Abstract
Background
South Asians have higher risk of diabetic complications compared with white Europeans. The aim of this study was to compare management of cardiovascular risk factors between Bangladeshis and white Europeans.
Methods
A retrospective survey of all diabetic patients attending an Inner London hospital diabetic clinic over one year was undertaken. Data were obtained from the hospital diabetes database: presence of macrovascular (myocardial infarction, angina, stroke, transient ischaemic attack, cardiac intervention) and microvascular disease (neuropathy, retinopathy, and nephropathy), glycated haemoglobin, blood pressure, lipids, smoking, and body mass index (BMI) were all determined.
Results
A total of 1162 white European and 912 Bangladeshi patients with full data available were included in the analyses. The groups were equivalent in age, sex, duration of diabetes. Compared with white Europeans, Bangladeshis had more macrovascular disease (19.5% v 11.9% p<0.01), sight threatening retinopathy (7.2% v 3.8%, p<0.01), and nephropathy (15.3% v 9.1%, p<0.01). In addition, Bangladeshis had significantly more male smokers (28.1% v 22.1%, p<0.01), poorer glycaemic control (mean HbA1c 8.6% v 8.1%, p = 0.039), greater proportion with uncontrolled hypercholesterolaemia (total cholesterol >5.0 mmol/l, 31.6% v 26% p = 0.05), and poorer control of blood pressure (proportion with BP >140/80 mm Hg, 43.2% v 32.1%, p<0.01).
Conclusions
South Asians with type 2 diabetes have poorer glycaemic, blood pressure, and lipid control than white Europeans. The reasons for this are probably multifactorial.
Keywords: diabetes, Bangladeshis, cardiovascular risk
South Asians (people originating from the India, Bangladesh, Pakistan, and Sri Lanka) are at high risk of developing type 2 diabetes.1 In the UK, South Asians, have a fourfold higher prevalence of type 2 diabetes, which also seems to present around 10 years earlier than in white Europeans.2 The reasons for this are unclear, although genetic, nutritional, lifestyle, and other factors have been suggested.3
Microvascular and macrovascular complications of diabetes, in particular cardiovascular disease,4 and renal disease,5 are more common in type 2 diabetic patients of South Asian origin. There is considerable evidence that intensive risk factor intervention can reduce complications of type 2 diabetes, including improving glycaemic, blood pressure, and lipid control.6
This study aimed to undertake a retrospective survey of the control of these factors over one year in a cohort of patients attending a large Inner London teaching hospital over the period of one year, and to compare the control of these factors between Bangladeshis (the predominant South Asian ethnic group in our area) and white Europeans.
Methods
Patients
Tower Hamlets is a deprived Inner London borough, with around one in four people of Bangladeshi origin. The borough has a standardised mortality ratio (SMR) of 145 for coronary heart disease (CHD), with type 2 diabetes an important contributor. Around 40% of patients attending the secondary care diabetes clinic run at the Royal London Hospital, are of Bangladeshi origin. Guidelines for referral to the secondary care diabetes clinics are well established and adhered to, and patients are requested to attend for blood tests before clinic attendance.
All patient consultations are recorded using the commercially available diabetes database, DIAMOND (Hicom, Surrey, UK), which was developed at the Royal London Hospital. The DIAMOND record contains all diabetes data relating to the patient, and a copy is sent to the GP from each visit, including clinical and blood test results, changes to therapy, and targets of therapy. Copies of the DIAMOND record are frequently sent to the patient. The survey retrospectively examined all the records of all patients who attended the clinic between 1 January 2003 and 1 January 2004 (that is, attended during 2003), identified from the database. Only patients with type 2 diabetes (diabetes diagnosed above the age of 30 years, and adequately controlled by diet and/or oral hypoglycaemic therapy for at least one year after diagnosis of diabetes) and with full data available were included in the analysis. All patients underwent a minimum of once yearly review of the following: macrovascular disease (new onset of angina, transient ischaemic attack (TIA) or claudication, previous known vascular disease), retinopathy (dilated fundoscopy or digital photography), neuropathy (foot ulceration, neuropathic symptoms, tuning fork vibration sense, fine touch perception with a 10 gram Semmes Weinstein monofilament), nephropathy (Albustix (Bayer Diagnostics, Newbury, UK) positive for albuminuria), blood pressure, body mass index, glycated haemoglobin (HbA1c, reference range 4.0%–6.0%), total cholesterol, and creatinine.
Data
Data gleaned included age, sex, ethnicity, and duration of diabetes. The presence of macrovascular disease was determined using ICD codes listed as myocardial infarction, angina, coronary artery bypass graft, peripheral vascular disease, stroke, TIA, and coronary intervention (bypass or angioplasty). Presence of neuropathy was defined as presence of neuropathic symptoms or loss of vibration, reduced fine touch perception with Semmes Weinstein monofilament, or foot ulceration. Presence of retinopathy was recorded as non‐sight threatening (background retinopathy) and sight threatening (pre‐proliferative, proliferative, and maculopathy). Presence of nephropathy was determined by the presence or absence of dipstick positive albuminuria. Other data obtained included most recent blood pressure, smoking status and BMI, most recent total cholesterol, and HbA1c. Pharmacotherapy for glycaemia, lipids, and blood pressure were also determined.
Statistics
Data are presented as mean (SD) or median (range). To examine differences in variables between the two groups, Student's t test was used. To compare the prevalence of complications or risk factors between the two groups, a χ2 test was performed for discrete variables, and Mann‐Whitney test for non‐normally distributed variables. Significance was achieved if Bonferroni corrected p = 0.05. Statistical methods were carried out using the statistical package Minitab (Minitab, PA, USA).
Results
During the survey period a total of 2610 individual patients with type 2 diabetes were seen in a total of 3983 consultations. No difference in number of clinic visits per year was seen between white Europeans and Bangladeshis. Full data were available for 2287 (87.6%) individual patients. Of the 323 patients without full data, ethnic origin was similar to that of patients with full data (Bangladeshi 40.5%, white European 50.7%, other 8.8%). Of the patients with incomplete data, the data missing were distributed as follows: HbA1c (45.8%, mostly attributable to haemoglobinopathy), total cholesterol (34.9%), blood pressure (31.8%).
A total of 912 patients (39.9%) were Bangladeshi in origin, and 1162 (50.8%) were white European in origin. Distribution of age and sex in the two groups was not significantly different (table 1). Duration of diabetes was longer in the Bangladeshi cohort, although this did not reach statistical significance (table 1).
Table 1 Demographic characteristics of patients surveyed.
| European | Bangladeshi | p Value | |
|---|---|---|---|
| Number | 1162 | 912 | – |
| Age (y) mean (SD) | 56.8 (5.4) | 52.1 (6.7) | 0.08 |
| Male n (%) | 604 (52.0) | 484 (53.1) | 0.88 |
| Duration of diabetes (y) mean (SD) | 12.2 (4.3) | 14.6 (4.3) | 0.14 |
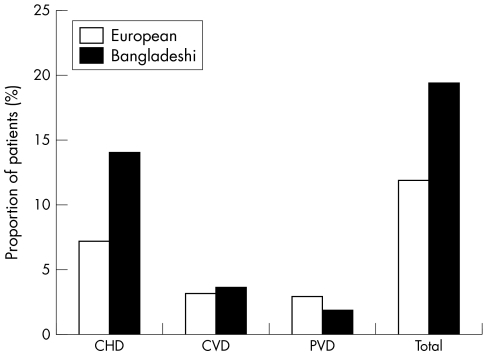
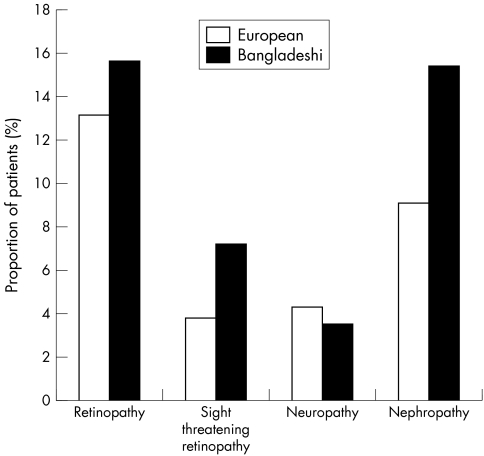
Figure 1 shows presence of known macrovascular disease. Significantly more Bangladeshi patients had known macrovascular disease (19.5% v 11.9%, p<0.01), in particular CHD (14.1% v 7.2% p<0.01). Figure 2 shows presence of microvascular complications of diabetes. There was no significant difference in presence of any grade of retinopathy (15.6% v 13.1% p = 0.12) or neuropathy (4.3% v 3.5% p = 0.26), but Bangladeshis had significantly more sight threatening retinopathy (7.2% v 3.8% p<0.01), probably as a result of significant more nephropathy, which is frequently associated with severe retinopathy (15.3% v 9.1%, p<0.01).
Figure 1 Presence of known macrovascular disease in the Bangladeshi and white European groups. CHD, coronary heart disease; CVD, cerebrovascular disease; PVD, peripheral vascular disease.
Figure 2 Presence of known microvascular disease in the Bangladeshi and white European groups.
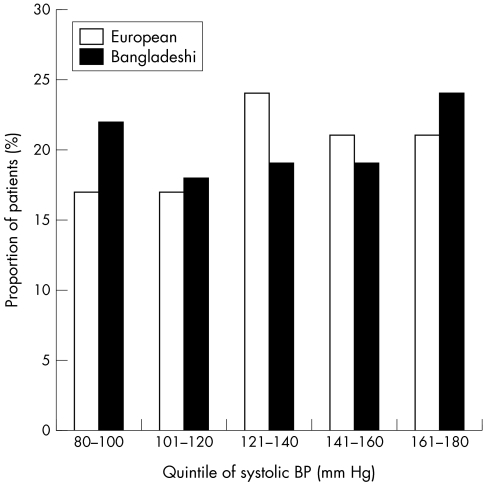
Table 2 shows the comparison of control of cardiovascular risk factors. Bangladeshis had significantly more male, and less female smokers. Median systolic and diastolic blood pressures were not significantly different (table 2). On examination of quintiles of systolic blood pressure, however (fig 3), the Bangladeshi group, had a significantly greater number of patients with systolic blood pressure in the lowest quintile (22% v 17%, p<0.01) and highest quintile (24% v 21%, p<0.01). Proportion of patients with poorly controlled blood pressure (>140/80 mm Hg) was higher in the Bangladeshi cohort (43.2% v 32.1%, p<0.01).
Table 2 Comparison of cardiovascular risk factors in Bangladeshi and white European patients with type 2 diabetes.
| European | Bangladeshi | Corrected p value | |
|---|---|---|---|
| Number | 1162 | 912 | – |
| Male smokers n (%) | 133 (22.1) | 136 (28.1) | <0.01 |
| Female smokers n (%) | 122 (21.8) | 6 (1.4) | <0.001 |
| Systolic blood pressure (mm Hg) median (range) | 129 (90–180) | 131 (80–180) | 0.76 |
| Diastolic blood pressure (mm Hg) median (range) | 79 (65–110) | 81 (65–110) | 0.56 |
| Total cholesterol (mmol/l) median (range) | 4.8 (3.2–7.2) | 5.1 (3.1–6.9) | 0.32 |
| Body mass index (kg/m2) median (range) | 27.2 (23.5–36.8) | 27.9 (22.4–30.4) | 0.81 |
| Height (m) median (range) | 1.70 (1.40–1.92) | 1.64 (1.43–1.87) | <0.01 |
| HbA1c (%) median (range) | 8.1 (5.6–12.3) | 8.6 (5.8–15.1) | <0.05 |
| HbA1c >7.5% n (%) | 605 (52.1) | 552 (58.7) | <0.01 |
| HbA1c >10.0% n (%) | 145 (12.5) | 161 (17.1) | <0.01 |
Figure 3 Plot of quintiles of systolic blood pressure in Bangladeshis and white European patients with type 2 diabetes.
Examination of total cholesterol showed no significant difference in the median total cholesterol between the two groups (table 2). Proportion of patients with known vascular disease receiving lipid lowering therapy was not significantly different between the two groups (87.6% v 80.9%, p = 0.18), and nor was the proportion of patients with absolute CHD risk greater than 15% who were receiving lipid lowering therapy (73.6% v 67.6%, p = 0.11). However, proportion of patients with uncontrolled total cholesterol (>5.0 mmol/l) was borderline significantly higher in the Bangladeshi group (31.6% v 26%, p = 0.05).
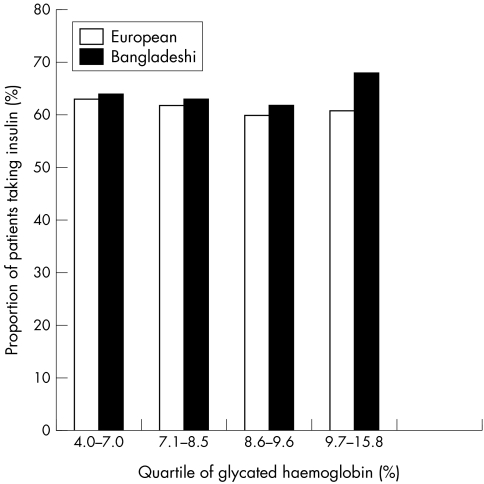
Bangladeshis had significantly poorer glycaemic control (table 2), with a median HbA1c of 8.6%, compared with a median of 8.1% in the white European group. Proportion of patients with inadequately controlled glycaemia (HbA1c >7.5%) and poorly controlled glycaemia (HbA1c >10%) was significantly higher in the Bangladeshi cohort. Examination of types of therapy used for glycaemia showed no significant difference in tablet or insulin use (insulin therapy in 67.4% of Bangladeshis v 65.1% of white Europeans). Dividing HbA1c into quartiles (fig 4), however, showed that significantly more Bangladeshi patients with HbA1c in the highest quartile were receiving insulin therapy (68% v 59%, p<0.01). Sub‐analysis of cholesterol, HbA1c, and blood pressure levels according to sex in the Bangladeshi and white European groups gave similar results to the combined analysis, suggesting that differences seen cannot be attributed to sex or smoking status.
Figure 4 Insulin therapy in pooled quartiles of glycated haemoglobin in Bangladeshis and white European patients with type 2 diabetes.
Discussion
Diabetes is common among South Asians living in South Asia, and in migrant populations living abroad.1,2 The excess risk of diabetes may be attributable to a greater propensity to insulin resistance at lower levels of excess adiposity. Insulin resistance is noted to be higher in South Asian children compared with white European children,7 and recently, the World Health Organisation criteria for overweight and obesity have been adjusted downwards in South Asians to recognise this greater risk.8 In addition, people of South Asian origin are at significantly higher risk of diabetic complications compared with white Europeans.4,5 Among the South Asian ethnic group, Bangladeshis (the predominant group in our area) are the most prone to premature cardiovascular disease, possibly because of particularly high rates of smoking.9
Our survey confirms that Bangladeshi patients with diabetes had higher rates of macrovascular and microvascular complications compared with white Europeans. Our results also suggest that Bangladeshis with type 2 diabetes had poorer control of risk factors contributing to the development of these complications, such as lipid, blood pressure, and glycaemic control, compared with white Europeans. These results are consistent with a previous survey in a separate population of South Asians, where we reported the finding of South Asians with young onset type 2 diabetes (diagnosed under age 40 years) having more complications and cardiovascular risk factors at diagnosis of type 2 diabetes, compared with white Europeans.10 Similar results have been reported in South Asian populations in Leicester, where renal and cardiovascular complications were more common in South Asians,11 despite no significant differences in cardiovascular risk factors. In a survey of a Birmingham diabetes clinic, significantly more white patients were taking insulin, but despite this, there was no significant difference in glycaemic control between the South Asian and Europeans cohorts.12
The reasons for poorer control of cardiovascular risk factors seen among Bangladeshis in our study are probably multifactorial. Type 2 diabetes occurs around 10 years earlier in patients of South Asian origin, although in our survey, the duration of diabetes was not significantly different between two cohorts. This suggests that there may have been a significant delay in the diagnosis of diabetes in the Bangladeshi cohort, although lower rates of referral of Bangladeshi patients from primary care is also possible. This is unlikely, however, as data from primary care suggest that a similar proportion of diabetic patients are of Bangladeshi origin attending primary care clinics, as there are attending secondary care. There is some recent evidence that diabetes in South Asians progresses more quickly than in white Europeans, which may account for the significantly poorer glycaemic control seen in the South Asian cohort.13 Concordance with lifestyle and pharmacotherapy in patients with diabetes may be different in South Asians. Previous surveys show that the knowledge of diabetes, cardiovascular disease, and their risk factors are poor among Bangladeshis.14 There is evidence that South Asians are more sedentary than white Europeans,15 but we know of no evidence that South Asians comply less with therapy than white Europeans, although it is possible that poorer compliance with oral hypoglycaemic, lipid lowering, or antihypertensive therapy may have contributed to our results. In our experience, reluctance to accept insulin therapy is commoner among Bangladeshi patients, and we are exploring the reasons for this in a separate qualitative study.
Poorer glycaemic control among Bangladeshis, despite similar insulin use, suggests inadequate escalation of dose, or poorer effect of insulin in Bangladeshi subjects. An interesting finding in our study is that a significant proportion of Bangladeshi patients with poor glycaemic control were taking insulin, and were not achieving adequate glycaemic control, suggesting that dose escalation, or adequate education in insulin management is not being achieved in these patients. The reasons for this need further exploration.
A further possibility is poorer awareness among health care professionals of the greater risks of complications in Bangladeshis. With language and cultural barriers, it may be the case that our Bangladeshi patients may not be being as aggressively treated at white Europeans. This seems unlikely, as we have been aware of the greater attrition rate of complications among Bangladeshis with diabetes in our area for two decades.16 In addition, there was no significant difference in types of treatment used for cholesterol, blood pressure, and glycaemic control, suggesting that appropriate therapies are being prescribed, but that appropriate escalation of therapy, or adequate concordance with therapy may not be being achieved.
Failure to achieve therapeutic targets is not uncommon in many aspects of chronic disease management. As in many secondary care diabetes clinics in the UK, we are unable to review patients more than one or twice per year for their diabetes, and in between clinic visits, we rely on primary care physicians and nurses to escalate therapy. Glycaemic, blood pressure, and lipid targets are suggested in our letter to the GP. Nevertheless, inadequate titration of therapy is common. Improvements in achieving cardiovascular risk modification may include the use of nurse led or pharmacist led protocols.17 Involving patients in goal setting may also be of benefit. We frequently send copies of clinic letters to patients, but plan to extend the routine copying of patients' letters to the patient. Patient held records are used in the clinic, although regrettably not widely adhered to. Increased educational support to patients and health care professionals is also required to highlight the need for careful cardiovascular risk modification in patients with diabetes, and we have established education sessions for patients in English and Bengali that tackle these issues. In addition, we run a diabetes education programme for primary health care professionals, along with support in primary care diabetes clinics from diabetes specialist nurses and a community diabetologist.
It is also possible that an as yet unknown, possibly genetic, factor may influence response to therapy. Similarly, a higher proportion of Bangladeshi patients with uncontrolled blood pressure, or lipids despite similar proportion on therapy also suggests inadequate titration of doses.
Previous studies have shown poorer risk factor control in other high risk populations with diabetes, such as Mexican Americans and African Americans.18,19,20 These findings are of concern, as patients who are at highest risk seem to be getting poorer outcomes. Studies have shown improved outcomes in high risk groups by undertaking education and algorithm based care for their patients.21 There is considerable evidence that culturally specific self management programmes can improve diabetes care in high risk populations,22,23,24 and a recent pilot study in the UK of the use of culturally tailored, community based interventions in South Asians has shown a small, but significant improvement in blood pressure and lipid control, but no improvement in glycaemic control.25
In summary, therefore, this study shows that Bangladeshis attending an Inner London diabetic clinic achieved poorer diabetes outcomes, and developed more complications than white Europeans. The reasons for this need to be better understood and tackled urgently to reduce the health inequalities that exist in this area.
Footnotes
Funding: none.
Conflicts of interest: none declared.
References
- 1.Anand S A, Yusuf S, Vuksan V.et al Difference in risk factors, atherosclerosis and cardiovascular disease between ethnic groups in Canada: the study of health assessment and risk in ethnic groups (SHARE). Lancet 2000356279–284. [DOI] [PubMed] [Google Scholar]
- 2.Mather H M, Keen H. The Southall diabetes survey: prevalence of known diabetes in Asians and Europeans. BMJ 19852911081–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bajaj M, Banerji M A. Type 2 diabetes in South Asians: a pathophysiologic focus on the Asian‐Indian epidemic. Curr Diab Rep 20044213–218. [DOI] [PubMed] [Google Scholar]
- 4.Mather H M, Chaturvedi N, Fuller J H. Mortality and morbidity from diabetes in South Asians and Europeans: 11 year follow‐up of the Southall diabetes survey, London, UK. Diabetic Med 19981553–59. [DOI] [PubMed] [Google Scholar]
- 5.Burden A C, McNally P G, Feehally J.et al Increased incidence of end stage renal failure from diabetes mellitus in Asian ethnic group in the United Kingdom. Diabet Med 19929641–645. [DOI] [PubMed] [Google Scholar]
- 6.Gaede P, Vedel P, Larsen N.et al Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. N Engl J Med 2003348383–393. [DOI] [PubMed] [Google Scholar]
- 7.Whincup P H, Gilg J A, Papacosta O.et al Early evidence of ethnic differences in cardiovascular risk: cross sectional comparison of British South Asian and white children. BMJ 2002324635–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.WHO Expert Consultation Appropriate body‐mass index for Asian populations and its implications for policy and intervention strategies. Lancet 2004363157–163. [DOI] [PubMed] [Google Scholar]
- 9.Bhopal R, Unwin N, White M.et al Heterogeneity of coronary heart disease risk factors in Indian, Pakistani, Bangladeshi, and European origin populations: cross sectional study. BMJ 1999319215–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chowdhury T A, Lasker S S. Complications and cardiovascular risk factors in South Asian and European patients with early onset type 2 diabetes. Q J Med 200295241–246. [DOI] [PubMed] [Google Scholar]
- 11.Samanta A, Burden A C, Jagger C. A comparison of the clinical features and vascular complications of diabetes between migrant Asians and Caucasians in Leicester, UK. Diabetes Res Clin Pract 199114205–213. [DOI] [PubMed] [Google Scholar]
- 12.Close C F, Lewis P G, Holder R.et al Diabetes care in South Asians and white European patients with type 2 diabetes. Diabet Med 199512619–621. [DOI] [PubMed] [Google Scholar]
- 13.Mukhopadhyay B, Fisher M, Kesson C M.et al South Asians develop type 2 diabetes earlier than Caucasians and have a more rapid deterioration in metabolic control over time. Diabet Med 200421(suppl 2)16 [Google Scholar]
- 14.Joint Health Surveys Unit Health survey for England 1999: the health of minority ethnic groups. London: The Stationery Office, 2001
- 15.Fischbacher C M, Hunt S, Alexander L. How physically active are South Asians in the United Kingdom: literature review. J Public Health 200426250–258. [DOI] [PubMed] [Google Scholar]
- 16.McKeigue P M, Marmot M G, Syndercombe Court Y D.et al Diabetes, hyperinsulinaemia, and coronary risk factors in Bangladeshis in east London. Br Heart J 198860390–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leal S, Glover J J, Herrier R N.et al Improving quality of care in diabetes through a comprehensive pharmacist‐based disease management program. Diabetes Care 2004272983–2984. [DOI] [PubMed] [Google Scholar]
- 18.Bonds D E, Zaccaro D J, Karter A J.et al Ethnic and racial differences in diabetes care: the insulin resistance atherosclerosis study. Diabetes Care 2003261040–1046. [DOI] [PubMed] [Google Scholar]
- 19.Gallegos‐Macias A R, Macias S R, Kaufman E.et al Relationship between glycemic control, ethnicity and socioeconomic status in Hispanic and white non‐Hispanic youths with type 1 diabetes mellitus. Pediatr Diabetes 2003419–23. [DOI] [PubMed] [Google Scholar]
- 20.Harris M I. Racial and ethnic differences in health care access and health outcomes for adults with type 2 diabetes. Diabetes Care 200124454–459. [DOI] [PubMed] [Google Scholar]
- 21.Fanning E L, Selwyn B J, Larme A C.et al Improving efficacy of diabetes management using treatment algorithms in a mainly Hispanic population. Diabetes Care 2004271638–1646. [DOI] [PubMed] [Google Scholar]
- 22.Banister N A, Jastrow S T, Hodges V.et al Diabetes self‐management training program in a community clinic improves patient outcomes at modest cost. J Am Diet Assoc 2004104807–810. [DOI] [PubMed] [Google Scholar]
- 23.Davidson M B. Effect of nurse‐directed diabetes care in a minority population. Diabetes Care 2003262281–2287. [DOI] [PubMed] [Google Scholar]
- 24.Brown S A, Garcia A A, Kouzekanani K.et al Culturally competent diabetes self‐management education for Mexican Americans: the Starr County border health initiative. Diabetes Care 200225259–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.O'Hare J P, Raymond N T, Mughal S.et al Evaluation of delivery of enhanced diabetes care to patients of South Asian ethnicity: the United Kingdom Asian diabetes study (UKADS). Diabet Med 2004211357–1365. [DOI] [PubMed] [Google Scholar]