Abstract
There are a number of pathophysiological processes underlying age related changes in the auditory system. The effects of hearing loss can have consequences beyond the immediate loss of hearing, and may have profound effects on the functioning of the person. While a deficit in hearing can be corrected to some degree by a hearing aid, auditory rehabilitation requires much more than simply amplifying external sound. It is important that those dealing with elderly people are aware of all the issues involved in age related hearing loss.
Keywords: ageing, auditory system, presbyacusis, age related hearing loss
Of all the changes in the senses that occur with age, deterioration in hearing is perhaps the most expected and indeed the most accepted decline. Hearing serves a number of functions: it permits communication through speech, provides warning of potentially injurious events occurring outside the visual field, and serves an aesthetic function as in the appreciation of music or nature. The wider impact of hearing loss may therefore be profound, with consequences for the social, functional, and psychological wellbeing of the person.1,2,3,4 Some of these problems can persist despite correction of the deficit with a hearing aid.5 This can result in the deaf person withdrawing from the everyday social interactions that are necessary for independent existence. The feeling of isolation may be subconsciously reinforced by friends, family, or carers in response to the increased effort required to communicate.
Hearing impairment is a common problem and the degree of impairment and its prevalence increase with age. The 1995 UK national study of hearing disorders found that 20% of adults had some degree of hearing impairment (audiometric threshold greater than 25 dB) in the better hearing ear, with 75% of these people aged over 60 years. Of those people with a moderately severe hearing loss (audiometric threshold greater than 45 dB), 84% were over 60 and 45% over 80.6 The 1998 UK general household survey found that 53% of men and 41% of women over the age of 75 reported some difficulty with hearing.7
Pathophysiology of age related hearing loss
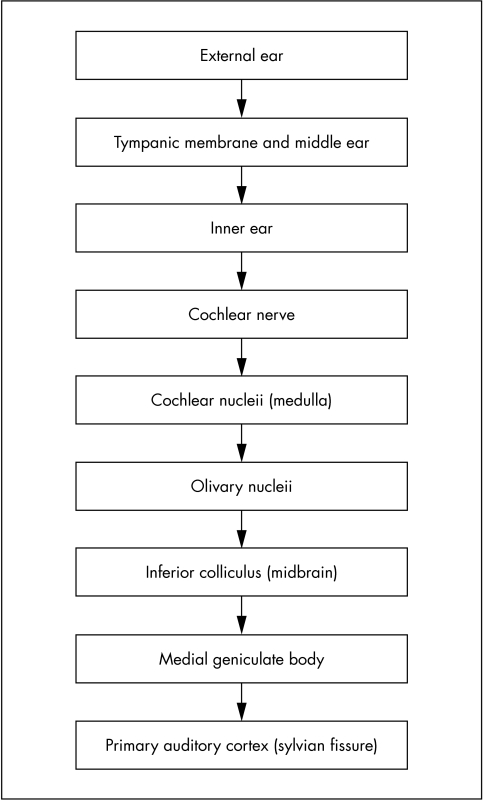
The auditory system acts to channel and transduce sound pressure waves into electrophysiological signals that can then be localised and interpreted by the higher cortical centres. There are a number of stages in this process (fig 1) and ageing can affect any stage from stimulus to perception, although the impact of the ageing process depends on the site of degeneration.
Figure 1 Stages in the hearing pathway.
The conductive mechanism of the ear
Sound from the external environment is channelled by the pinna and external auditory canal to the tympanic membrane. The external ear acts as an “acoustic resonator” to intensify sound in the range 2–5 KHz, those frequencies most important in speech. The lever action of the ossicles provides a mechanical advantage to increase sound pressure and the discrepancy in size between the tympanic membrane and the oval window further amplifies the sound pressure wave. The net pressure gain is thought to be by a factor of 17.8 Changes in the conductive component of the hearing apparatus associated with ageing have been described, including collapse of the cartilaginous external auditory canal9 and stiffening of the tympanic membrane10 and ossicular chain.11 However, the effect on the auditory threshold of these changes is minor and does not contribute significantly to the hearing loss associated with ageing.12
The transduction mechanism of the ear
The site of conversion of mechanical energy to electrophysiological signal is the cochlea. Vibrations of the basilar membrane of the cochlea are set up in response to vibration of the oval window. This in turn causes movement of the stereocilia of the hair cells lying on the basilar membrane resulting in an increase in permeability of the hair cell to potassium and a depolarisation of the hair cell. The consequence of this is the secretion of a neurotransmitter onto the afferent endings of the cochlear nerve with a resulting neurological signal.8
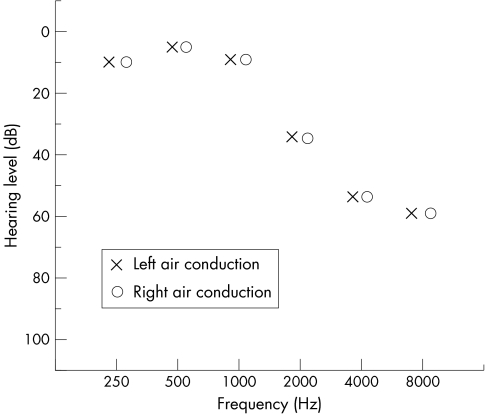
The age related changes in the auditory system that have the most significant effects on the hearing of the person occur in the cochlea with several interacting processes involved.13,14 Different principal lesions have been described (table 1). Although individually each of these gives a different clinical and audiological picture, these often combine in the ageing ear to give a resulting sensorineural hearing loss that is mild to moderate in nature affecting the mid to high frequencies bilaterally13 (fig 2). It is this clinical picture of a gradually progressive, bilaterally symmetrical hearing loss associated with age that has been termed presbyacusis (Greek Presbys—old man and Akousis—hearing) first used in 1874.19 This term, however, does not indicate the aetiology of the observed picture, which can have many underlying causes.14,20
Table 1 Principal changes in the cochlea associated with age13,15,16,17,18.
| Cochlea lesion | Pathology | Result |
|---|---|---|
| Sensory | Loss of sensory cells in basal turn of cochlea | Abruptly sloping high frequency loss above the speech frequency range |
| Neural | Loss of cochlear neurons | Progressive loss of speech discrimination in the presence of stable pure tone thresholds |
| Strial | Metabolic and vascular changes within cochlea | Slowly progressive hearing loss with flattening of audiogram and good speech discrimination |
| Conductive | Changes in the conduction or resonance of the cochlear duct | Linear descending pattern on audiogram |
| Indeterminate | No pathological correlate identified. Possibly impaired cellular function | Flat and/or abrupt high tone hearing loss |
| Mixed | Combination of above | Mild to moderate high frequency loss (fig 2) |
Figure 2 Audiogram showing typical age related hearing loss.
Despite extensive investigation the exact aetiology of the pathophysiological changes described in the cochlea is unclear. The hair cells within the cochlea are generated within the first trimester of development and are then required to survive for the lifetime of the person. Regeneration does not occur after loss of hair cells. As there is little redundancy within the cochlea, with each region in the cochlea transducing a particular frequency of sound, it follows that the loss of any of this small population of cells will have a noticeable effect on the person.
The central auditory system
From the cochlea afferent fibres pass to the cochlear nuclei and thence to the higher auditory centres via the lateral lemniscus and midbrain. The neuronal signal is converted to heard sound, which is then analysed by the cognitive centres of the brain to permit understanding of content and association with memory.8 There is strong bilateral representation of auditory information at all levels above the cochlea; for this reason hearing is rarely impaired after stroke, although interpretation of sound can be.
Box 1 Ototoxic drugs in common use27
Aminoglycosides
Quinine
β blockers
Diuretics
Non‐steroidal anti‐inflammatory agents
Salicylates
Tricyclic antidepressants
With ageing there is a decrease in the number of neurons in the cochlea nuclei and auditory centres of the brain.12,15,21 There is also a reduction in the size of cells and changes in the neurochemical make up of the cells.12 This is associated with a decline in the ability of the central auditory system to process sound.22 Some of the changes in the central auditory system may be attributable to the effects of the loss, or attenuation, of neural input from an impaired peripheral auditory system.12 These effects combine with general biological ageing within the brain to produce the difficulties seen. A number of pathologies that accompany ageing may affect the auditory pathways, including arteriosclerosis, anoxia and metabolic dysfunction of the liver or renal system, and free radical induced damage.20,23
A result of the changes in the central auditory system is that for many older people the difficulties in understanding speech are greater than would be expected solely from the audiometric threshold. A further difficulty is noticed when there is background noise requiring more complex auditory processing.22 A consequence of the changes in central auditory processing with ageing is that simple amplification of external sound with a hearing aid will not always fully correct the hearing disability experienced by the person. While changes in the central auditory system will contribute to age related hearing loss, it is thought that they have less of an impact on hearing in the elderly than the changes in the cochlea described above.24
Genetic basis of hearing loss
Studying the effects of genetic inheritance on age related hearing loss alone is difficult as most people have been exposed to other insults to the auditory system. There are a large number of genes coding for the cochlea. However, some evidence is emerging to support a genetic predisposition to both age related hearing loss and susceptibility to environmental damage.25 Mitochondrial DNA has been implicated in both of these types.26
Other causes of hearing loss in the elderly
While the ageing process itself is associated with degeneration of the auditory system, other insults to the cochlea from noise or ototoxic drugs (box 1) can accumulate over a lifetime and contribute to the decline in hearing experienced by older people. In addition, otological conditions such as Menières disease or otosclerosis that can affect patients of any age also need to be considered.
Ototoxicity as a cause of hearing loss may often be unrecognised—it has been estimated that up to 30% of elderly patients presenting with hearing impairment are taking potentially ototoxic drugs.28 Furthermore, in addition to the drugs listed in box 1 that are regularly associated with ototoxicity, there are many other drugs that are potentially ototoxic. The underlying mechanism of ototoxicity varies with different drugs. For example, aminoglycosides can cause loss of cochlea outer hair cells secondary to damage from free radicals and accumulation of calcium and potassium ions.29 This is dependent on the serum concentration of the drug. The clinical picture seen with cochleotoxic aminoglycosides such as neomycin is of a progressive high frequency sensorineural hearing loss associated with tinnitus occurring after a few days of treatment, although some aminoglycosides are predominantly vestibulotoxic.27 The effects are often permanent, therefore monitoring of serum drug levels in patients taking aminoglycosides, particularly the elderly is important to prevent damage occurring,30 although in the future protective agents my be available.31 Other drugs such as loop diuretics or β blockers may produce a reversible hearing loss.27 The clinical picture will depend on the drug taken. Drug induced ototoxicity in the elderly is thought to be more significant as a cause of hearing loss than in other groups for a number of reasons: high use of ototoxic drugs, impaired renal function or an increased susceptibility to ototoxic drugs are all potential explanations of why this is so.16
The histological features of cochlear damage from the cumulative effect of noise exposure through life, from ototoxic drugs, or from ageing may be indistinguishable.17,28
Systemic conditions contributing to hearing loss
A number of systemic diseases have been suggested as potentially contributing to hearing loss with age (box 2). Many of these are themselves associated with increasing age. However, the quality of the studies suggesting an association is generally low and further work is needed before any clear correlations can be identified.24
Clinical presentation and wider consequences of hearing loss
As an age related decline in hearing is usually gradual in onset, its presentation may be subtle in nature. Indeed, the median threshold of people seeking advice about amplification is about 45Db,40 although benefit may be obtained from using a hearing aid when there is as little as a 25–30 dB hearing loss. The person with the hearing loss may not be aware of the effects of their condition and often may not be the main instigator to seek advice on treatment of the problem. It is not uncommon for a person fitted with their first hearing aid to rediscover sounds long since forgotten. Tinnitus, frequently a cause of discomfort and distress, may accompany impaired hearing in the elderly person.41 Often patients present with difficulty in understanding speech rather than an inability to hear. In addition to the decline in central auditory processing discussed above, this is attributable to the preferential loss of hair cells at the basal turn of the cochlea, which transduce the high frequency sounds such as consonants and word endings that are required for decoding speech. The phenomenon of recruitment whereby adjacent cochlear hair cells are activated to compensate for damaged or dysfunctional cells, seen with age related hearing loss, may mean that a hearing impaired person paradoxically complains of sounds being uncomfortably loud.
Box 2 Diseases implicated in age related hearing loss
The impact of hearing impairment goes beyond its immediate effects. Apart from the expected problems with hearing and speech recognition, the elderly person may also have difficulty in processing and integrating hearing with other sensory modalities.1 This will have implications for the older person beyond the immediate effects of the loss of hearing and can cause difficulties with everyday tasks such as driving or interacting in complex social or physical environments.42 Loss of vision, also often associated with ageing, in particular can also exacerbate the effects of hearing impairment because of reduction in supportive cues that are crucial for communication.20 Hearing loss may also be associated with a decreased quality of life,43 depression,4 reduced functional status,44 and social isolation.45
The result of hearing loss in combination with impaired cognitive function should not be underestimated. An uncorrected deficiency in hearing can make cognitive testing, which is often dependent on verbal communication, difficult or even impossible; and cognitive dysfunction can make accurate audiological testing impossible. It may be that impairment of cognitive function in old age is directly linked to a diminished hearing capacity,3,39,46 although this has been disputed.47 One possibility is that the processes that lead to a decline in sensory ability lead to a decline in intellectual function,3 but it has also been suggested that sensory impairment is a causative factor in the decrease in intelligence through a lack of sensory intellectual stimulation.46 If there is pre‐existing dementia, deafness can be associated with the presence of delusions,2 possibly because patients with impaired cognitive capacity lack insight into the effect of the sensory impairment.
Management of hearing loss in the elderly
As hearing loss in the elderly may be atrributable to an underlying systemic or otological cause, any patient presenting with hearing loss needs to be thoroughly assessed before attributing their symptoms to age related changes. As is frequently the case, an accurate history is crucial. Decline in hearing with age is usually a gradual process and should affect both ears equally. While tinnitus is often present,14 the presence of other otological symptoms such as otalgia, otorrhoea, or vertigo suggests another aetiology for the hearing loss. Damage to the auditory system from previous noise exposure may exacerbate age related hearing loss, and is often only apparent when combined with the effects of ageing. Sudden hearing loss, either unilateral or bilateral, should prompt specialist investigation.48 Patients with unilateral tinnitus or asymmetrical hearing loss should have a retrocochlear cause for their symptoms such as a vestibular schwannoma (acoustic neuroma) ruled out.49 Evidence of systemic disease affecting hearing and the current or previous use of potentially ototoxic drugs should be sought. It has been suggested that the proportion of elderly patients presenting with deafness who show evidence of disease processes implicated in hearing loss my be as high as 80%, and over 50% of patients presenting with hearing loss may have evidence of previously unrecognised medical disorders.28
The otoscopic appearance of the ears of patients with age related hearing loss is unremarkable. Any abnormality on examination therefore suggests another pathological process. Clinical assessment of hearing by free field speech tests at 60 cm from the ear and then adjacent to the ear using whispered voice, conversational voice, and shouting may give an indication of the degree of hearing loss.14 Tuning fork tests will be compatible with a bilateral sensorineural hearing loss (Rinne positive bilaterally, Weber central). As previously discussed, the changes in the conductive mechanism of the ear occurring with age do not significantly affect the audiological threshold. Any evidence of a conductive deficit should therefore prompt a search for other causes such as tympanic membrane perforation or middle ear effusion. Usually these will be apparent from the history or on examination.
Managing patients with hearing loss is not just a case of providing a hearing aid. The rehabilitation of the hearing impaired needs to consider the function, activity, and participation of the person, not just the impairment.20 In addition to amplification of the auditory signal, auditory and listening skills, and the use of accessory listening devices are all important. It is well recognised that using a hearing aid may not overcome all hearing problems, with up to 59% of hearing aid users reporting hearing difficulties even with a hearing aid.7 Some of these problems may be overcome by improvements in signal processing technology in hearing aids, but it is important that attention is paid to education or counselling for the deaf patient and their family or carers.
A hearing aid acts to amplify sound; this is obviously an important starting point to treat age related hearing loss (table 2). Older, analogue hearing aids convert acoustic information to a constantly varying electrical signal. This type of aid may cause excessive amplification of loud sounds leading to subsequent discomfort for the user. Problems also arise in those patients with different degrees of hearing loss at different frequencies. Digital hearing aids represent sound mathematically. This signal is then processed by a microprocessor, permitting much greater manipulation of the signal than is possible with an analogue aid and greater comfort for the user.
Table 2 Types of hearing aid.
| Type of aid | Description | Advantages | Disadvantages |
|---|---|---|---|
| Behind the ear (BE) | Body of aid sits behind ear and connects to ear mould. Available on NHS | Widely available, good range of amplification | Cosmetic appearance sometimes unacceptable. Mould may predispose to external ear infection |
| In the ear (ITE)/In the canal (ITC) | Aid sits in conchal bowl (ITE) or ear canal (ITC) | Less obtrusive | Expensive. Limited range of amplification because of feedback problems caused by proximity of microphone and speaker |
| Body worn (BW) | Body of aid worn on strap around patient's chest or waist | Permits very high amplification for profound hearing loss | Cumbersome. May pick up rustling of patient's clothes |
| Bone conduction (BC) | Body of aid either on headband or body worn feeds to bone conductor worn on skull | Bypass the conductive mechanism of ear in patients with chronically discharging ears or ear canal stenosis | Cosmetic appearance. Bone conductor can be uncomfortable |
| Bone anchored (BAHA) | Osseointegrated percutaneous titanium abutment screwed into bone of mastoid connects to body of aid | As for bone conduction aids | Requires surgical implantation |
| Spectacle | Modification of standard spectacle frame to incorporate standard or bone conductor aid | Permits contralateral routing of signal in those with one profoundly deaf ear | Only suitable for glasses wearers. Require glasses to be worn constantly |
Patient resources
Royal National Institute for the Deaf web site (charity representing deaf and hard of hearing people in UK): http://www.rnid.org.uk
Modernising Hearing Aid Services web site (Department of Health programme to modernise hearing services in UK): http://www.mhas.info
Hearing Concern web site (charity providing support to hard of hearing adults in UK): http://www.hearingconcern.org.uk
It has been suggested that stimulation of the auditory system may reduce the degeneration in auditory function seen with age12—this would suggest that early provision of a hearing aid may be beneficial in modifying the ageing process on the auditory system. Many elderly people accept hearing impairment as part of the ageing process—it has been estimated that only one in five older people with hearing impairment seek assistance.6 Barriers to seeking help include negative attitudes to hearing impairment and hearing aids, held by both the hearing impaired and those around them, lack of knowledge of the options to treat hearing impairment, and problems accessing audiological services.20 Even with digital signal processing hearing aids tend to amplify background noise, which can limit their usefulness. In addition, limited manual dexterity and impaired cognitive function can make using a hearing aid difficult for many elderly users.
Auditory rehabilitation needs to teach the hearing impaired and those around them techniques to improve communication despite the disability. Close attention needs to be paid to the auditory environment for the older person with hearing loss.20 The use of visual clues can be maximised by ensuring adequate lighting and corrective devices for vision. The impaired person and those interacting with the person need to be aware of the importance of face to face communication, and, if possible, reducing background noise when attempting to communicate. The context in which a conversation occurs also provides supportive information to aid the hearing impaired, and simple conversational techniques such as stating the topic early in the conversation can be extremely useful.20
Many hearing aid users will not wear their aid at all times. Even with a hearing aid, devices such as alerting lights for telephones and doorbells and the use of the induction loop (telecoil) feature on the hearing aid in the home or in public areas can all make a contribution to improving quality of life for the hearing impaired.
The future
In the future management of age related auditory dysfunction will benefit from both advances in technology and increased knowledge of the molecular processes involved in the cochlea. Implantable devices already available such as the Vibrant Soundbridge (Symphonix) attach to the ossicular chain and increase the amplitude of natural ossicular vibration.50 While this still requires the user to wear an external microphone and battery, auditory feedback is eliminated and the ear canal is not occluded by a mould. In the future totally implantable devices may become available.
The holy grail of treating any age related degeneration would be to reverse the pathological changes involved. A recent trial of gene therapy in guinea pigs has done just that.51 Researchers used virally mediated expression of the Atoh1 gene to encode an important transcription factor for cochlea hair cell development, leading to the re‐growth of cochlea hair cells in adult animals with drug induced hair cell loss. The regenerated hair cells also showed a significant recovery of function with improvement in the test animals' hearing. This research is particularly exciting; as the newly functioning hair cells appeared to have both transdifferentiated from supporting supporting cells but also potentially have arisen by cell proliferation within the cochlea. A further promising area of gene therapy is the transplantation of pleuripotent neural stem cells into the inner ear where they can then differentiate into cochlear hair cells.52 While the genetic coding and molecular function of the human cochlea is complex, these results suggest that in the future gene therapy may be a viable treatment for age related hearing loss.
As the ageing ear will have been exposed to many insults during its lifetime, minimising damage from these events may reduce the impact of age related changes. Noise damage can clearly be minimised by ear protection, particularly in the workplace. It is also possible that protection from noise trauma or other ototoxic agents can be provided at a molecular level.29,31 Elucidation of the mechanism of damage allows therapies targeted to prevent damage from occurring. For example, damage attributable to free radical formation from aminoglycoside toxicity could be minimised by administration of free radical scavengers. This could be of particular benefit when using potentially ototoxic drugs in susceptible groups such as the elderly.
Summary
Hearing loss is a common problem associated with ageing, and is likely to become more of an issue with changing population demographics in the developed world. There is no one single underlying process causing age related deafness and those managing the elderly with hearing loss need to be aware of the potential for underlying systemic pathology. The implications of hearing loss go far beyond an inability to hear and can have significant effects on the quality of life and function of the person concerned. It must also be remembered that the person with hearing loss is not the only one affected. Optimal management of the condition requires early recognition and input from a range of health professionals and is much more than the simple provision of a hearing aid.
Acknowledgements
Dr Anne Freeman, consultant physician, Royal Gwent Hospital, for her advice, support, and patience.
Footnotes
Funding: none.
Competing interests: none declared.
This article is part of a series on ageing edited by Professor Chris Bulpitt.
References
- 1.Nusbaum N J. Aging and sensory senescence. South Med J 199992267–275. [DOI] [PubMed] [Google Scholar]
- 2.Ballard C, Bannister C, Graham C.et al Associations of psychotic symptoms in dementia sufferers. Br J Psychiatry 1995167537–540. [DOI] [PubMed] [Google Scholar]
- 3.Lindenberger U, Baltes P B. Sensory functioning and intelligence in old age: a strong connection. Psychol Aging 19949339–355. [DOI] [PubMed] [Google Scholar]
- 4.Jones D A, Victor C R, Vetter N J. Hearing difficulty and its psychological implications for the elderly. J Epidemiol Community Health 19843875–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tesch‐Römer C. Psychological effects of hearing aid use in older adults. J Gerontol B Psychol Sci 199752127–138. [DOI] [PubMed] [Google Scholar]
- 6.Davis A.Hearing in adults. London: Whurr, 1995
- 7.Bridgwood A.General household survey: living in Britain—results from the 1998 general household survey. London: The Stationery Office Books, 2000
- 8.Pocock G, Richards C D.Human physiology: the basis of medicine. Oxford: Oxford University Press, 1999131–136.
- 9.Chandler J R. Partial occlusion of the external auditory meatus: its effect upon air and bone conduction hearing acuity. Laryngoscope 19647422–45. [DOI] [PubMed] [Google Scholar]
- 10.Rosenwasser H. Otic problems in the aged. Geriatrics 19641911–17. [PubMed] [Google Scholar]
- 11.Belal A. Presbyacusis: physiological or pathological. J Laryngol Otol 1975891011–1025. [DOI] [PubMed] [Google Scholar]
- 12.Chisolm T H, Willott J F, Lister J L. The aging auditory system: anatomic and physiologic changes and implications for rehabilitation. Int J Audiol 2003422S3–310. [PubMed] [Google Scholar]
- 13.Schuknecht H F, Gacek M R. Cochlear pathology in presbyacusis. Ann Otol Rhinol Laryngol 19931021–16. [DOI] [PubMed] [Google Scholar]
- 14.Browning G G.Clinical otology and audiology. London: Arnold, 2001
- 15.Johnsson L, Hawkins J E. Vascular changes in the human inner ear associated with aging. Ann Otol Rhinol Laryngol 197281364–376. [DOI] [PubMed] [Google Scholar]
- 16.Belal A, Glorig A. The ageing ear a clinico‐pathological classification. J Laryngol Otol 19971011131–1135. [DOI] [PubMed] [Google Scholar]
- 17.Hawkins J E. Comparative otopathology: aging, noise, and ototoxic drugs. Adv Otorhinolaryngol 197320125–141. [DOI] [PubMed] [Google Scholar]
- 18.Schuknecht H F. Further observations on the pathology of presbyacusis. Arch Otolaryngol 196480369–382. [DOI] [PubMed] [Google Scholar]
- 19.ZwarardemakerH Der Verlaustau hohen Tonen mit zunehmendem Alter. Ein neues Gesetz. Arch Ohr Nas und Kehlkopfheilkunde 18743253–56. [Google Scholar]
- 20.Kiessling L, Pichora‐Fuller M K, Gatehouse S.et al Candidature for and delivery of audiological services: special needs of older people. Int J Audiol 2003422S92–3101. [PubMed] [Google Scholar]
- 21.Arnesen A R. Presbyacusis—loss of neurons in the human cochlear nuclei. J Laryngol Otol 198296503–511. [DOI] [PubMed] [Google Scholar]
- 22.Pichora‐Fuller M K, Souza P E. Effects of aging on auditory processing of speech. Int J Audiol 2003422S11–2S16. [PubMed] [Google Scholar]
- 23.Luxon L M. The anatomy and pathology of the central auditory pathways. Br J Audiol 19811531–40. [DOI] [PubMed] [Google Scholar]
- 24.Jennings C R, Jones N S. Presbyacusis. J Laryngol Otol 2001115171–178. [DOI] [PubMed] [Google Scholar]
- 25.Gates G A, Myers R H. Genetic associations in age‐related hearing thresholds. Arch Otolaryngol Head Neck Surg 1999125654–659. [DOI] [PubMed] [Google Scholar]
- 26.Ensink R J, Camp G V, Cremers C W. Mitochondrial inherited hearing loss. Clin Otolaryngol 1998233–8. [DOI] [PubMed] [Google Scholar]
- 27.Wright A, Forge A, Kotecha B. Ototoxicity. In: Booth JB, ed. Scott Browns otolaryngology. Vol 3. Otology. Oxford: Butterworth Heinemann, 1997. 3/20/1–2/20/31
- 28.Lim D P, Stephens S D G. Clinical investigation of hearing loss in the elderly. Clin Otolaryngol 199116288–293. [DOI] [PubMed] [Google Scholar]
- 29.Schacht J. Aminoglycoside ototoxicity: prevention in sight? Otolaryngol Head Neck Surg 1998118674–677. [DOI] [PubMed] [Google Scholar]
- 30.British Medical Association and Royal Pharmaceutical Society of Great Britain British National Formulary 50. Sep 2005. London: British Medical Association and Royal Pharmaceutical Society of Great Britain, 2005
- 31.Brors D, Bodmer D. New aspects of inner ear research. Br J Hosp Med 200465392–395. [DOI] [PubMed] [Google Scholar]
- 32.Gates G A, Cobb J L, D'Agostino R B.et al The relation of hearing in the elderly to the presence of cardiovascular risk factors. Arch Otolaryngol Head Neck Surg 1993119156–161. [DOI] [PubMed] [Google Scholar]
- 33.Brown C G, Gatehouse S, Lowe G D. Blood viscosity as a factor in sensorineural hearing impairment. Lancet 1986i121–123. [DOI] [PubMed]
- 34.Rosen S, Olin P. Hearing loss and coronary heart disease. Arch Otolaryngol Head Neck Surg 196582236–243. [DOI] [PubMed] [Google Scholar]
- 35.Rosen S, Olin P, Rosen H V. Dietary prevention of hearing loss. Acta Otolaryngol 197070242–247. [DOI] [PubMed] [Google Scholar]
- 36.Yamazaki T, Ogwa K, Imoto T.et al Senile deafness and metabolic bone disease. Am J Otol 19889376–382. [PubMed] [Google Scholar]
- 37.Tay H L, Ray N, Ohri R.et al Diabetes mellitus and hearing loss. Clin Otolaryngol 199520130–134. [DOI] [PubMed] [Google Scholar]
- 38.Hall S J, Kerr A G, Varghese M.et al Deafness in hypothyroidism. (Abstract). Clin Otolatyngol 198510292 [Google Scholar]
- 39.Gates G A, Karzon R K, Garcia P.et al Auditory dysfunction in aging and senile dementia of the Alzheimer's type. Arch Neurol 199552626–634. [DOI] [PubMed] [Google Scholar]
- 40.Anonymous Hearing problems in elderly people: implications for services. Lancet 1987i1181–1182. [PubMed]
- 41.Weinstein B E. Geriatric hearing loss: myths, realities, resources for physicians. Geriatrics 19894442–60. [PubMed] [Google Scholar]
- 42.Pichora‐Fuller M K. Cognitive aging and auditory information processing. Int J Audiol 2003422S26–2S32. [PubMed] [Google Scholar]
- 43.Mulrow C D, Aguilar C, Endicot J E.et al Quality of life changes and hearing impairment: a randomised trial. Ann Intern Med 1990113118–194. [DOI] [PubMed] [Google Scholar]
- 44.Keller B K, Morton J L, Thomas V S.et al The effect of visual and hearing impairments on functional status. J Am Geriatr Soc 1999471319–1325. [DOI] [PubMed] [Google Scholar]
- 45.Arlinger S. Negative consequences of uncorrected hearing loss—a review. Int J Audiol 2003;42 2S17–20. [PubMed]
- 46.Sekuler R, Blake R. Sensory underload. Psychology Today 19872148–53. [Google Scholar]
- 47.Gennis V, Garry P J, Haaland K Y.et al Hearing and cognition in the elderly. Arch Intern Med 19911512259–2264. [PubMed] [Google Scholar]
- 48.Fisch U. Management of sudden deafness. Otolaryngol Head Neck Surg 1983913–8. [DOI] [PubMed] [Google Scholar]
- 49.Kveton J F. Evaluation and management of acoustic neuroma. Current Opinion in Otolaryngology and Head and Neck Surgery 1993153–63. [Google Scholar]
- 50.Snik A F M, Mylanus E A M, Cremers C W R G. Implantable hearing devices for sensorineural hearing loss: a review of the audiometric data. Clin Otolaryngol 199823414–419. [DOI] [PubMed] [Google Scholar]
- 51.Izumikawa M, Minoda R, Kawamoto K.et al Auditory hair cell replacement and hearing improvement by Atoh1 gene therapy in deaf mammals. Nature Medicine . 2005;11: 3,271–276. [DOI] [PubMed]
- 52.Ito J, Kojima K, Kawaguchi S. Survival of neural stem cells in the cochlea. Acta Otolaryngol 2001121140–142. [DOI] [PubMed] [Google Scholar]