Abstract
Background
Information about long term outcomes of patients with acute coronary syndromes (ACS) who have clinically diagnosed heart failure is scarce.
Methods
In a UK registry, this study evaluated patients with non‐ST elevation ACS, recording treatment, and clinical outcomes for six months. In a subgroup, a four year mortality follow up was performed to estimate the impact of the clinical diagnosis of heart failure on survival.
Results
Of 1046 patients, 139 (13%) had a history of clinically diagnosed heart failure. At discharge, ACE inhibitors were prescribed for 58% and 28%, of those with and without a history of heart failure respectively (p<0.001). Rates of angiography, percutaneous intervention, and coronary artery bypass graft were 17.3% and 29.2% (p = 0.003), 5.0% and 8.4% (p = 0.17), and 5.0% and 7.5% (p = 0.3) for these groups respectively. Death or new myocardial infarction at six months occurred in 22% and 10% (p<0.001) and at four years death occurred in 60% and 20% of these groups respectively (p<0.001). In a multivariate analysis prior heart failure carried an odds ratio of 2.0 (p = 0.001) for death or myocardial infarction at six months and 2.4 (p<0.001) for death over four years. New heart failure was associated with an increased risk of death at six months (20% compared with 5%, p<0.001).
Conclusion
A clinical history of heart failure carries a substantial risk of death in patients admitted with ACS without ST elevation. Nearly 60% of those with prior heart failure are dead after four years. After adjustment for confounding factors, prior heart failure more than doubles the risk compared with those with no history.
Keywords: acute coronary syndromes, epidemiology, heart failure
The syndrome of heart failure is an important global health care problem. In Western Europe, with a population of about 300 million, it is estimated that 3 million people are affected. In the United Kingdom, heart failure accounts for 1%–2% of the total health care budget,1,2 and is associated with average five year death rates of 30%–40%.3,4 As coronary artery disease is one of the most common causes of heart failure, it is to be expected that a substantial proportion of patients will be admitted to the hospital with an episode of acute coronary syndrome with a history of heart failure.
We undertook the Prospective Registry of Acute Ischaemic Syndromes in the UK (PRAIS‐UK) to determine characteristics, practice patterns, outcomes, and important markers of risk of patients admitted to a wide range of UK hospitals with acute coronary syndromes (ACS) without ST elevation. We present data on the management and outcomes of patients with either pre‐existing or newly diagnosed clinical heart failure after an admission with an ACS, with a maximum of four years of follow up.
Methods
PRAIS‐UK was a prospective observational cohort registry of patients admitted to UK hospitals with ACS. The methods have been provided previously.5 In brief, 56 hospitals participated. Each hospital was asked to collect data on 20 consecutive eligible patients, irrespective of admission location or consultant team. Patients were enrolled from May 1998 to February 1999. Patients were followed up for six months after their index hospital admission. Patients with a clinical diagnosis of ACS without ST elevation (unstable angina or suspected non‐Q wave myocardial infarction (MI)) were eligible if they were admitted to the hospital through either the accident and emergency department or directly to the coronary care unit or other wards. A typical history of cardiac chest pain was required together with either ECG abnormalities consistent with myocardial ischaemia or a history of pre‐existing evidence of coronary artery disease (for example, prior MI, prior revascularisation). The exclusion criteria were ST elevation >1 mm in two or more contiguous leads on the ECG or planned or actual treatment with thrombolytic therapy on admission. Heart failure was defined by experienced physicians on clinical grounds, although echocardiography was not mandatory as part of this evaluation. The study had ethical approval from the multicentre and local research ethics committee and patients provided informed consent before entry. Collaborating sites and investigators are listed in Collinson et al.5
The decision to perform long term follow up was taken three months after starting enrolment in PRAIS‐UK. Although multicentre research ethics committee approval for long term follow up was obtained for all centres, local approvals could only be requested from hospitals that were still enrolling patients, or those that had not yet started enrolment. Local ethical approvals were eventually obtained in 34 of the 56 hospitals that enrolled a total of 653 patients with complete follow up for mortality at six months. Of these, 490 patients gave consent for long term mortality follow up. The Office of National Statistics (ONS) for England, Wales and Northern Ireland, and the General Register Office (GRO) of Scotland provided vital status as of 15 November 2002.
Statistical analysis
Baseline characteristics, treatments, and outcomes of all patients with and without a prior clinical history of heart failure were summarised. The main outcome at six months was death or non‐fatal MI (ST or non‐ST elevation), and for long term follow up was all cause mortality. Continuous and categorical data were compared with the use of Student's t and χ2 tests respectively. Time to event plots were drawn using the Kaplan Meier method and compared using the log‐rank test. Cox proportional regression model was used to calculate univariate and multivariate hazard ratios and their 95% confidence intervals (CI) for time to event outcomes. Univariate and multivariate relative hazards and 95% CIs for adverse outcomes were calculated using Cox regression models. Variables entered into the multivariate model included age, sex, diabetes, smoking status, chest pain or ischaemic ECG changes on admission, a history of any of the following: MI, heart failure, hypertension, hypercholesterolaemia (on treatment), stroke or coronary revascularisation (PTCA or CABG), and pre‐discharge angiography or revascularisation (PTCA or CABG). The ECG categories were: 1 = normal, 2 = ST depression or bundle branch block, and 3 = T wave inversion or non‐specific ST segment abnormalities. All statistical analyses were performed using Stata version 7.0.
Results
Patients with prior clinically diagnosed heart failure (see table 1)
Table 1 Characteristics, treatments, and outcomes of patients admitted with history of heart failure.
| With prior heart failure (n = 139) | No prior heart failure (n = 907) | p Value | |
|---|---|---|---|
| Age (SD) | 72.8 (9.7 | 64.7 (11.8 | <0.001 |
| >70 (%) | 65.5 | 36.3 | <0.001 |
| Female (%) | 50.4 | 37.6 | 0.004 |
| Diabetes (%) | 25.9 | 14.8 | 0.001 |
| Hypertension (%) | 43.9 | 36.1 | 0.08 |
| Prior MI (%) | 68.4 | 45.1 | <0.001 |
| Prior PTCA | 14.4 | 13.2 | 0.71 |
| Prior CABG | 20.9 | 12.5 | 0.007 |
| Prior angina | 91.4 | 71.9 | <0.001 |
| Admission ECG | |||
| ST depression/BBB (%) | 46.0 | 26.5 | <0.001 |
| Other (T, etc) (%) | 48.2 | 56.1 | |
| Normal (%) | 5.8 | 17.4 | |
| In‐hospital treatment | |||
| Aspirin (%) | 79.1 | 88.5 | 0.002 |
| LMWH (%) | 38.1 | 44.9 | 0.14 |
| IV UFH (%) | 33.6 | 33.6 | 1.0 |
| Either LMWH or IV UFH (%) | 70.5 | 77.8 | 0.08 |
| Treatment at six months | |||
| Aspirin (%) | 73.6 | 83.4 | 0.008 |
| β blockers (%) | 17.4 | 46.1 | <0.001 |
| Ca channel antagonists (%) | 45.5 | 43.3 | 0.65 |
| Nitrate (%) | 75.2 | 55.4 | <0.001 |
| K channel opener (%) | 31.4 | 18.8 | 0.001 |
| Statins (%) | 35.5 | 48.3 | 0.008 |
| Oral anticoagulants (%) | 20.7 | 8.9 | <0.001 |
| ACE inhibitors (%) | 58.7 | 28.2 | <0.001 |
| α2 antagonists (%) | 10.7 | 2.8 | <0.001 |
| In‐hospital investigation | |||
| Stress test (%) | 4.3 | 14.2 | 0.001 |
| Investigation at six months | |||
| Angiography (%) | 17.3 | 29.2 | 0.003 |
| PTCA (%) | 5.0 | 8.4 | 0.17 |
| CABG (%) | 5.0 | 7.5 | 0.3 |
| All events to follow up | |||
| Death (%) | 18.7 | 5.5 | <0.001 |
| Death/MI (%) | 23.8 | 10.8 | <0.001 |
| Death/MI/RFA/UA (%) | 42.8 | 27.8 | <0.001 |
| Heart failure | 42.5 | 6.5 | <0.001 |
| Major bleed | 4.3 | 0.8 | <0.001 |
At the time of the index hospital admission, 139 (13.3%) patients had a previous history of clinical heart failure (907 patients without). Mean age in patients with a prior history of heart failure was higher than those without (73 compared with 65 years, p<0.001). Patients with prior heart failure also had higher rates of diabetes, treated hypertension, and prior MI.
Patients with previous clinical heart failure were less likely to be treated with aspirin in hospital (79.1% compared with 88.5% for those without heart failure, p = 0.002). There were no significant differences in the use of unfractionated or low molecular weight heparin.
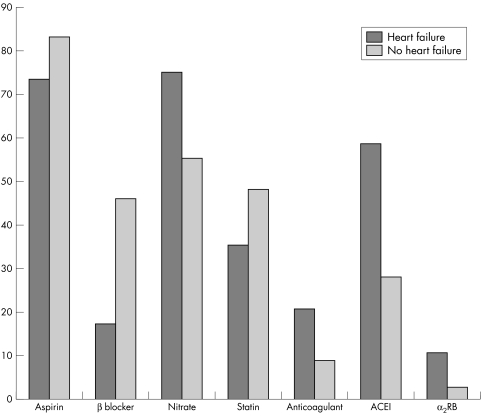
At the time of the six month follow up, aspirin was used in 73.6% and 83.4% (p = 0.008), β blockers in 17.4% and 46.1% (p<0.001), ACE inhibitors in 58.7% and 28.2% (p<0.001) and oral anticoagulants in 20.7 and 8.9% (p<0.001) of patients with and without a history of clinical heart failure respectively.
In hospital stress testing (including nuclear imaging) was performed in 4.3% of those with prior clinical heart failure compared with 14.2% of those without (p = 0.001). At six months, rates of angiography were 17.3% and 29.2% respectively (p = 0.003). Rates of PCI were 5.0% and 8.4% (p = 0.17), and for CABG were 5.0% and 7.5% (p = 0.3) for these groups respectively. Rates of revascularisation were not statistically different, therefore proportionately more of those patients with clinical heart failure who were invasively assessed proceeded to revascularisation.
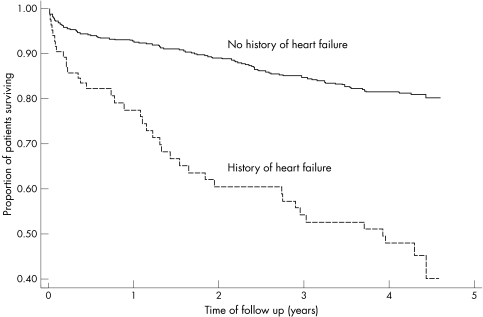
Figure 1 Unadjusted KM curves for long term rates of death for patients with and without history of heart failure.
Figure 2 Drug treatments at six months in patients with and without history of heart failure.
Unadjusted rates of death after six months were 18.7% compared with 5.5% and rates of death or new MI (ST or non‐ST elevation) 23.8% compared with 10.8% (p<0.001 for both comparisons) in the respective groups. Rates of major bleeds were 4.3% and 0.8% respectively (p<0.001).
After long term follow up, unadjusted rates of death were 59.6% and 19.5% for those with and without a history of clinical heart failure respectively (p<0.001). After adjusting for confounding factors, the hazard ratio was 2.41 (95% confidence intervals 1.60 to 3.63, p<0.001).
Patients developing new clinical heart failure (see table 2)
Table 2 Characteristics, treatments, and outcomes of patients developing new heart failure.
| New heart failure (n = 59) | No new heart failure (n = 848) | p Value | |
|---|---|---|---|
| >70 (%) | 54.2 | 35.0 | 0.003 |
| Female (%) | 44.1 | 37.2 | 0.29 |
| Diabetes (%) | 32.2 | 13.6 | <0.001 |
| Hypertension (%) | 44.1 | 35.5 | 0.19 |
| Smoker (%) | 18.6 | 24.7 | 0.30 |
| Prior MI (%) | 45.8 | 45.1 | 0.92 |
| Prior PTCA | 8.5 | 13.6 | 0.27 |
| Prior CABG | 15.3 | 12.3 | 0.5 |
| Prior angina | 72.9 | 71.7 | 0.85 |
| In‐hospital treatment | |||
| Aspirin (%) | 83.6 | 88.8 | 0.24 |
| LMWH (%) | 49.1 | 44.7 | 0.52 |
| IV UFH (%) | 41.8 | 33.0 | 0.18 |
| Either LMWH or IV UFH (%) | 83.1 | 77.5 | 0.32 |
| Treatment at six months | |||
| Aspirin (%) | 75.0 | 83.9 | 0.09 |
| β blockers (%) | 23.1 | 47.6 | 0.001 |
| Ca channel antagonists (%) | 23.1 | 44.6 | 0.002 |
| Nitrate (%) | 50.0 | 55.8 | 0.42 |
| K channel opener (%) | 21.2 | 18.7 | 0.66 |
| Statins (%) | 51.9 | 48.1 | 0.59 |
| Oral anticoagulants (%) | 25.0 | 7.9 | <0.001 |
| ACE inhibitors (%) | 51.9 | 26.7 | <0.001 |
| α2 antagonists (%) | 9.6 | 2.4 | 0.002 |
| Investigation at six months | |||
| Angiography (%) | 30.5 | 29.1 | 0.82 |
| PTCA (%) | 10.2 | 8.3 | 0.6 |
| CABG (%) | 11.1 | 5.4 | 0.08 |
| All events to follow up | |||
| Death (%) | 20.3 | 4.5 | <0.001 |
| Death/MI (%) | 25.4 | 9.8 | <0.001 |
| Death/MI/RFA/UA (%) | 49.2 | 31.6 | 0.06 |
| Major bleed | 5.1 | 0.5 | <0.001 |
After entry in the study, clinical heart failure developed in 59 (6.5%) of patients without a history of heart failure over six months of follow up. Compared with the group that did not develop clinical heart failure, these patients were older (proportion aged over 70, 54.2% versus 35.0%, p = 0.003) and had more diabetes (32.2% versus 13.6%, p<0.001), but otherwise there were no significant differences in baseline characteristics. The in‐hospital use of antiplatelet agents and antithrombotic treatments did not differ between the groups.
At six months, those developing new heart failure were less likely to be treated with β blockers (23.1% compared with 47.6%, p = 0.001) and calcium channel antagonists (23.1% compared with 44.6%, p = 0.002) but more likely to be treated with oral anticoagulants (25.0% compared with 7.9%, p<0.001), ACE inhibitors (51.9% compared with 26.7%, p<0.001), and angiotensin 2 antagonists (9.6% compared with 2.4%, p = 0.002). There were no differences in the use of angiography or PTCA, although there was a trend to the use of more CABG in the new heart failure group (11.1% compared with 5.4%, p = 0.08).
At six month follow up, rates of death were 20.3% and 4.5% and rates of death or new MI 25.4% and 9.8%, for those developing new heart failure and those not respectively (p<0.001 for both comparisons). Rates of major bleeding were 5.1% and 0.5% respectively (p<0.001).
Discussion
Our study shows that one in eight patients with non‐ST elevation ACS have a history of heart failure, but by four years, 60% of these patients are dead. Development of new heart failure in those with no such history also predicts poor prognosis. Although ACE inhibitors and α2 blockers are used more frequently in those with heart failure, rates are still suboptimal at 60%. Use of β blockers in these patients was low, but our study was conducted in the period before the introduction of β blockers for heart failure.6,7,8
Results from the Global Registry of Acute Coronary Events (GRACE) showed that patients with heart failure were significantly older and less likely to be male or smokers. They also were less likely to undergo cardiac catheterisation procedures and to receive β blockers and statins than patients without heart failure.9 These findings are consistent with our results. Heart failure has been consistently shown to be an independent marker of important clinical end points in several randomised clinical trials and observational studies.9,10,11,12,13,14,15,16,17 However, these studies frequently mixed all ACS groups (non‐ST and ST elevation ACS), thus our results confirm these findings specifically for patients with non ST‐elevation ACS and also extend the duration of follow up to four years.
Studies such as HOPE18 and EUROPA18 suggest that ACE inhibitor use should be high in patients with ACS, irrespective of heart failure and this may reduce the burden of developing heart failure. Given the poor outcomes of patients developing heart failure, aggressive strategies to reduce future risk are clearly warranted. Thus the use of ACE inhibitors in patients with ACS and heart failure should be mandatory, unless there are overriding contraindications. Pre‐discharge echocardiography should be mandatory both for patients with clinical heart failure as well as being a routine strategy to identify patients with asymptomatic left ventricular dysfunction. This would allow targeting of treatments to patients at higher risk of future events. With more recent evidence on the efficacy of β blockers in heart failure, it is also essential to ensure that patients with heart failure and ACS are treated with a β blocker.6,7,8 The prognosis of patients with significant coronary artery disease and left ventricular dysfunction are generally improved after coronary revascularisation, even though the risk of the procedure may be higher than in similar patients without left ventricular dysfunction.20,21 The selection of patients with left ventricular dysfunction who may benefit most from revascularisation is under investigation.22
The study is limited by not including formal assessment of left ventricular function with the classification of heart failure being made by experienced clinical investigators. Heart failure is a clinical diagnosis, however, in the absence of formal left ventricular function assessment, it is possible that some patients were mis‐classified. In addition, we did not record diuretic use.
Heart failure associated with ACS without ST elevation carries a very high rate of death and other adverse events. This excess risk continues at long term follow up. Drug therapies known to improve prognosis in patients with heart failure are under‐used and an increase in the use of ACE inhibitors and β blockers would probably have an important clinical impact. It is also probable that these high risk patients may gain from early angiography and revascularisation. Any patient with a history of heart failure or who develops heart failure in the context of an ACS needs to be treated as high risk.
Abbreviations
CABG - coronary artery bypass graft
ACS - acute coronary syndrome
PCI - percutaneous intervention
MI - myocardial infarction
Footnotes
Funding: none.
Conflicts of interest: none declared.
References
- 1.McGuire A J, Stewart S, Jenkins A.et al The current cost of heart failure to the National Health Service in the UK. European Journal of Heart Failure 20024361–371. [DOI] [PubMed] [Google Scholar]
- 2.Kannel W B. Heart failure. In: Poole‐Wilson PA, Colucci WS, Massie BM, et al, eds. New York: Churchill Livingstone, 1997279–288.
- 3.Ho K K, Anderson K M, Kannel W B.et al Survival after the onset of congestive heart failure in Framingham heart study subjects. Circulation 199388107–115. [DOI] [PubMed] [Google Scholar]
- 4.Cowie M R, Wood D A, Thompson S G.et al Incidence and aetiology of heart failure. A population‐based study. Eur Heart J 199920421–428. [DOI] [PubMed] [Google Scholar]
- 5.Collinson J, Flather M D, Fox K A.et al Clinical outcomes, risk stratification and practice patterns of unstable angina and myocardial infarction without ST elevation: Prospective Registry of Acute Ischaemic Syndromes in the UK (PRAIS‐UK). Eur Heart J 2000211450–1457. [DOI] [PubMed] [Google Scholar]
- 6.CIBIS‐II Investigators and Committees The cardiac insufficiency and bisoprolol study II (CIBIS‐II): a randomized trial. Lancet 19993539–13. [PubMed] [Google Scholar]
- 7.MERIT‐HF Study Group Effect of metoprolol CR/XL in chronic heart failure: Metoprolol CR/XL randomised intervention rrial in congestive heart failure (MERIT‐HF). Lancet 19993532001–2007. [PubMed] [Google Scholar]
- 8.Packer M, Fowler M B, Roecker E B.et al Effect of carvedilol on the morbidity of patients with severe chronic heart failure: results of the carvedilol prospective randomized cumulative survival (COPERNICUS) study. Circulation 20021062194–2199. [DOI] [PubMed] [Google Scholar]
- 9.Steg P G, Dabbous O H, Feldman O J.et al Determinants and prognostic impact of heart failure complicating acute coronary syndromes. Observations from the Global Registry of Acute Coronary Events (GRACE). Circulation 2004109494–499. [DOI] [PubMed] [Google Scholar]
- 10.Marchioli R, Avanzini F, Barzi F.et al Assessment of absolute risk of death after myocardial infarction by use of multiple risk‐factors assessment equations: GISSI‐Prevenzione mortality risk chart. Eur Heart J 2001222085–2103. [DOI] [PubMed] [Google Scholar]
- 11. Reference withdrawn
- 12.Newby L K, Bapkhar M V, White H.et al Predictors of 90 day outcome in stabilized patients after acute coronary syndromes. Eur Heart J 20032172–181. [DOI] [PubMed] [Google Scholar]
- 13.Richards A M, Nichols M G, Troughton R W.et al Antecendent hypertension and heart failure after myocardial infarction. J Am Coll Cardiol 2002391182–1188. [DOI] [PubMed] [Google Scholar]
- 14.de Kam P J, Nicolosi G L, Voors A A.et al Prediction of 6 months left ventricular dilatation after myocardial infarction in relation to cardiac morbidity and mortality. Application of a new dilatation model to GISSI 3 data. Eur Heart J 20023536–542. [DOI] [PubMed] [Google Scholar]
- 15.Zornoff L A, Skali H, Pfeffer M A.et al Right ventricular dysfunction and risk of heart failure and mortality after myocardial infarction. J Am Coll Cardiol 2002391450–1455. [DOI] [PubMed] [Google Scholar]
- 16.Boersma E, Pieper K S, Steyerberg E W.et al Predictors of outcome in patients with acuter coronary syndromes without persistent ST‐segment elevation. Results from an international trial of 9461 patients. The PURSUIT investigators. Circulation 20001012557–2567. [DOI] [PubMed] [Google Scholar]
- 17.Herlitz J, Karlson B W, Lindqvist J.et al Prognosis and risk indicators of death during a period of 10 years for women admitted to the emergency department with a suspected acute coronary syndrome. Int J Cardiol 200282259–268. [DOI] [PubMed] [Google Scholar]
- 18.Yusuf S, Sleight P, Pogue J.et al Effects of an angiotensin‐converting‐enzyme inhibitor, ramipril, on cardiovascular events in high‐risk patients. Heart Outcomes Prevention Evaluation Study Investigators. N Engl J Med 2000342145–153. [DOI] [PubMed] [Google Scholar]
- 19.EURopean trial On reduction of cardiac events with perindopril in stable coronary artery disease investigators Efficacy of perindopril in reduction of cardiovascular events among patients with stable coronary artery disease: randomised, double‐blind, placebo‐controlled, multicentre trial (the EUROPA study). Lancet 2003362782–788. [DOI] [PubMed] [Google Scholar]
- 20.Argenziano M, Spitritz H M, Whay W.et al Risk stratification for coronary bypass surgery in patients with left ventricular dysfunction. Analysis of the CABG patch trial database. Circulation 1999100II119–II124. [DOI] [PubMed] [Google Scholar]
- 21.Alderman E L, Bourassa M G, Cohen L S.et al Ten‐year follow‐up of survival and myocardial infarction in the randomized Coronary artery surgery study. Circulation 1990821629–1646. [DOI] [PubMed] [Google Scholar]
- 22.Cleland J G, Freemantle N, Ball S G.et al The heart failure revascularisation trial (HEART): rationale, design and methodology. European Journal of Heart Failure 20035295–303. [DOI] [PubMed] [Google Scholar]