Abstract
The side effects of conventional non‐steroidal anti‐inflammatory drugs (NSAIDs) on the stomach is undoubtedly a serious public health problem contributing significantly to the morbidity and mortality of patients receiving these drugs. However, the damage of NSAIDs is not confined to the stomach. Indeed the short term and long term damage of NSAIDs on the small bowel (NSAID enteropathy) is more frequent than NSAID gastropathy. Furthermore, NSAID enteropathy is associated with complications (bleeding and protein loss). While many of these are mild, the serious events (significant bleeding, perforation, obstruction, and sudden death) are frequent as that reported for NSAID gastropathy. The diagnosis of NSAID enteropathy has been greatly aided by the introduction of wireless capsule enteroscopy.
Keywords: NSAIDs, intestinal damage, toxicity, inflammation
The deleterious effects of non‐steroidal anti‐inflammatory drugs (NSAIDs) on the stomach and duodenum are well established and reported. However, the effects of these agents on the more distal portions of the small intestine (NSAID enteropathy) is not as widely publicised despite the fact that the small bowel is more susceptible to the damaging effects of NSAIDS than the stomach.1,2 Furthermore, the prevalence of NSAID enteropathy is greater than NSAID gastropathy and the serious outcomes are similar.1 Nevertheless NSAID enteropathy has not been perceived as an important clinical problem whereas NSAID gastropathy was the driving force for the development of the so called COX‐2 selective agents. There are many reasons for the low awareness of NSAID enteropathy. The condition is usually asymptomatic and diagnosis has until recently, only been possible with the use of tests that are not widely available.3 However, the most important factor is that pharmaceutical companies with invested interest in “gastroprotective” agents drove the recognition of the side effects of NSAIDs on the gastrointestinal tract. The same companies groomed certain clinical research workers who became “opinion” leaders that perpetuated the myth of a site selective damaging effect of NSAIDs. These same “opinion” leaders seem to have deliberately misinterpreted some of the safety data for the COX‐2 selective agents, not least the cardiovascular adverse events and this has caused a dramatic fall in their sales. However, serious as these allegations are for the investigators and patients alike, the Food and Drug Administration has now requested a cardiovascular safety warning on all NSAIDs irrespective of their COX‐2 selectivity. Therefore, it seems probable that the gastrointestinal side effects will again become the central focus by which we prescribe these drugs.
This paper aims to provide a review of the literature evidence, which corroborates the existence of NSAID enteropathy. In addition, the diagnosis, clinical features, and possible treatment options of this condition will be discussed.
Epidemiology
Necropsy findings have shown that non‐specific small intestinal ulcerations were detected in 8.4% of patients who recently used NSAIDs compared with 0.6% of those who had no history of NSAID use.4 It has also been reported that after enteroscopy, jejunal or ileal ulcerations were detected in 47% of patients receiving NSAIDs for rheumatoid arthritis.5 Furthermore, a retrospective study in the USA found that 4% of all small bowel resections carried out over a three year period were attributable to small bowel side effects of NSAIDs.6 More recent data come from a prospective double blind study of 8076 rheumatoid patients who were randomised to receive either naproxen or rofecoxib. The results showed that in the naproxen group, the rate of serious lower gastrointestinal events (beyond the duodenum) was 0.89/100 patient years, which accounted for 39.4% of all serious gastrointestinal events.7 These epidemiological studies suggest that NSAID induced small intestinal damage may be associated with significant mortality and morbidity.
Animal studies and mechanisms of NSAID enteropathy
Kent et al found a dose dependent relation between NSAIDs and penetrating longitudinal ulcers in the distal jejunum and ileum accompanied by an overgrowth of caecal‐type organisms.8 The pathogenesis of NSAID enteropathy was initially thought to be simply attributable to COX‐1 inhibition. However, it has now been shown to be multifactorial, involving a combination of biochemical events (COX‐1 and COX‐2 inhibition and the topical effect) that compromise mucosal cell integrity, which translates to increased epithelial permeability. Increased intestinal permeability permits mucosal exposure to luminal aggressors (bacteria and their degradation products, bile acids, etc) with a predictable inflammatory reaction.9,10 This inflammation varies in intensity from mild to that producing erosions and ulcers.
As with other inflammatory diseases of the gastrointestinal tract NSAID induced damage is associated with bleeding and protein loss and the ulcers may heal with a degree of fibrosis causing obstruction.
It is important to note that it has now been shown beyond any reasonable doubt that selective COX‐1 inhibition (or absence) does not lead to gastrointestinal damage.11 In contrast with prevailing theories about the “housekeeping” function of COX‐1 and role of COX‐2 in inflammation it is clear that selective COX‐2 inhibition (or absence) (not COX‐1 inhibition/absence) leads to small bowel damage (stomach is apparently unaffected), but this damage differs from NSAID enteropathy, being localised to the ileocaecal region.11 The pathogenesis of NSAID induced gastrointestinal damage can now be viewed as the combination of COX‐1 inhibition (restricting mucosal blood flow), COX‐2 inhibition (through an unknown “immunological” mechanism), and the topical effect of NSAIDs (NSAID‐phospholipid interaction and uncoupling of mitochondrial oxidative phosphorylation).11,12 Together these cause a breach in mucosal integrity. In the stomach this exposes the mucosa to acid and pepsin causing erosions and ulcers with Helicobacter pylori playing an additional part. In the small bowel the permeability increase exposes the mucosa to bile acids and bacteria (and their degradation products), which results in inflammation, erosions, and ulcers.
It is therefore evident that the premise by which the COX‐2 selective agents were developed is incorrect (that is, the idea of the distinctively different roles and biochemical consequences for COX‐1 and COX‐2) although fortunately and perhaps somewhat fortuitously they have in some respects been shown to be much safer than conventional NSAIDs.
NSAID enteropathy in humans
Diagnosis of NSAID enteropathy
The single most important reason for underestimating the clinical importance of NSAID enteropathy is the difficulty in making a diagnosis. A significant number of studies have used the various techniques described below to detect the small intestinal side effects of NSAIDs in humans.
Small bowel permeability assessment
It was a fortuitous discovery to find increased small bowel permeability in patients with rheumatoid and osteoarthritis associated with NSAID use.13 It is now clear that most conventional NSAIDs except aspirin and nabumetone increase intestinal permeability.13,14,15,16 The rationale for using permeability tests in the diagnosis of NSAID enteropathy is based on the knowledge that NSAIDs disrupt the intercellular integrity of intestinal epithelial cells. The choice of intestinal permeability tests for clinical testing is wide although their availability in hospitals and clinical biochemical laboratories is quite limited. Increased intestinal permeability attributable to NSAIDs can be detected with the use of 51Cr‐EDTA or the differential urinary excretion of 51Cr‐EDTA or lactulose/mannitol or rhamnose given with or without osmotic fillers. These tests show increased intestinal permeability after all conventional NSAIDs within 24 hours of ingestion. Prevalence rates are variably between 60% and 80% depending on the sensitivity of the method. Long term ingestion of conventional NSAIDs are also associated with increased intestinal permeability and there is no noticeable difference between the various NSAIDs, all showing an abnormality in 50%–70% of patients.16 The drawback of using intestinal permeability tests for the diagnosis of NSAID enteropathy is that these tests are non‐specific and abnormal in a variety of other conditions.17
Assessment of intestinal inflammation
Intestinal inflammation is the defining feature of NSAID enteropathy. Demonstration of the inflammation can therefore act as a diagnostic procedure. NSAID enteropathy was initially shown in humans by 111indium labelled leucocyte technique,13,14,18 which entails abdominal scintigraphy as well as a four day faecal collection.
Similar to the permeability studies the 111indium labelled leucocyte technique showed the presence of NSAID enteropathy in 50%–70% of patients taking long term NSAIDs with all conventional NSAIDs except with aspirin and nabumetone.16 The method is however prohibitively expensive (more than £500 per patient), which excludes it from routine use.
Calprotectin is a protein that is selective for the cytosol of neutrophils, monocytes, and macrophages. The amount in faeces reflects the traffic of these cells into the intestine. Single stool faecal concentrations of calprotectin correlate with the four day faecal excretion of 111indium labelled leucocytes and histopathological parameters of inflammation in inflammatory bowel diseases as well as with gastrointestinal mucosal inflammation secondary to NSAID use.19,20 NSAID enteropathy is evident within seven days of NSAID ingestion in volunteers.21 The prevalence of NSAID enteropathy in long term users, using this method, is variously reported as 44%–70%.16,19
Faecal calprotectin is eminently suitable for diagnosing NSAID enteropathy. However, as with the intestinal permeability tests this method is not disease specific (it is specific for inflammation) and raised levels of faecal calprotectin are consistently evident in inflammatory bowel disease and colorectal cancer.22
Nevertheless, they may have a role as first line non‐invasive investigations before more invasive measures.
Enteroscopy
Enteroscopy is a method used to visualise limited areas of the small intestine. Using Sonde enteroscopy, Morris et al found small bowel erosions and ulceration in 47% of patients with rheumatoid arthritis on NSAIDs.5 It is not used as a first line investigation because of its invasive nature.23 Push enteroscopy may not reach the main pathology, which is in the mid‐small bowel.
Capsule endoscopy
Most of the small intestine is outside the range of normal endoscopic examination. The advent of capsule endoscopy, a wireless probe propelled by peristalsis has been shown to provide painless and superior visualisation of the small intestine compared with enteroscopy.23,24,25 In the comparatively short time since it has been available it has outperformed all diagnostic modalities for detecting small bowel bleeding.25
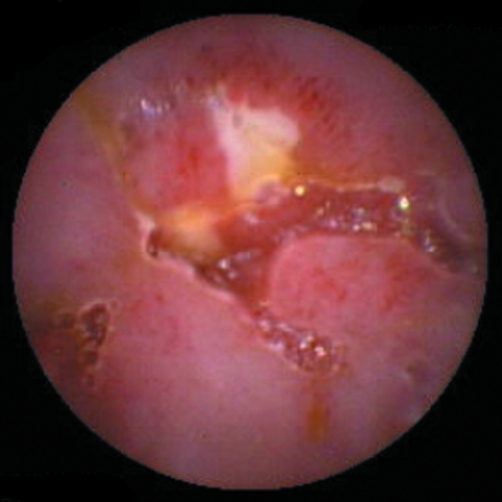
Capsule endoscopy has also proved to be of significant value in the diagnosis of intestinal inflammation including NSAID enteropathy. A recent study compared faecal calprotectin levels with capsule endoscopic findings after two weeks of NSAID therapy. Endoscopic abnormalities were detected in 68% of patients, while 75% of patients had high faecal calprotectin concentrations. Although there was not a significant correlation between faecal calprotectin levels and capsular endoscopy results, both methods showed the high prevalence of NSAID enteropathy.26 The most striking finding was the very high small bowel ulcer rates seen with the capsule (fig 1) and some of these were actively bleeding. Furthermore, Graham et al recently showed the same high prevalence of ulcers in long term NSAID users.27
Figure 1 Capsule image from a volunteer taking diclofenac 75 mg twice a day for two weeks showing a large small bowel ulcer.
The major potential complication associated with capsule endoscopy is capsule impaction, which may require surgical retrieval. Nevertheless, this technique is now increasingly widely available to clinicians and is increasingly becoming the test of choice to diagnose NSAID enteropathy.24,25
Radiological studies
Radiological studies rarely assist in the diagnosis of NSAID enteropathy, but occasionally a carefully performed small bowel enema is helpful to show the multiple, diaphragm‐like strictures.3,28
Complications of NSAID enteropathy
NSAID enteropathy is by itself comparatively unimportant, but for the complications that may originate from the inflammation or ulcers. Some of the complications are subclinical and hence go unnoticed. Other complications are clinically relevant and these can be subtle or potentially life threatening.
Chronic iron deficiency anaemia
NSAID enteropathy is associated with continuous and mild bleeding. In the longer term this may result in iron deficiency anaemia. Evidence to support this is derived from a number of studies. Using a dual radioisotopic method (111indium labelled leucocyte and technetium‐99 m labelled red blood cells) it was shown that the white and red cell markers appeared at the same place at the same time.14 Hayllar showed a significant correlation between the faecal excretion of labelled white and red cells, neither of which correlated with endoscopic appearances of the stomach and duodenum.29 Furthermore, treatment of the enteropathy with sulphasalazine or metronidazole reduces the gastrointestinal bleeding.29,30 Finally, using enteroscopy, Morris et al investigated the site of blood loss in patients on NSAIDs for rheumatoid arthritis with, chronic iron deficiency anaemia, who had negative endoscopy and colonoscopy findings. They showed that 47% of these patients had small bowel ulcerations and concluded that this contributed to their anaemia.5 The amount of blood loss from the small intestine is in most cases modest between 2–10 ml/day.
Protein loss
Patients with NSAID enteropathy have a protein losing enteropathy, which can lead to hypoalbuminaemia. Low serum albumen is found in about 10% of hospitalised patients with rheumatoid arthritis.14
Serious outcomes
Serious outcome studies (defined as a life threatening complication) are important in the assessment of the safety of NSAIDs. Before considering the serious outcomes from the small bowel it is important to put this damage in context of the same complications from the stomach. The three serious outcome studies that justified COX‐2 selective agents as safer alternatives to conventional NSAIDs were CLASS, VIGOR, and TARGET.31,32,33 The number of patients in each study was greater than 8000.
The CLASS criteria for “ulcer complications” included perforation and obstruction as well as gastroduodeal ulcers or erosions associated with (1) heamatemesis or maelena, (2) endoscopy findings (carried out because of dyspeptic symptoms) where stigmata of bleeding were present, or (3) occult blood positive stool with pre‐specified criteria for serious bleeding. However the perforations (0 v 0 cases, celecoxib and NSAIDs, respectively), obstruction (1 v 0 cases), and ulcer bleeds (10 v 20 cases) did not differ significantly between celecoxib and NSAIDs. When patients taking aspirin were excluded 5 of 1143 on celecoxib and 14 of 1104 patients taking NSAIDs had “ulcer complications” (p = 0.04).29
The VIGOR criteria for gastrointestinal events were perforations and obstruction, bleeding (more complex definitions than in the CLASS study, but included erosions as well as ulcers), and symptomatic ulcers. There were no significant differences between rofecoxib and naproxen with regards to perforation (3 of 4047 taking rofecoxib and 4 of 4029 taking naproxen) or obstruction (1 and 0, respectively). There was however significantly less bleeding with rofecoxib (n = 12) than naproxen (n = 32), but the bleeding was often not attributable to ulcers.30
Similar to the above studies there were no significant differences between lumiracoxib and NSAIDs with regards to perforation (1 of 9117 taking lumiracoxib and 2 of 9127 taking NSAIDs) or obstruction (0 and 1, respectively). Bleeding episodes were significantly less common with lumiracoxib (n = 28) than with NSAIDs (n = 80),33 but again many bleedings were not associated with ulcers. The overall conclusions from these trials are that the COX‐2 selective agents reduce bleeding episodes (which are in any case rare), many of which are non‐ulcer related. There is no evidence that these drugs reduce obstruction or perforation rates, which are in any case so rare as to be classified as medical curiosities. The number of deaths attributable to gastrointestinal complications in these trials is not stated.
Specifically designed studies to assess the prevalence of acute NSAID induced small bowel bleeds are not available. A retrospective study reviewing 283 cases of small bowel resection performed from 1991 to 1994 by Kessler et al identified 11 patients with NSAID related small bowel complications.6 Fifty per cent of these cases had gastrointestinal haemorrhage. Langman et al also showed that patients taking NSAIDs were at significant risk of small bowel or colonic bleeds.34 Re‐analyses of the VIGOR study showed that serious lower gastrointestinal events (predominantly bleeding) occurred at a rate of 0.9% per year in rheumatoid arthritis patients taking the non‐selective NSAID naproxen, accounting for nearly 40% of the serious gastrointestinal events that developed in these patients.7 Careful analysis of the MUCOSA study shows the same if not slightly higher bleeding rates from the small bowel.35 Hence the prevalence of clinically significant bleeding from the stomach and small bowel in NSAID users seem to be comparable.
The association between intestinal perforation and NSAID use in humans has been most noticeably seen in before term infants treated with indomethacin for closure of a patent ductus arteriosus. Bowel perforation occurred in 30% of patients taking high dose indomethacin.36 Further evidence of the link between NSAID use and bowel perforation is derived from the studies of Kessler et al and Langman et al, but the prevalence of these complications seems to be low.6,34
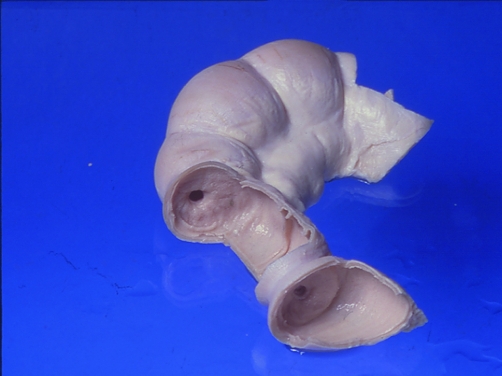
Chronic ulceration of the small intestine associated with NSAID use results in fibrosis and the formation of diaphragm strictures. The diaphragm‐like septae are about 2 mm to 4 mm thick and are usually found in the mid‐small bowel and can significantly reduce the small bowel lumen diameter (see fig 2). This can potentially result in sub‐acute obstruction. Kessler et al reported that 17% of patients with NSAID induced small bowel ulceration developed intestinal obstruction.6 There are also numerous case reports of NSAID induced small bowel and colonic strictures, the number of strictures found with the capsule enteroscopy technique is rising and these are certainly much more common that the gastric obstruction that occurs with these drugs.
Figure 2 Diaphragm strictures of the small intestine caused by NSAIDs.
It is noteworthy that although the COX‐2 selective agents were developed in part to reduce deaths, the three important trials simply state that there was no significant difference in deaths attributable to gastrointestinal events. While accepting that these studies looked at comparatively low risk patients it nevertheless suggests that the epidemic of gastric ulcer deaths attributable to NSAIDs may be illusory.
The mortality rates from NSAID enteropathy are also difficult to assess. A necropsy study from Scotland investigated deaths directly. The authors studied 713 patients at postmortem examination (249 had NSAIDs prescribed during the six months before death and 464 patients had not). Three patients who were long term users of NSAIDs were found to have died of perforated small intestinal ulcers.4 Accepting the selection bias for carrying out necropsies and the small number of patients dying from small bowel perforations, these data, if extrapolated over to national figures, suggests that 2000 patients die (undiagnosed) annually from NSAID induced small bowel perforations in the UK annually. The one study that reports a death among patients taking NSAIDs at significant risk of a serious gastrointestinal complication showed that the patient died from a small bowel perforation.37 The notion that NSAIDs related gastrointestinal complications are an important cause of deaths requires further and appropriately designed studies.
Management
The mainstay of treatment for patients with NSAID enteropathy is the withdrawal of NSAIDs. However, unless you are an epidemiologist, this is an unfeasible solution for the vast majority of patients as they rely on NSAIDs for the symptomatic relief of their medical conditions. Instead, a more realistic option is to consider prophylactic treatment, treat the inflammation in established cases, and switch over to anti‐inflammatory analgesics that cause less damage.
Key references
Laine L, Connors LG, Reicin A, et al. Serious lower gastrointestinal clinical events with nonselective NSAID or coxib use. Gastroenterology 2003;124:288–92.
Sigthorsson G, Simpson RJ, Walley M, et al. COX‐1 and 2, intestinal integrity and pathogenesis of NSAID‐enteropathy in mice. Gastroenterology 2002;122:1913–23.
Whittle BJR. Mechanisms underlying intestinal injury induced by anti‐inflammatory COX inhibitors. Eur J Pharmacol 2004;500:427–39.
Graham DY, Opekun AR, Willingham FF, et al. Visible small‐intestinal mucosal injury in chronic NSAID users. Clinical Gastroenterology and Hepatology 2005;3:55–9.
Maiden L, Thjodleifsson B, Theodors A, et al. A quantitative analysis of NSAID‐induced small bowel pathology by capsule enteroscopy. Gastroenterology 2005;128:1172–8.
The literature suggests that misoprostol, metronidazole, or sulphasalazine may be beneficial in the treatment of NSAID enteropathy.29,30,38 However these are not controlled studies.
If NSAID enteropathy is to be prevented it is reasonable to coadminister misoprostol with the NSAID as this consistently reduces the permeability changes caused by NSAIDs.38 Misoprostol may also be effective in established cases of NSAID enteropathy.39 Other studies have reported that misoprostol has limited effects in the treatment of NSAID enteropathy. Davies et al carried out a double blind, placebo controlled, randomised study that showed that there was no reduction in intestinal permeability of 51Cr‐EDTA after treatment with indomethacin and misoprostol,40 but they used comparatively low doses of misoprostol.
Treatment with the antimicrobial agent metronidazole has also been suggested to be of potential benefit in the management of NSAID enteropathy. A dose of 800 mg metronidazole with indomethacin resulted in a significant reduction in NSAID induced intestinal permeability in volunteers.40 Another study showed that metronidazole significantly reduced intestinal inflammation and bleeding in established cases of NSAID enteropathy, there was no effect on intestinal permeability.30
Pathological similarities between NSAID enteropathy and inflammatory bowel disease led to the suggestion that sulphasalazine may be a possible therapeutic option in NSAID enteropathy. Hayllar et al assessed the use of disease modifying antirheumatic drugs including sulphasalazine in patients taking NSAIDs. Sulphasalazine significantly reduced intestinal inflammation and blood loss, while other second line antirheumatic drugs did not.29
As all conventional NSAIDs appear to cause NSAID enteropathy there is little sense to switch from one to another. However COX‐2 selective agents are consistently found to be safe when used short term in volunteers and may be a much better option for patients requiring anti‐inflammatory treatment long term. However, their long term safety does require appropriate study as the data from long term COX‐2 inhibition in experimental animals do suggest some damage that is however distinctively different from NSAID enteropathy.11
Of note is the suggestion from the manufactures of proton pump inhibitors that coadministration of these agents with conventional NSAIDs is the way forward to reduce gastrointestinal damage (especially in view of the perceived cardiovascular risks of COX‐2 selective agents). By now we should have woken up to the workings of these companies. Proton pump inhibitors will certainly reduce the gastric damage attributabl to NSAIDs, but leaves the small bowel equally vulnerable to the damage. The gastric damage of NSAIDs is of course only half of the problem as detailed above.
Other treatments may be required for some of the complications of NSAID enteropathy (surgery for massive bleedings). Endoscopic balloon dilatation can be used for accessible strictures, but most cases of obstruction or perforation require surgical intervention.
Conclusion
There is no doubt that NSAIDs cause small bowel damage in humans and that this injury is common. However, a low index of clinical suspicion and the sparse availability of diagnostic tools makes the diagnosis of this condition difficult. The advent of wireless capsule enteroscopy will facilitate the diagnosis in patients taking NSAIDs with obscure bleeding, but there is a need for the development and trials of effective prevention and healing regimens.
Self test questions (true/(T)/false (F); answers after the references)
NSAIDs cause gastrointestinal damage predominantly by virtue of their action to inhibit COX‐1.
Treatment with COX‐2 selective agents is associated with significant reductions in gastric ulcer perforation, obstruction, and death as compared with conventional NSAIDs.
Clinically significant NSAID induced gastrointestinal bleeds from the stomach and duodenum are often non‐ulcer related.
Wireless capsule enteroscopy is used for the diagnosis of NSAID enteropathy.
Indomethacin and piroxicam cause more severe small bowel damage that ibuprofen and diclofenac when taken long term.
Bacteria are important in the pathogenesis of NSAID enteropathy.
Sulphasalazine is one of the drugs used in the treatment of NSAID enteropathy.
The prevalence of serious events (bleeding, perforation, obstruction, and death) resulting from the side effects of NSAIDs on the stomach is significantly greater than that of the small bowel.
ANSWERS
1. F; 2. F; 3. T; 4. T; 5. F; 6. T; 7. T; 8. F.
Footnotes
Conflicts of interest: Professor Bjarnason has received research grants and lecture honorariums from a number of pharmaceutical companies that market conventional NSAIDs and COX‐2 selective agents.
References
- 1.Smale S, Tibble J, Sigthorsson G.et al Epidemiology and differential diagnosis of NSAID‐induced injury to the mucosa of the small intestine. Best Pract Res Clin Gastroenterol 200115723–738. [DOI] [PubMed] [Google Scholar]
- 2.Fortun P J, Hawkey C J. Nonsteriodal anti‐inflammatory drugs and the small intestine. Curr Opin Gastroenterol 200521169–175. [DOI] [PubMed] [Google Scholar]
- 3.Bjarnason I, Hayllar J, Macpherson A J.et al Side effects of nonsteroidal anti‐inflammatory drugs on the small and large intestine. Gastroenterology 19931041832–1847. [DOI] [PubMed] [Google Scholar]
- 4.Allison M C, Howatson A G, Torrance C J.et al Gastrointestinal damage associated with the use of nonsteroidal anti‐inflammatory drugs. N Engl J Med 1992327749–754. [DOI] [PubMed] [Google Scholar]
- 5.Morris A J, Madhok R, Sturrock R D.et al Enteroscopic diagnosis of small bowel ulceration in patients receiving non‐steroidal antiinflammatory drugs. Lancet 1991337520. [DOI] [PubMed] [Google Scholar]
- 6.Kessler W F, Shires G T, Fahey T J. Surgical complications of non‐steroidal anti‐inflammatory drug‐induced small bowel ulceration. J Am Coll Surg 1997185250–254. [DOI] [PubMed] [Google Scholar]
- 7.Laine L, Connors L G, Reicin A.et al Serious lower gastrointestinal clinical events with nonselective NSAID or coxib use. Gastroenterology 2003124288–292. [DOI] [PubMed] [Google Scholar]
- 8.Kent T H, Cardeli R M, Stanler F U. Small intestinal ulcers and intestinal flora in rats given indomethacin. Am J Pathol 196954237–245. [PMC free article] [PubMed] [Google Scholar]
- 9.Davies N M, Saleh J Y, Skjodt N M. Detection and prevention of NSAID‐induced enteropathy. J Pharm Pharmaceut Sci 20003137–155. [PubMed] [Google Scholar]
- 10.Reuter B K, Davies N M, Wallace J L. Nonsteroidal anti‐inflammatory drug enteropathy in rats: Role of permeability, bacteria, and enterohepatic circulation. Gastroenterology 1997112109–117. [DOI] [PubMed] [Google Scholar]
- 11.Sigthorsson G, Simpson R J, Walley M.et al COX‐1 and 2, intestinal integrity and pathogenesis of NSAID‐enteropathy in mice. Gastroenterology 20021221913–1923. [DOI] [PubMed] [Google Scholar]
- 12.Whittle B J R. Mechanisms underlying intestinal injury induced by anti‐inflammatory COX inhibitors. Eur J Pharmacol 2004500427–439. [DOI] [PubMed] [Google Scholar]
- 13.Bjarnason I, Williams P, So A.et al Intestinal permeability and inflammation in rheumatoid arthritis; effects of non‐steroidal anti‐inflammatory drugs. Lancet 1984ii1171–1174. [DOI] [PubMed]
- 14.Bjarnason I, Zanelli G, Prouse P.et al Blood and protein loss via small intestinal inflammation induced by nonsteroidal anti‐inflammatory drugs. Lancet 1987ii711–714. [DOI] [PubMed]
- 15.Smecuol E, Bai J C, Sugai E.et al Acute gastrointestinal permeability responses to different non‐steroidal anti‐inflammatory drugs. Gut 200149650–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sigthorsson G, Tibble J, Hayllar J.et al Intestinal permeability and inflammation in patients on NSAIDs. Gut 199843506–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bjarnason I, Takeuchi K, Bjarnason A.et al The G.U.T. of gut. Scand J Gastroenterol 200439807–815. [DOI] [PubMed] [Google Scholar]
- 18.Bjarnason I, Zanelli G, Smith T.et al Nonsteroidal antiinflammatory drug induced intestinal inflammation in humans. Gastroenterology 198793480–489. [DOI] [PubMed] [Google Scholar]
- 19.Tibble J, Sigthorsson G, Foster R.et al Faecal calprotectin: A simple method for the diagnosis of NSAID‐induced enteropathy. Gut 199945362–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roseth A G, Schmidt P N, Fagerhol M K. Correlation between faecal excretion of indium‐111‐labelled granulocytes and calprotectin, a granulocyte marker protein, in patients with inflammatory bowel disease. Scand J Gastroenterol 19993450–54. [DOI] [PubMed] [Google Scholar]
- 21.Meling T R, Aabakken L, Roseth A.et al Faecal calprotectin shedding after short‐term treatment with non‐steroidal anti‐inflammatory drugs. Scand J Gastroenterol 199631339–344. [DOI] [PubMed] [Google Scholar]
- 22.Tibble J A, Bjarnason I. Faecal calprotectin as an index of intestinal inflammation. Drugs of Today 20013785–96. [DOI] [PubMed] [Google Scholar]
- 23.Rabenstein T, Krauss N, Hahn E G.et al Wireless capsule endoscopy – beyond the frontiers of flexible gastrointestinal endoscopy. Med Sci Monit 20028128–132. [PubMed] [Google Scholar]
- 24.Swain P. Wireless capsule endoscopy. Gut 200352(suppl 4)48–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mylonaki M, Fritscher‐Ravens A, Swain P. Wireless capsule endoscopy: a comparison with push enteroscopy in patients with gastroscopy and colonoscopy negative gastrointestinal bleeding. Gut 2003521122–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maiden L, Thjodleifsson B, Theodors A.et al A quantitative analysis of NSAID‐induced small bowel pathology by capsule enteroscopy. Gastroenterology 20051281172–1178. [DOI] [PubMed] [Google Scholar]
- 27.Graham D Y, Opekun A R, Willingham F F.et al Visible small‐intestinal mucosal injury in chronic NSAID users. Clinical Gastroenterology and Hepatology 2005355–59. [DOI] [PubMed] [Google Scholar]
- 28.Levi S, DeLacey G, Price A B.et al ‘Diaphragm like' strictures of the small bowel in patients treated with non‐steroidal anti‐inflammatory drugs. Br J Radiol 199063186–189. [DOI] [PubMed] [Google Scholar]
- 29.Hayllar J, Price A B, Smith T.et al Nonsteroidal antiinflammatory drug‐induced small intestinal inflammation and blood loss: effect of sulphasalazine and other disease modifying drugs. Arthritis Rheum 1994371146–1150. [DOI] [PubMed] [Google Scholar]
- 30.Bjarnason I, Hayllar J, Smethurst P.et al Metronidazole reduces inflammation and blood loss in NSAID enteropathy. Gut 1992331204–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Silverstein F E, Faich G, Goldstein J L.et al Gastrointestinal toxicity with celecoxib vs nonsteroidal anti‐inflammatory drugs for osteoarthritis and rheumatoid arthritis: the CLASS study: a randomised controlled trial. Celecoxib long‐term arthritis study. JAMA 20002841247–1255. [DOI] [PubMed] [Google Scholar]
- 32.Bombardier C, Laine L, Reicin A.et al Comparison of upper gastrointestinal toxicity of rofecoxib and naproxen in patients with rheumatoid arthritis. VIGOR Study Group. N Engl J Med 20003431520–1528. [DOI] [PubMed] [Google Scholar]
- 33.Schnitzer T J, Burmester G R, Mysler E.et al Comparison of lumiracoxib with naproxen and ibuprofen in the therapeutic arthritis research and gastrointestinal event trial (TARGET), reduction in ulcer complications: randomised controlled trial. Lancet 2004364665–674. [DOI] [PubMed] [Google Scholar]
- 34.Langman M J S, Morgan L, Worrall A. Use of anti‐inflammatory drugs by patients with small or large bowel perforation and haemorrhage. BMJ 1985290347–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Silverstein F E, Graham G Y, Senior J R.et al Misoprostol reduces serious gastrointestinal complications in patients with rheumatoid arthritis receiving nonsteroidal anti‐inflammatory drugs. Ann Intern Med 1995123241–249. [DOI] [PubMed] [Google Scholar]
- 36.Grosfeld J L, Chaet M, Molinari F.et al Increased risk of necrotising enterocolitis in premature infants with patent ductus arteriosus treated with indomethacin. Ann Surg 1966224350–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chan F K L, Hung L C T, Suen B Y.et al Celecoxib versus diclofenac and omeprazole in reducing the risk of recurrent ulcer bleeding in patients with arthritis. N Engl J Med 20023472104–2110. [DOI] [PubMed] [Google Scholar]
- 38.Bjarnason I, Smethurst P, Fenn G C.et al Misoprostol reduces indomethacin induced changes in human small intestinal permeability. Dig Dis Sci 198934407–411. [DOI] [PubMed] [Google Scholar]
- 39.Morris A J, Murray L, Sturrock R D.et al Short report: the effect of misoprostol on the anaemia of NSAID enteropathy. Aliment Pharmacol Ther 19948343–346. [DOI] [PubMed] [Google Scholar]
- 40.Davies G R, Wilkie M E, Rampton D S. Effects of metronidazole and misoprostol on indomethacin‐induced changes in intestinal permeability. Dig Dis Sci 199338417–425. [DOI] [PubMed] [Google Scholar]