Med 1996154394–399. [DOI] [PubMed] [Google Scholar]
- 47.Cerra F B, Benitez M R, Blackburn G L.et al Applied nutrition in ICU patients. A consensus statement of the American College of Chest Physicians. Chest 1997111769–778. [DOI] [PubMed] [Google Scholar]
- 48.Nseir S, Di Pompeo C, Pronnier P.et al Nosocomial tracheobronchitis in mechanically ventilated patients: incidence, aetiology and outcome. Eur Respir J 2002201483–1489. [DOI] [PubMed] [Google Scholar]
- 49.Wunderink R G. Radiologic diagnosis of ventilator‐associated pneumonia Chest2000117188–90S. [DOI] [PubMed] [Google Scholar]
- 50.Meduri G U, Mauldin G L, Wunderink R G.et al Causes of fever and pulmonary densities in patients with clinical manifestations of ventilator‐associated pneumonia. Chest 1994106221–235. [DOI] [PubMed] [Google Scholar]
- 51.Fabregas N, Ewig S, Torres A.et al Clinical diagnosis of ventilator associated pneumonia revisited: comparative validation using immediate post‐mortem lung biopsies. Thorax 199954867–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jordi Rello, Jose Artur Paiva, Jorge Baraibar et al International conference for the development of consensus on the diagnosis and treatment of ventilator‐associated pneumonia. Chest 2001120955–970. [DOI] [PubMed] [Google Scholar]
- 53.Luna C M, Videla A, Mattera J.et al Blood cultures have limited value in predicting severity of illness and as a diagnostic tool in ventilator‐associated pneumonia. Chest 19991161075–1084. [DOI] [PubMed] [Google Scholar]
- 54.Gibot S, Cravoisy A, Levy B.et al Soluble triggering receptor expressed on myeloid cells and the diagnosis of pneumonia. N Engl J Med 2004350451–458. [DOI] [PubMed] [Google Scholar]
- 55.Sanchez‐Nieto J M, Torres A, Garcia‐Cordoba F.et al Impact of invasive and noninvasive quantitative culture sampling on outcome of ventilator‐associated pneumonia. Am J Respir Crit Care Med 1998157371–376. [DOI] [PubMed] [Google Scholar]
- 56.Marquette C H, Copin M C, Wallet F.et al Diagnostic tests for pneumonia in ventilated patients: prospective evaluation of diagnostic accuracy using histology as a diagnostic gold standard. Am J Respir Crit Care Med 19951511878–1888. [DOI] [PubMed] [Google Scholar]
- 57.Sauaia A, Moore F A, Moore E E.et al Diagnosing pneumonia in mechanically ventilated trauma patients: endotracheal aspirate versus bronchoalveolar lavage. J Trauma 199335512–517. [DOI] [PubMed] [Google Scholar]
- 58.Ruiz M, Torres A, Ewig S.et al Noninvasive versus invasive microbial investigation in ventilator‐associated pneumonia: evaluation of outcome. Am J Respir Crit Care Med 2000162119–125. [DOI] [PubMed] [Google Scholar]
- 59.Fagon J Y, Chastre J, Wolff M.et al Invasive and noninvasive strategies for management of suspected ventilator‐associated pneumonia. A randomized trial. Ann Intern Med 2000132621–630. [DOI] [PubMed] [Google Scholar]
- 60.Guerra L F, Baughman R P. Use of bronchoalveolar lavage to diagnose bacterial pneumonia in mechanically ventilated patients. Crit Care Med 199018169–173. [DOI] [PubMed] [Google Scholar]
- 61.Sole Violan J, Fernandez J A, Benitez A B.et al Impact of quantitative invasive diagnostic techniques in the management and outcome of mechanically ventilated patients with suspected pneumonia. Crit Care Med 2000282737–2741. [DOI] [PubMed] [Google Scholar]
- 62.Shorr A F, Sherner J H, Jackson W L.et al Invasive approaches to the diagnosis of ventilator‐associated pneumonia: a meta‐analysis. Crit Care Med 20053346–53. [DOI] [PubMed] [Google Scholar]
- 63.Torres A, El‐Ebiary M. Bronchoscopic BAL in the diagnosis of ventilator‐associated pneumonia. Chest 2000117198–202S. [DOI] [PubMed] [Google Scholar]
- 64.Wimberley N, Faling L J, Bartlett J G. A fiberoptic bronchoscopy technique to obtain uncontaminated lower airway secretions for bacterial culture. Am Rev Respir Dis 1979119337–343. [DOI] [PubMed] [Google Scholar]
- 65.Cook D J, Fitzgerald J M, Guyatt G H.et al Evaluation of the protected brush catheter and bronchoalveolar lavage in the diagnosis of nosocomial pneumonia. J Intensive Care Med 19916196–205. [DOI] [PubMed] [Google Scholar]
- 66.Wearden P D, Chendrasekhar A, Timberlake G A. Comparison of nonbronchoscopic techniques with bronchoscopic brushing in the diagnosis of ventilator‐associated pneumonia. J Trauma 199641703–707. [DOI] [PubMed] [Google Scholar]
- 67.Ibrahim E H, Ward S, Sherman G.et al A comparative analysis of patients with early‐onset vs late‐onset nosocomial pneumonia in the ICU setting. Chest 20001171434–1442. [DOI] [PubMed] [Google Scholar]
- 68.Rello J, Torres A, Ricart M.et al Ventilator‐associated pneumonia by Staphylococcus aureus. Comparison of methicillin‐resistant and methicillin‐sensitive episodes. Am J Respir Crit Care Med 19941501545–1549. [DOI] [PubMed] [Google Scholar]
- 69.Mehta R, Niederman M S. Adequate empirical therapy minimizes the impact of diagnostic methods in patients with ventilator‐associated pneumonia. Crit Care Med 2000283092–3094. [DOI] [PubMed] [Google Scholar]
- 70.Iregui M, Ward S, Sherman G.et al Clinical importance of delays in the initiation of appropriate antibiotic treatment for ventilator‐associated pneumonia. Chest 2002122262–268. [DOI] [PubMed] [Google Scholar]
- 71.Luna C M, Vujacich P, Niederman M S.et al Impact of BAL data on the therapy and outcome of ventilator‐associated pneumonia. Chest 1997111676–685. [DOI] [PubMed] [Google Scholar]
- 72.Fowler R A, Flavin K E, Barr J.et al Variability in antibiotic prescribing patterns and outcomes in patients with clinically suspected ventilator‐associated pneumonia. Chest 2003123835–844. [DOI] [PubMed] [Google Scholar]
- 73.Wunderink R G, Rello J, Cammarata S K.et al Analysis of two double‐blind studies of patients with methicillin‐resistant Staphylococcus aureus nosocomial pneumonia. Chest 20031241789–1797. [PubMed] [Google Scholar]
- 74.Kollef M H. Inadequate antimicrobial treatment: an important determinant of outcome for hospitalized patients. Clin Infect Dis 200031S131–S138. [DOI] [PubMed] [Google Scholar]
- 75.Ibrahim E H, Ward S, Sherman G.et al Experience with a clinical guideline for the treatment of ventilator‐associated pneumonia. Crit Care Med 2001291109–1115. [DOI] [PubMed] [Google Scholar]
- 76.Torres A, Ewig S. Diagnosing ventilator‐associated pneumonia. N Engl J Med 2004350433–435. [DOI] [PubMed] [Google Scholar]
- 77.Chastre J, Wolff M, Fagon J Y.et al PneumA Trial Group. Comparison of 8 vs 15 days of antibiotic therapy for ventilator‐associated pneumonia in adults: a randomized trial, JAMA 20032902588–2598. [DOI] [PubMed] [Google Scholar]
- 78.Kollef M H. Prevention of hospital‐associated pneumonia and ventilator‐associated pneumonia. Crit Care Med 2004321396–1405. [DOI] [PubMed] [Google Scholar]
- 79.Rumbak M J, Newton M, Truncale T.et al A prospective, randomized, study comparing early percutaneous dilational tracheotomy to prolonged translaryngeal intubation (delayed tracheotomy) in critically ill medical patients. Crit Care Med 2004321689–1694. [DOI] [PubMed] [Google Scholar]
- 80.Kress J P, Pohlman A S, O'Connor M F.et al Daily interruption of sedative infusions in critically ill patients undergoing mechanical ventilation. N Engl J Med 20003421471–1477. [DOI] [PubMed] [Google Scholar]
- 81.Sirvent J M, Torres A, El‐Ebiary M.et al Protective effect of intravenously administered cefuroxime against nosocomial pneumonia in patients with structural coma. Am J Respir Crit Care Med 19971551729–1734. [DOI] [PubMed] [Google Scholar]