Abstract
Coatomer is the soluble precursor of the COPI coat (coat protein I) involved in traffic among membranes of the endoplasmic reticulum and the Golgi apparatus. We report herein that neomycin precipitates coatomer from cell extracts and from purified coatomer preparations. Precipitation first increased and then decreased as the neomycin concentration increased, analogous to the precipitation of a polyvalent antigen by divalent antibodies. This suggested that neomycin cross-linked coatomer into large aggregates and implies that coatomer has two or more binding sites for neomycin. A variety of other aminoglycoside antibiotics precipitated coatomer, or if they did not precipitate, they interfered with the ability of neomycin to precipitate. Coatomer is known to interact with a motif (KKXX) containing adjacent lysine residues at the carboxyl terminus of the cytoplasmic domains of some membrane proteins resident in the endoplasmic reticulum. All of the antibiotics that interacted with coatomer contain at least two close amino groups, suggesting that the antibiotics might be interacting with the di-lysine binding site of coatomer. Consistent with this idea, di-lysine itself blocked the interaction of antibiotics with coatomer. Moreover, di-lysine and antibiotics each blocked the coating of Golgi membranes by coatomer. These data suggest that certain aminoglycoside antibiotics interact with di-lysine binding sites on coatomer and that coatomer contains at least two of these di-lysine binding sites.
INTRODUCTION
The transfer of components from one membrane compartment to another during membrane trafficking occurs when a vesicle buds from a donor membrane and then fuses with a specific target membrane. Budding almost always involves a protein complex that coats the membrane at the site of bud formation and envelops the nascent vesicle. The coat eventually dissociates from the mature vesicle, leaving an exposed membrane that can fuse with a target. Different vesicles are coated with different protein complexes depending on the pathway of membrane traffic (Kreis and Pepperkok, 1994; Barlowe, 1995; Harter, 1995; Rothman and Wieland, 1996; Schekman and Orci, 1996). Two types of coat protein complexes have been described for the secretory pathway, COPI and COPII (coat protein), differing in their protein subunits. Coatomer, the soluble form of COPI, is the subject of this report and contains seven subunits (α, β, β′, γ, δ, ε, and ζ) that as a unit assemble on membranes and disassociate from vesicles in a cycle that involves the GTP-binding protein adenosine diphosphate-ribosylation factor 1 (ARF1; Malhotra et al., 1989; Waters et al., 1991; Orci et al., 1993; Ostermann et al., 1993). ARF1-GDP in the cytosol is recruited to a membrane by a mechanism dependent on a guanine nucleotide exchange factor that catalyzes exchange of GDP for GTP. Membrane binding of ARF1-GTP activates phospholipase D (Ktistakis et al., 1995, 1996) and ultimately results by an unknown mechanism in the binding of coatomers to the membrane and formation of a COPI-coated vesicle. For a COPI-coated vesicle to uncoat, ARF1 hydrolyzes GTP, assisted by a GTPase activating factor (Cukierman et al., 1995; Makler et al., 1995).
Vesicles coated with COPI bud from Golgi membranes and from the endoplasmic reticulum (ER; Ostermann et al., 1993; Bednarek et al., 1995). The functions of COPI-coated vesicles are not entirely understood, but it has been suggested that they operate in both retrograde and anterograde transport between the Golgi complex and the ER (Fiedler et al., 1996; Rothman and Wieland, 1996; Schekman and Orci, 1996). Evidence for a function in retrograde transport is particularly strong and includes the observation that coatomer binds the motif KKXX (where K is lysine and X is any amino acid), and closely related sequences, at the carboxyl end of the cytoplasmic domains of many ER-resident membrane proteins (Cosson and Letourneur, 1994). The KKXX motif is a retrieval signal for retrograde transport back to the ER for many ER-resident membrane proteins that have escaped to the Golgi complex (Jackson et al., 1990, 1993). Moreover, mutations in three coatomer subunits in yeast, α-COP, β′-COP, and γ-COP, result in defective retrograde transport of membrane proteins displaying the KKXX signal (Letourneur et al., 1994). It has also been shown that a partial coatomer complex containing α-COP, β′-COP, and ε-COP interacts with KKXX-containing proteins in vitro (Cosson and Letourneur, 1994; Lowe and Kreis, 1995).
We report herein that certain aminoglycoside antibiotics (e.g., neomycin) precipitate coatomer in solution, strongly suggesting that they bind to and cross-link coatomer. The properties of the precipitation reaction imply that coatomer has two or more binding sites for the antibiotics. Several other aminoglycoside antibiotics that do not themselves precipitate coatomer (e.g., Geneticin), nevertheless inhibit the precipitation by neomycin. Antibiotics that interact with coatomer also block the binding of coatomer to Golgi membranes. A structural feature in common among the antibiotics that interact with coatomer is one or more clusters of two amino groups that could be mimicking the di-lysine component of the KKXX signal. We found that di-lysine itself blocked the interaction of selected antibiotics with coatomer, suggesting that the antibiotics interact with di-lysine binding sites on coatomer.
MATERIALS AND METHODS
Materials
HEPES, sucrose, magnesium acetate, KCl, ATP, GTP, creatine phosphate, creatine phosphokinase, palmitoyl CoA, protease inhibitors, neomycin, paromomycin, lividomycin, sisomicin, hygromycin B, gentamicin, Geneticin, di-lysine, and horseradish peroxidase conjugated to either goat anti-mouse IgG or to protein A were purchased from Sigma (St. Louis, MO). Neamine was from Biomol Research Laboratories (Plymouth Meeting, PA). 2-Deoxystreptamine was a gift from Dr. Julian Davies (University of British Columbia, Vancouver, British Columbia, Canada). Anti-β-COP monoclonal antibody M3A5 was obtained from the supernatant of hybridoma cells. The cells were provided by Dr. Thomas Kreis (Université de Genéve, Geneva, Switzerland). Anti-β′-COP antibodies raised against a β′-COP peptide were also provided by Dr. Kreis (Lowe and Kreis, 1995). Purified bovine coatomer (Waters et al., 1992) was the generous gift of Dr. Gerard Waters (Princeton University).
Preparation of Golgi and Cytosol
Chinese hamster ovary (CHO) cells were grown in culture as previously described (Kao and Draper, 1992; Bau and Draper, 1993). Golgi-enriched membranes and cytosol from CHO cells were prepared as described by Balch et al. (1984) with minor modifications. Briefly, 20 plates (15 cm in diameter) of CHO cells were grown to confluency and trypsinized. The cells were isolated by centrifugation, washed once with PBS and once with homogenization buffer (25 mM HEPES, pH 7.4, and 250 mM sucrose) and resuspended in five times the cell pellet volume with ice-cold homogenization buffer. The cells were homogenized on ice with a 15-ml stainless-steel Dounce homogenizer. The homogenate was then split into two parts, one for making cytosol and the other for making Golgi membranes. To prepare cytosol, the homogenate was centrifuged at 100,000 × g for 1 h at 4°C in a Beckman SW50.1 rotor. The supernatants were pooled and recentrifuged under the same conditions. The final supernatant was then desalted over a Sephadex G-25 column by using homogenization buffer. To prepare Golgi membranes, the homogenate was brought to 1.4 M sucrose, 1 mM EDTA, and 25 mM HEPES, pH 7.4. The homogenate was placed in centrifuge tubes and overlaid with 5 ml of 1.2 M and 5 ml of 0.8 M sucrose, both in 25 mM HEPES, pH 7.4. This was then centrifuged in a Beckman SW28.1 rotor for 16 h at 25,000 rpm at 4°C. The 1.2 M/0.8 M sucrose interface, which contained the Golgi-enriched membranes, was collected and pooled. Protein was quantitated by the method of Bradford (1976).
Precipitation of Coatomer by Antibiotics and Immunodetection of β-COP and β′-COP
CHO cell cytosol was incubated with the compounds indicated in the figures for 2 h at 4°C in a 100-μl volume of reaction buffer containing 25 mM HEPES, pH 7.4, 50 mM KCl, and 2.5 mM Mg(OAc)2 at a final cytosol protein concentration of 1.5 mg/ml. The mixture was centrifuged at 100,000 × g in a Beckman TLA120.1 rotor for 30 min at 4°C to separate supernatants from precipitates. The pellets were suspended in 100 μl of sample buffer and equivalent volumes of the supernatant and suspended pellet were electrophoresed in a 7% polyacrylamide gel with SDS. Proteins were transferred to nitrocellulose by electrophoresis and the nitrocellulose was incubated with 0.1% Tween 20 as a blocking agent, followed by incubation with either monoclonal antibody M3A5 to β-COP or with the rabbit antiserum raised against a β′-COP peptide (Lowe and Kreis, 1995). The secondary antibody for detecting β-COP was horseradish peroxidase-conjugated goat anti-mouse IgG, and horseradish peroxidase-conjugated protein A was used to detect β′-COP. The blots were developed with the Pierce Biochemical Supersignal ECL system (Pierce Chemical, Rockford, IL). Films were digitized using an LKB densitometer and the intensity of bands was quantitated by using ImageQuant analysis software.
Figure 2.
Neomycin precipitates purified bovine coatomer. Purified bovine coatomer (12 μg per 100-μl reaction) was incubated with the indicated concentrations of neomycin for 8 h at 4°C and precipitates were collected by centrifugation at 16,000 × g for 30 min in an Eppendorf centrifuge with an F-45–18-11 rotor. After electrophoresis, coatomer subunits were detected by staining with Coomassie blue.
In some experiments, mixtures of neomycin and coatomer were centrifuged at 16,000 × g (14,000 rpm for 30 min at room temperature in an Eppendorf F-45–18-11 rotor) rather than at 100,000 × g. This was done to determine whether the precipitates were large enough to pellet at the lower speed. The sedimentation coefficient for a particle is given by:
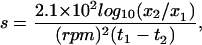 |
where x1 (in cm) is the distance of the particle from the axis of rotation at time t1 (in seconds) and x2 is the distance that the particle has traveled at time t2 (Sheeler, 1981). For the type of rotor, reaction volumes (100 μl), and tubes (0.5-ml conical tubes) used in these experiments, x1 was measured as 5.75 cm and x2, the distance to the bottom of the tube, was 6.48 cm. Thus, for 30 min of centrifugation at 14,000 rpm, a particle with a minimum sedimentation coefficient of about 300 S would be completely cleared from the solution and appear in the pellet.
When purified bovine coatomer was used instead of CHO cell cytosol, the conditions of precipitation were the same as with CHO cell cytosol except that neomycin and bovine coatomer (12 μg per 100-μl reaction) were incubated for 8 h at 4°C. The samples were centrifuged at 16,000 × g for 30 min at room temperature in an Eppendorf centrifuge. After the pellets were electrophoresed, protein was detected by staining with Coomassie blue.
Binding of Coatomer to Golgi Membranes
Golgi-enriched membranes (5 μg) and cytosol (60 μg) were added to a mixture containing 1 mM ATP, 1 mM GTP, 5 mM creatine phosphate, 8 U of creatine phosphokinase, 15 μM palmitoyl CoA, and protease inhibitors (1 μg/ml pepstatin A, 1 μg/ml leupeptin, 1 μg/ml aprotinin, 1 μg/ml antipain, and 1 μg/ml benzamidine) in a total volume of 100 μl for 20 min at 34°C. Reactions were stopped by immediately chilling them to 4°C in an ice bath. Membranes were collected by centrifugation at 16,000 × g for 10 min at 4°C in a microcentrifuge. The pelleted membranes were dissolved in sample buffer and electrophoresed in 7% polyacrylamide gels with SDS, and β-COP was detected by immunoblotting as described in the preceding paragraph.
RESULTS
Precipitation of Coatomer by Neomycin
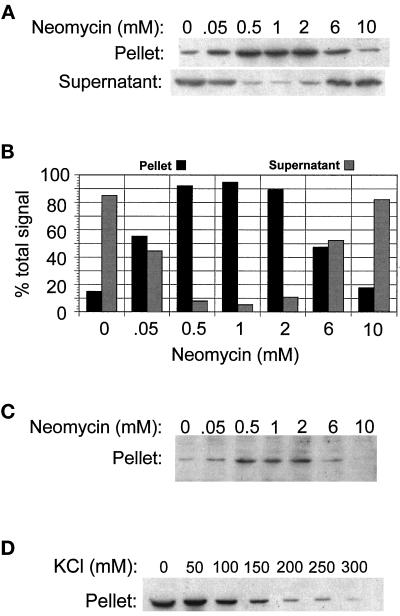
While investigating the possible effects of neomycin on the binding of coatomer to Golgi membranes, we noticed that control samples containing neomycin and crude cytosol from CHO cells (without Golgi membranes) generated a form of β-COP that appeared in the pellet after centrifugation at 100,000 × g for 30 min, conditions that do not normally sediment soluble coatomer. This suggested that neomycin was causing coatomer to form high molecular weight aggregates. To characterize the aggregation, cytosol was incubated with different concentrations of neomycin and centrifuged, and the presence of β-COP in the pellets and supernatants was assayed by immunoblotting. As the concentration of neomycin increased, the amount of β-COP in the pellet increased, reaching the half-maximal level at about 50 μM neomycin and the maximal level at about 1 mM (Figure 1, A and B). At neomycin concentrations greater than 1 mM, β-COP in the pellet declined with increasing neomycin. The amount of β-COP in the supernatant was inversely related to that in the pellet. This dependence of precipitation on concentration is reminiscent of antibody–antigen reactions and suggests that neomycin cross-links coatomer. Neomycin also precipitated coatomer from yeast cytosol (our unpublished results).
Figure 1.
Neomycin precipitates β- and β′-COP in cytosol from CHO cells. Cytosol from CHO cells was incubated with the indicated concentrations of neomycin for 2 h at 4°C in 100 μl of reaction buffer containing 25 mM HEPES, pH 7.4, 50 mM KCl, and 2.5 mM Mg(OAc)2 at a final cytosol protein concentration of 1.5 mg/ml. The mixture was centrifuged and β-COP or β′-COP in the pellets or supernatants were assayed by immunoblotting as described in MATERIAL AND METHODS. (A) Detection of β-COP in pellets and supernatants by immunoblotting. (B) Quantitation of the β-COP bands in A. (C) Detection of β′-COP in pellets by immunoblotting. (D) Detection of β-COP precipitated from cytosol that was adjusted to the indicated concentrations of KCl prior to adding 1 mM neomycin.
If neomycin precipitated intact coatomer, then the precipitates should contain other coatomer subunits in addition to β-COP. The presence of β′-COP was assayed by immunoblotting with a rabbit antibody to β′-COP (Lowe and Kreis, 1995). As seen in Figure 1C, β′-COP was also precipitated. This argues against the possibility that neomycin is somehow aggregating free β-COP subunits that are not assembled into coatomer.
Neomycin is positively charged at neutral pH, and to see whether there might be an ionic component to the interaction of neomycin with coatomer, the influence of salt concentration on the precipitation of coatomer by neomycin was investigated. Cytosol from CHO cells was adjusted to different concentrations of KCl and 1 mM neomycin was added. The samples were centrifuged and the amount of β-COP in the pellets was measured by immunoblotting. As seen in Figure 1D, β-COP in the pellets declined as the salt increased but was still present in significant amounts at 150 mM KCl. This suggests that there is an ionic component to the interaction of neomycin and coatomer and also demonstrates that neomycin still interacts with coatomer at physiological salt concentrations, albeit less so than at low salt.
The precipitates in Figure 1, A and C, were collected after centrifugation at 100,00 × g. To see whether the precipitates were large enough to sediment in a much lower centrifugal field, the experiment was repeated with centrifugation at 16,000 × g for 30 min. The distribution of β-COP in pellets and supernatants was nearly identical to that seen in Figure 1, A and C (our unpublished results, but see also Figure 2). This demonstrated that β-COP was in precipitates that were quite large because particles with a minimum sedimentation coefficient of about 300 S will be cleared from the solution under these conditions of centrifugation (see MATERIALS AND METHODS).
The precipitation of coatomer from CHO cell cytosol by neomycin in Figure 1 could be due to direct interaction of neomycin with coatomer or it could involve accessory proteins present in the cytosol in addition to coatomer. To see whether the interaction was direct, purified bovine coatomer was incubated with different concentrations of neomycin and the reaction mixture was centrifuged at 16,000 × g for 30 min. The pellets were collected and electrophoresed in a 10% polyacrylamide gel with SDS, and proteins were stained with Coomassie blue. As seen in Figure 2, a band for α-COP, unresolved bands corresponding to β′-, β-, and γ-COP, plus a light band corresponding to δ-COP, were detected. A faint band of ε-COP was also visible but is not shown in this figure. The intensity of all the bands first increased and then declined in the pellet as the concentration of neomycin increased, similar to results with β- and β′-COP from CHO cell cytosol (Figure 1, A and C). These data suggest that no proteins other than coatomer are required for precipitation by neomycin. In addition, the results confirm that neomycin extensively cross-links coatomer into large aggregates because precipitates of bovine coatomer also sedimented at 16,000 × g for 30 min.
Dilysine Inhibits the Precipitation of Coatomer by Neomycin
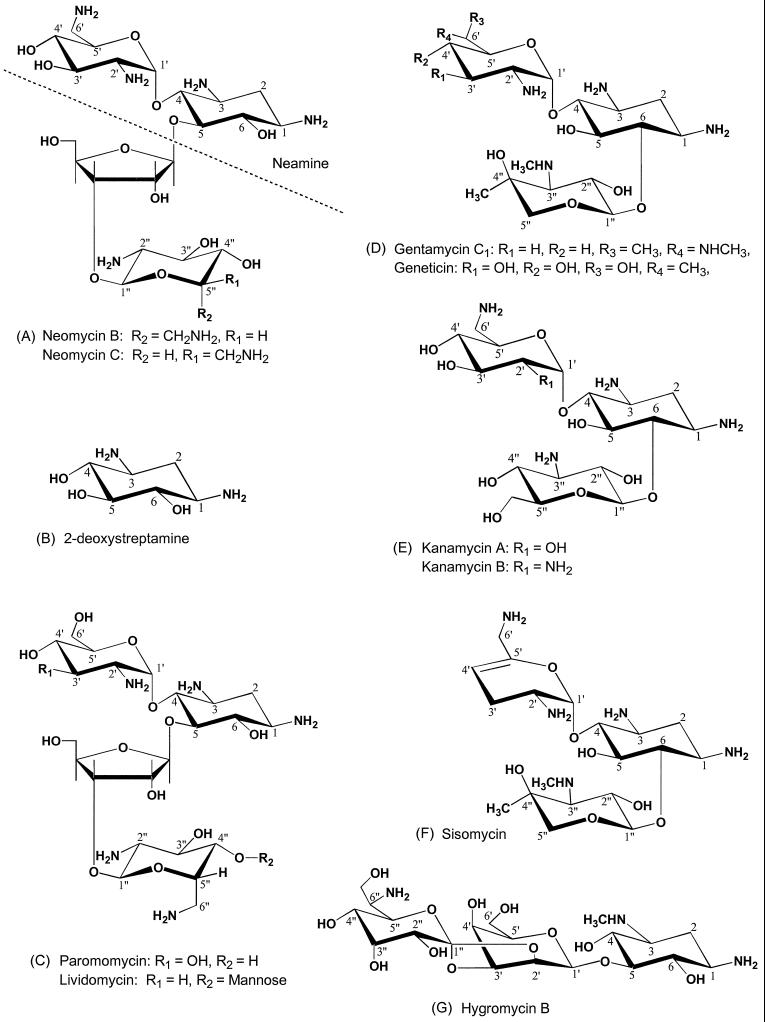
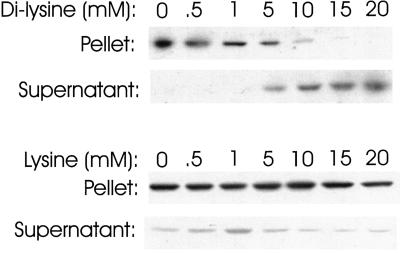
Commercially available neomycin is a mixture of neomycins B and C whose structures differ only in the stereochemistry of a methylamine group at carbon position 5′ (Figure 3A). Neomycin belongs to a family of antibiotics that all have the 2-deoxystreptamine moiety (Figure 3B), and the structures of some other members of the family are shown in Figure 3, C–G. Neomycin contains a pair of amino groups in three separate locations, one pair in 2-deoxystreptamine itself, another at positions 2′ and 6′ in 2,6-diaminodideoxyglucose attached to the 4 position of 2-deoxystreptamine, and a third pair on carbons 2" and 6" (Figure 3A, R1 or R2) in the distal amino sugar attached to the ribose ring. These clusters of amino groups might be important for interaction with coatomer because Cosson and Letourneur (1994) reported that coatomer bound to KKXX, the di-lysine-containing motif that is a retrieval signal for ER-resident membrane proteins. It is possible, therefore, that coatomer contains a binding site that accommodates two amino groups, including those in neomycin. To test this idea, we assessed the ability of di-lysine itself to inhibit the precipitation of coatomer by neomycin. Cytosol from CHO cells was incubated with 1 mM neomycin (which results in maximum precipitation) in the presence of various concentrations of di-lysine, and the cytosol was centrifuged to pellet aggregates. As seen in Figure 4, top, di-lysine at greater than 10 mM completely blocked the precipitation, supporting the idea that neomycin interacts with di-lysine binding sites on coatomer. Lysine itself did not interfere with aggregation by neomycin (Figure 4, bottom), demonstrating that the reaction was specific for di-lysine.
Figure 3.
Structures of antibiotics that contain 2-deoxystreptamine. Structures from Price et al. (1977).
Figure 4.
Effect of di-lysine and lysine on precipitation of coatomer by neomycin. CHO cell cytosol was incubated with 1 mM neomycin in the presence of the indicated concentrations of either di-lysine or lysine for 2 h at 4°C and centrifuged as in Figure 1. β-COP in the pellets and supernatants was detected by immunoblotting.
Interaction of Other Antibiotics with Coatomer
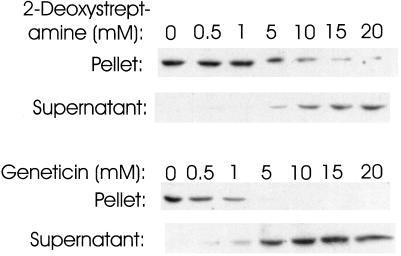
We tested a variety of other antibiotics for the ability to precipitate coatomer. If they did not precipitate coatomer, we determined whether they could inhibit the precipitation by neomycin. The approach is illustrated for 2-deoxystreptamine and Geneticin in Figure 5. 2-Deoxystreptamine did not itself precipitate coatomer (our unpublished results) but did inhibit the precipitation by neomycin (Figure 5, top). This confirms that some structural feature of 2-deoxystreptamine interacts with coatomer, most likely the two amino groups. Geneticin also did not produce high molecular weight coatomer yet effectively blocked the precipitation by neomycin (Figure 5, bottom). This is evidence that Geneticin contains only one binding site that interacts with coatomer; if there were more than one, cross-linking and precipitation should have occurred. Geneticin (structure in Figure 3D) contains 2-deoxystreptamine that should interact with coatomer but has only one amino group, not a pair, in each of the sugar residues attached to 2-deoxystreptamine. The fact that the amino sugar containing only one amine in Geneticin apparently does not interact with coatomer further emphasizes the importance of paired amino groups in binding to coatomer.
Figure 5.
Effect of 2-deoxystreptamine and Geneticin on precipitation of coatomer by neomycin. CHO cell cytosol was incubated with 1 mM neomycin in the presence of the indicated concentrations of either 2-deoxystreptamine or Geneticin for 2 h at 4°C and centrifuged as in Figure 1. β-COP in the pellets and supernatants was detected by immunoblotting.
Table 1 summarizes data on the interaction of other selected antibiotics with coatomer. The neamine moiety of neomycin (structure above the dotted line in Figure 3A) also precipitated coatomer, although not as well as neomycin. This suggests that the two amino groups in 2-deoxystreptamine in conjunction with the amino groups at positions 2′ and 6′ of 2,6-diaminodideoxyglucose are sufficient to cross-link coatomer. Paromomycin (structure in Figure 3C) precipitated coatomer even though it has only a single amino group on carbon 2′ of the carbohydrate attached to position 4 of 2-deoxystreptamine. However, paromomycin contains another residue of 2,6-diaminodideoxyglucose attached to the ribose ring. This suggests that the amino groups at positions 2" and 6" of paromomycin, plus the two primary amines in 2-deoxystreptamine, can together cross-link coatomer. Thus, it is likely that neomycin C itself has three pairs of amino groups able to bind to coatomer: one pair in 2-deoxystreptamine, another in the two amino groups of 2,6-diaminodideoxyglucose on position 4 of 2-deoxystreptamine, and the third pair in 2,6-diaminodideoxyglucose attached to the ribose ring. Kanamycin B (structure in Figure 3E) and sisomicin (structure in Figure 3F) also precipitated coatomer, and both have two foci of double amino groups, consistent with the idea that two clusters of double amino groups are essential for cross-linking coatomer. Note that kanamycin A, which has only one amine in the sugar attached to position 4 of 2-deoxystreptamine, did not precipitate, further supporting the importance of paired amino groups in coatomer binding.
Table 1.
Interaction of antibiotics with coatomer
| Compund | Precipitates coatomera | Inhibits precipitation by neomycinb |
|---|---|---|
| Neomycin | Yes (0.05) | |
| Neamine | Yes (0.7) | |
| Paromomycin | Yes (0.7) | |
| Kanamycin B | Yes (0.4) | |
| Sisomycin | Yes (0.3) | |
| 2-Deoxystreptamine | No | Yes (8.0) |
| Geneticin (G418) | No | Yes (2.0) |
| Gentamycin C1 | No | Yes (0.5) |
| Kanamycin A | No | Yes (2.0) |
| Hygromycin B | No | Yes (5.0) |
| Lividomycin | No | Yes (1.0) |
Precipitation was measured as in Figure 1. Values in parenthesis are the approximate concentrations (mM) to precipitate 50% of the coatomer.
Inhibition of coatomer precipitation by neomycin was measured as in Figure 5. Values in parenthesis are the approximate concentrations (mM) to inhibit precipitation by 50%.
All the other antibiotics in Table 1 contain unmodified 2-deoxystreptamine, with the exception of hygromycin B (structure in Figure 3G), and all failed to precipitate; nevertheless, they all inhibited precipitation by neomycin, including hygromycin B. The result with hygromycin B is interesting because 2-deoxystreptamine is modified by methylation of the amino group at position 3. This suggests that an amine pair in which at least one of the amines is a secondary amine might interact with the di-lysine binding site on coatomer; however, there is also a primary amine on the 6" carbon of hygromycin B that could conceivably be the second amine of a pair with the amine at carbon 1, provided the sugar rings can be oriented such that the two primary amines are closely opposed. Lividomycin (structure in Figure 3C) did not precipitate although it is similar to paromomycin. A likely explanation for this is that lividomycin has a bulky mannose group on carbon 4" that is absent in paromomycin and the mannose group might sterically interfere with the ability of the amino groups at positions 2" and 6" to interact with coatomer. It might have been expected that gentamicin C1 (structure in Figure 3D) would have aggregated coatomer considering that there are two amines in the amino sugar attached to position 4 of 2-deoxystreptamine, one at position 2′ and a methylamine at 6′. However, in the context of the rest of the molecule, this particular configuration of amino groups apparently does not bind coatomer with sufficient affinity to cross-link.
Inhibition of Coatomer Binding to Golgi-enriched Membranes
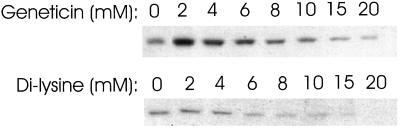
If antibiotics were interacting with the di-lysine binding site of coatomer, they should also block binding to Golgi membranes. We tested this with Geneticin, which blocks precipitation by neomycin but does not precipitate coatomer itself, and found that coatomer binding to membranes was inhibited (Figure 6, top). Di-lysine also interfered with coatomer binding to Golgi (Figure 6, bottom). Thus, the site on coatomer to which antibiotics and di-lysine bind is functionally important in the binding of coatomer to Golgi membranes.
Figure 6.
Effect of Geneticin and di-lysine on binding of coatomer to Golgi-enriched membranes. CHO cell cytosol and Golgi-enriched membranes were incubated under conditions that promote binding of coatomer to the membranes as described in MATERIALS AND METHODS. Either Geneticin or di-lysine was added at the concentrations indicated. Membranes were collected by centrifugation, and the amount of β-COP associated with the membranes was assayed by immunoblotting.
DISCUSSION
The precipitation of coatomer by neomycin had three salient features. First, at a fixed concentration of coatomer, the amount of coatomer in the precipitate increased and then declined as the concentration of neomycin increased, similar to the precipitin reaction that occurs between antibodies and polyvalent antigens (Eisen, 1980). Second, subunits from purified bovine coatomer were also precipitated, suggesting that the interaction between neomycin and coatomer was direct, not requiring any other protein factors that may have been in crude cytosol preparations. Third, at neomycin concentrations to produce optimal precipitation, coatomer in precipitates sedimented at a rate indicating a minimum sedimentation coefficient of approximately 300 S. Thus, neomycin cross-linked coatomer into aggregates that were much larger than the coatomer itself, which is reported to have a sedimentation coefficient in the range of 11 S (Waters et al., 1991) to 14 S (Duden et al., 1991). The aggregates were also much larger than would be expected for a coatomer dimer. These features suggest that there are at least two sites on neomycin (and other precipitating antibiotics) that bind to and cross-link coatomer units and that there must also be at least two antibiotic binding sites on each coatomer to result in the extensive cross-linking of multiple coatomers into large aggregates. Maximum aggregation should occur when the ratio of antibiotic concentration to coatomer concentration is such to maximize the number of cross-links, analogous to the equivalence point in antibody–antigen reactions. If there is insufficient antibiotic, there should be unoccupied binding sites on coatomer and maximum aggregation should not occur, as was observed. At excess antibiotic, the binding sites on coatomer should all be occupied by antibiotic that is not cross-linked to another coatomer, resulting in less aggregation at higher antibiotic concentrations, also as was observed.
An alternative explanation for the data is that there is a single di-lysine binding site on coatomer that when occupied induces the oligomerization of coatomer into large aggregates by direct protein–protein contacts between coatomers. However, two lines of evidence argue strongly against this possibility. First, it does not in any simple way explain why precipitation should decline at high concentration of ligand. Second, ligand-induced oligomerization of coatomer would predict that all antibiotics, and even di-lysine, should aggregate coatomer, but this did not occur.
A variety of antibiotics did not precipitate coatomer, but they did inhibit the precipitation of coatomer by neomycin. For an antibiotic to precipitate coatomer, it should have at least two sites that bind to and cross-link coatomers. Comparing the structures of those antibiotics that precipitated coatomer with those that did not helped elucidate the structural parameters important for binding. One coatomer binding site is in the 2-deoxystreptamine moiety because 2-deoxystreptamine inhibited precipitation by neomycin. Neamine also precipitated coatomer but lacks the ribose ring and attached amino sugar of neomycin. This argues that a second coatomer binding site in neamine (and therefore also in neomycin) contains the 2,6-diaminodideoxyglucose moiety attached to position 4 of 2-deoxystreptamine. In neomycin C, the sugar attached to the ribose ring is also 2,6-diaminodideoxyglucose and should provide a third site able to interact with coatomer. Evidence that 2,6-diaminodideoxyglucose attached to the ribose ring does bind coatomer comes from the observation that paromomycin, which contains 2-deoxystreptamine plus 2,6-diaminodideoxyglucose attached to the ribose ring, also precipitated coatomer. These considerations lead to the conclusion that 2-deoxystreptamine and 2,6-diaminodideoxyglucose can each interact with coatomer. This conclusion is consistent with the generalization that those antibiotics able to precipitate coatomer contained 2-deoxystreptamine and at least one 2,6-diaminodideoxyglucose unit but those that did not precipitate contained only one or the other of these determinants.
Because a common structural feature among di-lysine, the KKXX motif, 2-deoxystreptamine, and 2,6-diaminodideoxyglucose is the presence of two primary amino groups, it is likely that paired amino groups are a critical determinant for interaction with coatomer. The simplest explanation for the fact that di-lysine blocked the aggregation of coatomer by neomycin and that both di-lysine and an antibiotic inhibited the binding of coatomer to Golgi membranes is that the antibiotics and di-lysine compete for the same binding sites on coatomer, although we have not formally proven that the inhibition is competitive. However, the presence of two close amino groups in all of the compounds that interact with coatomer favors the idea that they all bind to the same site on coatomer. Additional evidence consistent with a role for the amino groups of the antibiotics in interacting with coatomer is the observation that the precipitation decreased as the salt concentration increased. The amino groups are positively charged at neutral pH and any ionic interactions between the charged amino groups and coatomer should be reduced at high salt concentrations.
A model of neomycin shows that the two amino groups of 2-deoxystreptamine are about 5 Å apart when in equatorial positions of the chair conformation. This suggests that the di-lysine binding site on coatomer accommodates amines that are at least within 5 Å. The two amino groups in 2,6-diaminodideoxyglucose can approach within a distance of about 5.6 Å when the ring is in the chair conformation, but if there is distortion in the ring to bring the 5′ carbon to a boat conformation, the amino groups can come even closer. Thus, it is reasonable that the two amino groups of 2,6-diaminodideoxyglucose could fit into the same site that binds 2-deoxystreptamine. It is also interesting that neamine is at most about 12 Å in length and yet cross-links coatomer, suggesting that the di-lysine binding sites of two coatomer units can come within about 12 Å of one another.
Previous work by Cosson and Letourneur (1994) revealed that a partial coatomer complex consisting of α-, β′-, and ε-COP could bind to a protein containing the KKXX motif, suggesting that a di-lysine binding site was contained in the α-, β′-, and ε-COP trimer. A similar conclusion was also reached by Lowe and Kreis (1995). Thus, it is possible that the α-, β′-, and ε-COP trimer contains two sites that bind di-lysine and that participate in cross-linking by antibiotics. However, Harter et al. (1996) reported that a peptide containing KKXX and a photoactivatible analog of tyrosine cross-linked to the γ subunit, suggesting that a KKXX binding site could be in or near the γ subunit. It is conceivable, therefore, that one di-lysine binding site is associated with α-, β′-, and ε-COP trimer and a second site is associated with the γ subunit and that these two sites account for the cross-linking of coatomer by precipitating antibiotics.
Why coatomer should have two (or more) di-lysine binding sites is uncertain, but one possible reason is that structural differences between the sites would allow for different functions. For example, one type of site may initially promote interaction of coatomer with KKXX-containing proteins in membranes, and another site may subsequently recruit KKXX-containing cargo proteins to patches of COPI already on the membrane. A second possible reason is the enhanced affinity of coatomer for target membranes that would result from the simultaneous binding of two or more membrane proteins displaying the KKXX motif. This could be especially important if some transmembrane proteins containing KKXX in their cytoplasmic domains were aggregated into clusters in the membrane independent of the KKXX motif to become founding receptors for coatomer binding. Aggregation of founding receptors could be the downstream consequence of ARF action or related to events on the lumenal side of the membrane or could result simply from protein–protein binding between receptors at high concentrations in the membrane. Potential candidates for the founding receptors could be members of the p24 family of putative cargo proteins, some of which contain the KKXX motif (or related sequences) and are present in Golgi membranes at high concentrations (Stamnes et al., 1995; Sohn et al., 1996). It is also interesting that Fiedler et al. (1996) recently found that the β, δ, and ζ subunits of coatomer interact with cytoplasmic tails of some proteins in the p24 family containing two adjacent phenylalanine residues, defining another peptide-binding domain in coatomer distinct from the di-lysine binding site. What relationships exist among the different peptide-binding sites in coatomer remains to be determined.
Regardless of their functional roles, the di-lysine binding sites on coatomer have a substrate specificity that is not restricted to only the KKXX motif. Simple di-lysine was an effective inhibitor of coatomer binding to Golgi membranes, indicating that di-lysine does not have to be in the context of a larger peptide to interact with coatomer. Moreover, the di-lysine binding site appears not to require any features of a peptide in its ligands since it apparently accommodates antibiotics in addition to di-lysine. Thus, other dibasic or polybasic compounds in the cell might also interact with the di-lysine site and conceivably modulate coatomer activity.
Aminoglycoside antibiotics are best known for inhibiting protein synthesis and for causing mistranslation on prokaryotic ribosomes (Price et al., 1977). Neomycin is also known to bind phosphoinositides (Schacht, 1976; Gabev et al., 1989) and intercede in biochemical and physiological processes that involve phosphoinositide metabolism. It has been more recently shown that many aminoglycoside antibiotics inhibit self-splicing of group I intron RNA in vitro, including splicing of the rRNA intron from a eukaryote, Tetrahymena (von Ahsen et al., 1991, 1992). To this list of effects is now added the interaction of aminoglycoside antibiotics with coatomer and the potential to interfere with membrane traffic.
ACKNOWLEDGMENTS
We acknowledge C. Shao for early contributions to this work. We thank M. Corboy for helpful discussions and C. Mikoryak and P. Colbaugh for reading the manuscript. We gratefully acknowledge Dr. J. Davies for providing 2-deoxystreptamine, Dr. T. Kreis for providing antibodies, and Dr. G. Waters for providing purified bovine coatomer. This research was supported in part by grants from the National Institutes of Health (GM-34297) and the National Science Foundation (MCB9513244). Work by R.T. Hudson partially fulfills the requirements for the Ph.D. degree in Molecular and Cell Biology.
Footnotes
Abbreviations used: ARF, ADP-ribosylation factor; CHO, Chinese hamster ovary; ER, endoplasmic reticulum.
REFERENCES
- Balch WE, Glick BS, Rothman JE. Sequential intermediates in the pathway of intercompartmental transport in a cell-free system. Cell. 1984;39:525–536. doi: 10.1016/0092-8674(84)90459-8. [DOI] [PubMed] [Google Scholar]
- Barlowe C. COPII: a membrane coat that forms endoplasmic reticulum-derived vesicles. FEBS Lett. 1995;369:93–96. doi: 10.1016/0014-5793(95)00618-j. [DOI] [PubMed] [Google Scholar]
- Bau M-Y, Draper RK. Ricin intoxicates End4 mutants that have an aberrant Golgi complex. J Biol Chem. 1993;268:19939–19942. [PubMed] [Google Scholar]
- Bednarek SY, Ravazzola M, Hosobuchi M, Amherdt M, Perrelet A, Schekman R, Orci L. COPI- and COPII-coated vesicles bud directly from the endoplasmic reticulum in yeast. Cell. 1995;83:1183–1196. doi: 10.1016/0092-8674(95)90144-2. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Cosson P, Letourneur F. Coatomer interaction with di-lysine endoplasmic reticulum retention motifs. Science. 1994;263:1629–1631. doi: 10.1126/science.8128252. [DOI] [PubMed] [Google Scholar]
- Cukierman E, Huber I, Rotman M, Cassel D. The ARF1 GTPase-activating protein: zinc finger motif and Golgi complex localization. Science. 1995;270:1999–2002. doi: 10.1126/science.270.5244.1999. [DOI] [PubMed] [Google Scholar]
- Duden R, Griffiths G, Frank R, Argos P, Kreis TE. β-COP, a 110 kd protein associated with non-clathrin-coated vesicles and the Golgi complex, shows homology to β-adaptin. Cell. 1991;64:649–665. doi: 10.1016/0092-8674(91)90248-w. [DOI] [PubMed] [Google Scholar]
- Eisen HN. Immunology. 2nd ed. Hagerstown, MD: Harper and Row; 1980. [Google Scholar]
- Fiedler K, Veit M, Stamnes MA, Rothman JE. Bimodal interaction of coatomer with the p24 family of putative cargo receptors. Science. 1996;273:1396–1399. doi: 10.1126/science.273.5280.1396. [DOI] [PubMed] [Google Scholar]
- Gabev E, Kasianowicz J, Abbott T, McLaughlin S. Binding of neomycin to phosphatidylinositol 4,5-bisphosphate (PIP2) Biochim Biophys Acta. 1989;979:105–112. doi: 10.1016/0005-2736(89)90529-4. [DOI] [PubMed] [Google Scholar]
- Harter C. COP-coated vesicles in intracellular protein transport. FEBS Lett. 1995;369:89–92. doi: 10.1016/0014-5793(95)00621-f. [DOI] [PubMed] [Google Scholar]
- Harter C, Pavel J, Coccia F, Draken E, Wegehingel S, Tschochner H, Wieland F. Nonclathrin coat protein Ó, a subunit of coatomer, binds to the cytoplasmic dilysine motif of membrane proteins of the early secretory pathway. Proc Natl Acad Sci USA. 1996;93:1902–1906. doi: 10.1073/pnas.93.5.1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson MR, Nilsson T, Peterson PA. Identification of a consensus motif for retention of transmembrane proteins in the endoplasmic reticulum. EMBO J. 1990;9:3153–3162. doi: 10.1002/j.1460-2075.1990.tb07513.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson M, Nilsson T, Peterson PA. Retrieval of transmembrane proteins to the endoplasmic reticulum. J Cell Biol. 1993;121:317–333. doi: 10.1083/jcb.121.2.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao C-Y, Draper RK. Retention of secretory proteins in an intermediate compartment and disappearance of the Golgi complex in an END4 mutant of Chinese hamster ovary cells. J Cell Biol. 1992;117:701–715. doi: 10.1083/jcb.117.4.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreis TE, Pepperkok R. Coat proteins in intracellular membrane transport. Curr Opin Cell Biol. 1994;6:533–537. doi: 10.1016/0955-0674(94)90073-6. [DOI] [PubMed] [Google Scholar]
- Ktistakis N, Brown A, Sternweis PC, Roth MG. Phospholipase D is present on Golgi-enriched membranes and its activation by ADP ribosylation factor is sensitive to brefeldin A. Proc Natl Acad Sci USA. 1995;92:4952–4956. doi: 10.1073/pnas.92.11.4952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ktistakis NT, Brown HA, Waters MG, Sternweis PC, Roth MG. Evidence that phospholipase D mediates ADP ribosylation factor-dependent formation of Golgi coated vesicles. J Cell Biol. 1996;134:295–306. doi: 10.1083/jcb.134.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letourneur F, Gaynor EC, Hennecke S, Demolliere C, Duden R, Emr SD, Riezman H, Cosson P. Coatomer is essential for retrieval of dilysine-tagged proteins to the endoplasmic reticulum. Cell. 1994;79:1199–1207. doi: 10.1016/0092-8674(94)90011-6. [DOI] [PubMed] [Google Scholar]
- Lowe M, Kreis TE. In vitro assembly and disassembly of coatomer. J Biol Chem. 1995;270:31364–31371. doi: 10.1074/jbc.270.52.31364. [DOI] [PubMed] [Google Scholar]
- Makler V, Cukierman E, Rotman M, Admon A, Cassel D. ADP-ribosylation factor-directed GTPase-activating protein. J Biol Chem. 1995;270:5232–5237. doi: 10.1074/jbc.270.10.5232. [DOI] [PubMed] [Google Scholar]
- Malhotra V, Serafini T, Orci L, Shepard JC, Rothman JE. Purification of a novel class of coated vesicles mediating biosynthetic protein transport through the Golgi stack. Cell. 1989;58:329–336. doi: 10.1016/0092-8674(89)90847-7. [DOI] [PubMed] [Google Scholar]
- Orci L, Palmer DJ, Amherdt M, Rothman JE. Coated vesicle assembly in the Golgi requires only coatomer and ARF proteins from the cytosol. Nature. 1993;364:732–734. doi: 10.1038/364732a0. [DOI] [PubMed] [Google Scholar]
- Ostermann J, Orci L, Tani K, Amherdt M, Ravazzola M, Elazar Z, Rothman JE. Stepwise assembly of functionally active transport vesicles. Cell. 1993;75:1015–1025. doi: 10.1016/0092-8674(93)90545-2. [DOI] [PubMed] [Google Scholar]
- Price KE, Godfrey JC, Kawaguchi H. Structure–Activity Relationships among the Semisynthetic Antibiotics. 1st ed. D. Perlman, New York: Academic Press; 1977. Effect of structural modifications on the biological properties of aminoglycoside antibiotics that contain 2-deoxystreptamine; pp. 239–355. [Google Scholar]
- Rothman JE, Wieland FT. Protein sorting by transport vesicles. Science. 1996;272:227–234. doi: 10.1126/science.272.5259.227. [DOI] [PubMed] [Google Scholar]
- Schacht J. Inhibition by neomycin of polyphosphoinositide turnover in subcellular fractions of guinea-pig cerebral cortex in vitro. J Neurochem. 1976;27:1119–1124. doi: 10.1111/j.1471-4159.1976.tb00318.x. [DOI] [PubMed] [Google Scholar]
- Schekman R, Orci L. Coat proteins and vesicle budding. Science. 1996;271:1526–1533. doi: 10.1126/science.271.5255.1526. [DOI] [PubMed] [Google Scholar]
- Sheeler P. Centrifugation in Biology and Medical Science. New York: John Wiley and Sons; 1981. [Google Scholar]
- Sohn K, Orci L, Ravazzola M, Amherdt M, Bremser M, Lottspeich F, Fiedler K, Helms JB, Wieland FT. A major transmembrane protein of Golgi-derived COPI-coated vesicles involved in coatomer binding. J Cell Biol. 1996;135:1239–1248. doi: 10.1083/jcb.135.5.1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamnes MA, Craighead MW, Hoe MH, Lampen N, Geromanos S, Tempst P, Rothman JE. An integral membrane component of coatomer-coated transport vesicles defines a family of proteins involved in budding. Proc Natl Acad Sci USA. 1995;92:8011–8015. doi: 10.1073/pnas.92.17.8011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Ahsen U, Davies J, Schroeder R. Antibiotic inhibition of group I ribozyme function. Nature. 1991;353:368–370. doi: 10.1038/353368a0. [DOI] [PubMed] [Google Scholar]
- von Ahsen U, Davies J, Schroeder R. Non-competitive inhibition of group I intron RNA self-splicing by aminoglycoside antibiotics. J Mol Biol. 1992;226:935–941. doi: 10.1016/0022-2836(92)91043-o. [DOI] [PubMed] [Google Scholar]
- Waters MG, Beckers CJM, Rothman JE. Purification of coat protomers. Methods Enzymol. 1992;219:331–337. doi: 10.1016/0076-6879(92)19033-3. [DOI] [PubMed] [Google Scholar]
- Waters MG, Serafini T, Rothman JE. “Coatomer”: a cytosolic protein complex containing subunits of non-clathrin-coated Golgi transport vesicles. Nature. 1991;349:248–251. doi: 10.1038/349248a0. [DOI] [PubMed] [Google Scholar]