Abstract
Introduction
An outbreak of echovirus meningitis occurred in the north west of England in 2001. This paper reviewed the clinical features and the role of different diagnostic methods.
Methods
This was a prospective study of adults admitted to a regional infectious disease unit with a probable diagnosis of meningitis, March to August 2001.
Results
Half the 40 cases were male; median age was 28 (range 16–51) years. Fifteen of 38 (39.5%) were smokers, and 20 of 24 (83.3%) had close contact with children. Median (range) duration of symptoms was 1.1 (0.25–7) days. Symptoms included headache (100%), photophobia (87.5%), and nausea (67.5%), and severity ranged from minimal signs to those consistent with a meningoencephalitis. The diagnosis was confirmed virologically in 29 of 40 (72%); echovirus 30 was isolated from six. Cerebrospinal fluid (CSF) enterovirus polymerase chain reaction (PCR) was positive in 26 of 32 (81%), and CSF virus culture in 3 of 16 (19%). Thirty one per cent of CSF samples had a neutrophil predominance, and 3 of 29 (10%) virologically confirmed cases had normal CSF microscopy and biochemistry.
Conclusion
CSF microscopy may be normal or suggest bacterial meningitis in a substantial minority of cases of echovirus meningitis. CSF PCR for enterovirus seems to be more sensitive than virus culture of CSF, although PCR does not yield information on circulating virus type. Early and accurate diagnosis could reduce both use of parenteral antibiotics and length of hospital stay with both morbidity and cost implications. Close contact with children may be a risk factor, particularly if good hygiene measures are not practised.
Keywords: aseptic meningitis, viral, enterovirus, polymerase chain reaction, cerebrospinal fluid
Enteroviruses are RNA viruses belonging to the picornaviridae family, and include echoviruses, coxsackieviruses A and B, enteroviruses 68 to 71, and poliovirus. Coxsackieviruses and echoviruses are major aetiological agents of viral meningitis, and account for about 80% of cases in children and adults.1,2,3 A total of 64 enterovirus serotypes have been identified, and 30 echovirus serotypes. Clinical manifestations of enterovirus infection range from asymptomatic carriage to life threatening disease. Other disease manifestations include mild febrile illness, respiratory disease (pharyngitis, bronchitis, bronchilitis), rash, hand foot and mouth disease, myocarditis, encephalitis, and neonatal sepsis.4,5
In the northern hemisphere, enterovirus infections predominate in summer and autumn, although sporadic cases occur throughout the year. Young children are most susceptible, with viral meningitis being about five to eight times more common in children than adults.6 Enteroviruses are spread predominantly by the faecal‐oral route, although infection may also occur through the oral‐oral route and by upper respiratory tract infections.2 No specific prevention or control measures are available for the non‐polio enteroviruses, however good hygiene practices such as thorough hand washing after nappy changes, disinfection of contaminated surfaces, and avoidance of shared utensils may help interrupt transmission.7
In clinical practice, rapid diagnostic confirmation of viral meningitis is advantageous, and avoids unnecessary treatment with antibiotics, reduces the length of hospitalisation, and ultimately reduces costs. Virus culture, the standard technique for enterovirus detection, is time consuming, and has poor sensitivity, but provides serotype data on the virus causing disease.8 While the identification of a specific enterovirus may be of limited interest to clinicians, it is of considerable epidemiological and public health importance in identifying outbreaks and understanding circulation patterns. Molecular methods of diagnosis such as polymerase chain reaction (PCR) and sequencing have increasingly become available, and serotype specific primers have been developed for several enteroviruses. A rapid, sensitive RT‐nested PCR assay based on VP1 has been used directly on clinical samples in an outbreak of aseptic meningitis, to type the group B enteroviruses causing aseptic meningitis.9
Echovirus 30 is one of the most frequently isolated enteroviruses in Europe, and North America.10,11,12 It follows an epidemic mode of transmission, causing large outbreaks, and then becomes quiescent for a period of several years.13,14 This quiescence is probably attributable to the development of population immunity that occurs in a high infection rate epidemic. Echovirus 30 was also the commonest enterovirus identified in a prospective study of 61 children with enteroviral meningitis.3
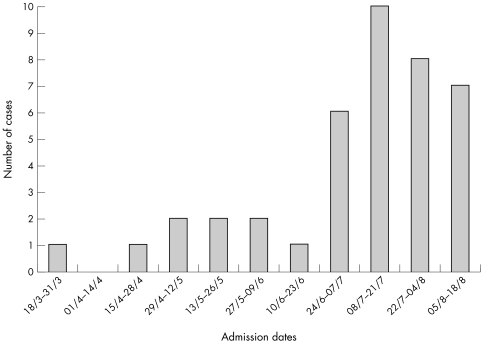
Between March and August 2001, an outbreak of echovirus 30 meningitis occurred in the north west of England (fig 1). This was mirrored by above normal levels of confirmed echovirus elsewhere in England and Wales.15,16 The clinical features and different diagnostic methods are reviewed here.
Figure 1 Fortnightly distribution of cases of viral meningitis presenting to a regional infectious disease unit.
Methods
All adult patients admitted to University Hospital Aintree with a diagnosis of viral meningitis were recruited. Patients were identified from the laboratory records of all cerebrospinal (CSF) samples processed for possible meningitis between 30 March 2001 and 16 August 2001, throat swabs or faeces samples for culture for enteroviruses, and of serum samples sent for pathogens implicated in viral meningitis. These searches were supplemented by a review of the prospectively maintained ward based diagnostic index on the infectious disease unit and review of unit discharge letters covering the same period. All patients were admitted to the infectious disease unit either directly or from the accident and emergency unit. Clinical and laboratory parameters were recorded on a specifically designed form. CSF cell total and differential counts and biochemistry were done in all patients. Virus was cultured using two cell lines, secondary rhesus monkey kidney (RMK) and MRC‐5 (human embryo lung) cells. After inoculation the cells were examined daily for 10 days for evidence of cytopathic effects. Enteroviruses were initially identified by the characteristic cytopathic effect produced, and individual serotypes identified by fluorescent antibody testing using polyvalent and individual fluorescein labelled antibodies. Detection of enterovirus by nucleic acid amplification used a two step reverse transcriptase Taqman real time PCR.17 The assay is specific for enterovirus and paraechovirus, and does not amplify other viruses. Throat swabs were sent for viral culture, as were faecal samples in a minority of patients (although universally requested).
The following definitions were used;
Probable case: symptoms of meningitis (fever, headache, neck stiffness, vomiting, photophobia).
Confirmed case: symptoms of meningitis (fever, headache, neck stiffness, vomiting, photophobia) and either positive enterovirus PCR, positive CSF viral culture or CSF pleocytosis (>5 WCC/ml) and positive serology.
Statistical analysis was performed using SPSS version 11 for Windows.
Results
Forty adults with viral meningitis were identified during the period March to August 2001 inclusive. Twenty (50%) were male, and the age range was 16–51 years, median age 28 years. Fifteen of 38 (39.5%) were smokers, and 20 of 24 (83.3%) had close contact with young children (parent, grandparent, school teacher, paediatric nurse). Symptom duration ranged from six hours to seven days, median 1.1 days. The most frequent symptoms were headache (100%), photophobia (87.5%), nausea (67.5%), vomiting (65%), fever (65%), and neck stiffness (62.5%). Specific gastrointestinal symptoms (abdominal pain and diarrhoea) only occurred in 2 of 40 (5%) of patients. Myalgia was specifically inquired about, and reported in 7 of 40 (17.5%).
Twenty three of 40 (58%) had fever on admission, 30 of 38 (79%) had clinical photophobia, and 27 (68%) had meningism on examination. Kernig's sign was positive in 3 of 22 (14%), and fundoscopy was abnormal in 2 of 30 (7%). Nine of 40 patients had a CT scan of the head performed, all of which were normal.
All patients had a CSF cell count performed, but a differential count was only recorded in 32 of 40 (80%) cases. Table 1 shows a breakdown of CSF characteristics in culture positive and negative cases and PCR positive and negative cases. There was a lymphocyte predominance in 22 (69%) and a neutrophil predominance in 10 (31%). A total of six patients had a CSF white cell count of ⩽5, 3 of whom had a positive CSF enterovirus PCR. CSF glucose was normal in all patients. Three of 40 (7.5%) had normal CSF cell count and biochemistry, but positive virology (positive CSF enterovirus PCR). There was a trend suggesting lower CSF glucose in culture positive compared with culture negative cases (p = 0.05), and in PCR positive compared with PCR negative cases (p = 0.31), but these differences were not significant.
Table 1 CSF characteristics in patients with echovirus meningitis.
| Range Median (95% CI) | All patients (n = 40) | Culture positive (n = 3) | Culture negative (n = 13) | PCR positive (n = 26) | PCR negative (n = 6) |
|---|---|---|---|---|---|
| White cell count (/mm3) | 1–405 | 14–211 | 1–365 | 2–378 | 1–194 |
| 38 (20 to 140) | 26 (14 to 211) | 55 (26 to 187) | 36 (18–112) | 29 (1–167) | |
| % Lymphocytes | 2–100 | 5–85 | 5–100 | 2–100 | 5–100 |
| 85 (33 to 95) | 40 (5 to 85) | 85 (27 to 95) | 88 (38 to 95) | 85 (5 to 100) | |
| % Neutrophils | 0–98 | 15–95 | 0–95 | 15–95 | 0–95 |
| 15 (5 to 68) | 60 (15 to 95) | 15 (5 to 73) | 60 (15 to 95) | 15 (0 to 95) | |
| Protein (g/l) | 0.25–1.6 | 0.4–1.4 | 0.3–1.4 | 0.3–1.4 | 0.3–0.9 |
| 0.7 (0.5 to 0.8) | 1.2 (0.4 to 1.4) | 0.7 (0.5 to 0.9) | 0.6 (0.5 to 0.8) | 0.7 (0.6 to 0.9) | |
| % Abnormal protein | 31/39 (79.5%) | 2/3 (66.7 %) | 11/13 (84.6%) | 20/26(76.9 %) | 5/6 (83.3%) |
| Glucose (mmol/l) | 2.5–7.3 | 2.6–3.2 | 2.6–4.7 | 2.6–7.3 | 3.0–4.2 |
| 3.4(3.1 to 3.6) | 3.0 (2.6 to 3.2) | 3.6 (3.3 to 4.0) | 3.3 (3.1 to 3.6) | 3.6 (3.2 to 4.0) |
Table 2 shows a comparison of CSF culture with PCR. All CSF culture positive cases were also PCR positive. Overall, the diagnosis was confirmed virologically in 29 patients (72%). Echovirus 30 was isolated from six patients (three throat swab, three CSF). CSF enterovirus PCR was positive in 26 of 32 (81%) compared with CSF viral culture 3 of 16 (19%), (p<0.0001, Fisher's exact test) or 3 of 31 (9.7%) throat swab cultures (p = 0.65).
Table 2 CSF culture compared with CSF PCR in patients with echovirus meningitis.
| CSF virology | CSF PCR | |||
|---|---|---|---|---|
| Positive | Negative | Not done | Total | |
| Positive | 3 | 0 | 0 | 3 |
| Negative | 8 | 4 | 1 | 13 |
| Not done | 15 | 2 | 7 | 24 |
| Total | 26 | 6 | 8 | 40 |
As CSF and PCR positivity may be related to duration of symptoms, we examined the relation between duration of symptoms and diagnostic confirmation and found no relation.
Table 3 compares the performance characteristics of CSF PCR against the gold standard, CSF virus culture, in several published studies. Our study showed low specificity and positive predictive values, which is probably because of the small number of culture positive cases. Our results are similar to some of the other studies.
Table 3 Comparison of performance characteristics of RT‐PCR with CSF virus culture (gold standard) from previous studies.
| Reference | Sensitivity (%) | Specificity (%) | Positive predictive value (%) | Negative predictive value (%) |
|---|---|---|---|---|
| Verstrepen et al18 | 100 | 91 | 69 | 100 |
| Corless et al17 | 100 | 19 | 28 | 100 |
| Pozo et al19 | 100 | 11 | 28 | 100 |
| Guney et al20 | 89 | 66 | 74 | 84 |
| Gorgievski‐Hrisoho et al21 | 100 | 19 | 28 | 100 |
| Buxbaum et al22 | 68 | 54 | 52 | 70 |
| Carrol et al (this study) | 100 | 33 | 27 | 100 |
Discussion
This prospective observational study took place in response to a developing viral meningitis outbreak. The symptoms and signs were similar to other those seen elsewhere.16,23 However, in a study from Munich, preceding signs of gastrointestinal infection were reported in 14 of 19 (79%) patients with acute aseptic meningitis secondary to enterovirus infection.24 Our study failed to support such an association, as only 2 of 40 patients (5%) patients had specific gastrointestinal symptoms. We consider that the nausea and vomiting that occurred in most of the patients were symptoms of meningeal irritation and meningitis, and should not be regarded as separate gastrointestinal symptoms secondary to enterovirus infection. In a larger study from Marseilles, only 11% of patients had diarrhoea as a presenting symptom.23 Our study found that nearly a fifth of patients (17.5%) reported myalgia, which was more commonly reported than from another recent study of an echovirus 30 outbreak (3.8%).23 Myalgia as a presenting symptom of sepsis, if not specifically inquired about, can often be missed.25
As there is no control group, it is impossible to comment on the possible significance of smoking as a risk factor. In a study carried out on Greek military recruits, meningococcal carriage was significantly associated with smoking,26 but there are no studies confirming such an association in viral meningitis. Eighty three per cent of our patients had close contact with small children as parents, grandparents, schoolteachers, or paediatric nurses. As the disease is usually spread by the faecal‐oral or oral‐oral routes, one could speculate that failure to observe thorough hand washing after nappy changes, assisting young children with toileting, and kissing small children might facilitate transmission to adult carers. Previous community based studies have shown high attack rates for enteroviral illness in household members of day care centre children (13%) and day care centre employees (5%). Household members of ill day care centre children were 15 times more likely to have met the case definition for enteroviral illness than those of non‐ill day care centre children.27 Another study reporting an echovirus 30 outbreak confirmed that contact with an ill household member (odds ratio, OR, 6.3), day care attendance (OR 2.6), and playground use either two or three times a week (OR 3.7) or daily (OR 4.3) were risk factors for transmission, and therefore, illness.28
The echovirus 30 serotype has been responsible for numerous outbreaks in the Rhone‐Alps region of France between 1976 and 2000, and was partly responsible for the outbreak of viral meningitis seen in that region in 2000 (together with echovirus 6 and echovirus 13). The echovirus 30 serotype mainly affected patients between the ages of 15 and 49 years.29 This is similar to our cohort, most of whom were between 16 and 40 years old. Possible reasons for the predilection of the virus for this age group are decreasing levels of neutralising antibody in adolescents and adults, or genomic variation in echovirus 30.29,30
This study confirms that CSF parameters are not reliable markers for excluding viral meningitis, and may be normal in nearly 10% of confirmed cases. This is in keeping with previous studies, which have shown that in routine medical and laboratory practice, quantitative and qualitative CSF examination results are of little value in ruling out viral meningitis.3,23 Verstrepen et al18 found that of 41 patients with positive enterovirus PCR, 8 (19.5%) had a WCC <10/mm3. A CSF neutrophil predominance was seen in 31% of patients in our study, which is similar to the CSF neutrophil predominance of 42%,3 41%,31 and 55%23 reported by others.
Only 3 of 31 (9.7%) throat swab samples were positive by virus culture, and only three patients submitted samples for stool virus culture (all of which were negative). Attempts to isolate enterovirus from CSF are frequently less successful because of the lower viral titre in clinical specimens, and also because some serotypes grow slowly,19 this was less sensitive in our series compared with previous reports.3,32 This is a limitation of the usefulness of cell culture techniques.20 In an echovirus 30 associated outbreak of aseptic meningitis in Taiwan, virus was more frequently isolated from throat swab (85%), and to a lesser extent stool (76%) or CSF (70%).33 In another study from Germany, more isolates of enterovirus were isolated from stool samples than from CSF samples over three consecutive years.22
CSF PCR for enterovirus is more sensitive than CSF viral culture, and increased diagnostic success about threefold in our hands. This contrasts with experience during an epidemic of echovirus 30 meningitis in Marseilles in 2000, where CSF PCR had similar sensitivity to cultures of faeces or of throat swabs.23 We had access to a two step reverse transcriptase real time PCR method that has been optimised to produce rapid results, with excellent sensitivity in CSF and throat swab samples.17 RT‐PCR is more rapid and sensitive than virus culture, which is traditionally used as the “gold standard”. This makes it difficult to assess the performance of RT‐PCR against a true gold standard,21 and RT‐PCR should now be used as the gold standard.
Several authors have argued that performing CSF PCR for enteroviruses on patients with probable viral meningitis may affect clinical decision making, reduce hospitalisation times, and reduce diagnostic or therapeutic interventions including prolonged administration of unnecessary antibiotics or dexamethasone.3,8,16,34,35 It has been estimated that rapid availability of PCR results on CSF specimens from adults could result in cost savings for England and Wales of over £250 000 ($450 000) per year,16 and the savings would be further increased if processing of specimens from children was taken into account.35 We would support this view, particularly if it can be introduced as a rapid routine test, because our results confirm that PCR significantly improves the ability to specifically diagnose enteroviral meningitis, irrespective of CSF parameters.
Unfortunately, specific antiviral agents such as pleconaril that showed initial promise,36,37,38,39,40 are not licensed for routine clinical use, and there is therefore no effective treatment available, even if confirmatory diagnostic tests results were available rapidly.
Conclusion
This prospective study of an echovirus outbreak in the north west of England confirms that headache, photophobia, nausea, vomiting, and temperature are the most common symptoms seen in adults. CSF parameters alone cannot be reliably used to confirm or exclude viral meningitis. CSF PCR for enterovirus is more sensitive than viral culture, although PCR does not yield information on circulating type. The implications of early and accurate confirmation of viral meningitis using PCR are to reduce parenteral antibiotic use and length of in‐patient stay. Both these factors have implications for patient morbidity and the cost of patient management. Finally, close contact with children could be a significant risk factor, especially if good hygiene measures are not practised.
Abbreviations
PCR - polymerase chain reaction
CSF - cerebrospinal fluid
Footnotes
Funding: none.
Conflicts of interest: none declared.
This research was presented in part at the Federation of Infection Societies eighth conference, Manchester, 28–30 November 2001 (poster).
References
- 1.Rotbart H A. Enteroviral infections of the central nervous system. Clin Infect Dis 199520971–981. [DOI] [PubMed] [Google Scholar]
- 2.Rotbart H A. Viral meningitis. Semin Neurol 200020277–292. [DOI] [PubMed] [Google Scholar]
- 3.Henquell C, Chambon M, Bailly J L.et al Prospective analysis of 61 cases of enteroviral meningitis: interest of systematic genome detection in cerebrospinal fluid irrespective of cytologic examination results. J Clin Virol 20012129–35. [DOI] [PubMed] [Google Scholar]
- 4.Cherry J.Enteroviruses, coxsackieviruses, echoviruses, and polioviruses. 4th ed. Philadelphia: Saunders, 1998
- 5.Pichichero M E, McLinn S, Rotbart H A.et al Clinical and economic impact of enterovirus illness in private pediatric practice. Pediatrics 19981021126–1134. [DOI] [PubMed] [Google Scholar]
- 6.Nicolosi A, Hauser W A, Beghi E.et al Epidemiology of central nervous system infections in Olmsted County, Minnesota, 1950–1981. J Infect Dis 1986154399–408. [DOI] [PubMed] [Google Scholar]
- 7.Centers for Disease Control. Prevention (CDC) Outbreaks of aseptic meningitis associated with echoviruses 9 and 30 and preliminary surveillance reports on enterovirus activity—United States, 2003. MMWR Morb Mortal Wkly Rep 200352761–764. [PubMed] [Google Scholar]
- 8.Sawyer M H. Enterovirus infections: diagnosis and treatment. Pediatr Infect Dis J 1999181033–1039. [DOI] [PubMed] [Google Scholar]
- 9.Thoelen I, Lemey P, Van Der Donck I.et al Molecular typing and epidemiology of enteroviruses identified from an outbreak of aseptic meningitis in Belgium during the summer of 2000. J Med Virol 200370420–429. [DOI] [PubMed] [Google Scholar]
- 10.Nairn C, Clements G B. A study of enterovirus isolations in Glasgow from 1977 to 1997. J Med Virol 199958304–312. [PubMed] [Google Scholar]
- 11.Schumacher J D, Chuard C, Renevey F.et al Outbreak of echovirus 30 meningitis in Switzerland. Scand J Infect Dis 199931539–542. [DOI] [PubMed] [Google Scholar]
- 12.Rice S K, Heinl R E, Thornton L L.et al Clinical characteristics, management strategies, and cost implications of a statewide outbreak of enterovirus meningitis. Clin Infect Dis 199520931–937. [DOI] [PubMed] [Google Scholar]
- 13.Oberste M S, Maher K, Kennett M L.et al Molecular epidemiology and genetic diversity of echovirus type 30 (E30): genotypes correlate with temporal dynamics of E30 isolation. J Clin Microbiol 1999373928–3933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chambon M, Bailly J L, Beguet A.et al An outbreak due to echovirus type 30 in a neonatal unit in France in 1997: usefulness of PCR diagnosis. J Hosp Infect 19994363–68. [DOI] [PubMed] [Google Scholar]
- 15.Public Health Laboratory Service (PHLS) Viral meningitis in England and Wales associated with an increase of echovirus type 30. CDR Weekly 2001111–3. [Google Scholar]
- 16.Chadwick D R, Lever A M. The impact of new diagnostic methodologies in the management of meningitis in adults at a teaching hospital. QJM 200295663–670. [DOI] [PubMed] [Google Scholar]
- 17.Corless C E, Guiver M, Borrow R.et al Development and evaluation of a ‘real‐time' RT‐PCR for the detection of enterovirus and parechovirus RNA in CSF and throat swab samples. J Med Virol 200267555–562. [DOI] [PubMed] [Google Scholar]
- 18.Verstrepen W A, Bruynseels P, Mertens A H. Evaluation of a rapid real‐time RT‐PCR assay for detection of enterovirus RNA in cerebrospinal fluid specimens. J Clin Virol 200225(suppl 1)S39–S43. [DOI] [PubMed] [Google Scholar]
- 19.Pozo F, Casas I, Tenorio A.et al Evaluation of a commercially available reverse transcription‐PCR assay for diagnosis of enteroviral infection in archival and prospectively collected cerebrospinal fluid specimens. J Clin Microbiol 1998361741–1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guney C, Ozkaya E, Yapar M.et al Laboratory diagnosis of enteroviral infections of the central nervous system by using a nested RT‐polymerase chain reaction (PCR) assay. Diagn Microbiol Infect Dis 200347557–562. [DOI] [PubMed] [Google Scholar]
- 21.Gorgievski‐Hrisoho M, Schumacher J D, Vilimonovic N.et al Detection by PCR of enteroviruses in cerebrospinal fluid during a summer outbreak of aseptic meningitis in Switzerland. J Clin Microbiol 1998362408–2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Buxbaum S, Berger A, Preiser W.et al Enterovirus infections in Germany: comparative evaluation of different laboratory diagnostic methods. Infection 200129138–142. [DOI] [PubMed] [Google Scholar]
- 23.Bernit E, de Lamballerie X, Zandotti C.et al Prospective investigation of a large outbreak of meningitis due to echovirus 30 during summer 2000 in Marseilles, France. Medicine (Baltimore) 200483245–253. [DOI] [PubMed] [Google Scholar]
- 24.Nowak D A, Boehmer R, Fuchs H H. A retrospective clinical, laboratory and outcome analysis in 43 cases of acute aseptic meningitis. Eur J Neurol 200310271–280. [DOI] [PubMed] [Google Scholar]
- 25.Carrol E D, Thomson A P, Mobbs K J.et al Myositis in children with meningococcal disease: a role for tumour necrosis factor‐alpha and interleukin‐8? J Infect 20024417–21. [DOI] [PubMed] [Google Scholar]
- 26.Blackwell C C, Tzanakaki G, Kremastinou J.et al Factors affecting carriage of Neisseria meningitidis among Greek military recruits. Epidemiol Infect 1992108441–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vieth U C, Kunzelmann M, Diedrich S.et al An echovirus 30 outbreak with a high meningitis attack rate among children and household members at four day‐care centers. Eur J Epidemiol 199915655–658. [DOI] [PubMed] [Google Scholar]
- 28.Reintjes R, Pohle M, Vieth U.et al Community‐wide outbreak of enteroviral illness caused by echovirus 30: a cross‐sectional survey and a case‐control study. Pediatr Infect Dis J 199918104–108. [DOI] [PubMed] [Google Scholar]
- 29.Chomel J J, Antona D, Thouvenot D.et al Three ECHOvirus serotypes responsible for outbreak of aseptic meningitis in Rhone‐Alpes region, France. Eur J Clin Microbiol Infect Dis 200322191–193. [DOI] [PubMed] [Google Scholar]
- 30.Oberste M S, Maher K, Kilpatrick D R.et al Typing of human enteroviruses by partial sequencing of VP1. J Clin Microbiol 1999371288–1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang Q S, Carr J M, Nix W A.et al An echovirus type 33 winter outbreak in New Zealand. Clin Infect Dis 200337650–657. [DOI] [PubMed] [Google Scholar]
- 32.Rotbart H A, Ahmed A, Hickey S.et al Diagnosis of enterovirus infection by polymerase chain reaction of multiple specimen types. Pediatr Infect Dis J 199716409–411. [DOI] [PubMed] [Google Scholar]
- 33.Wang J R, Tsai H P, Huang S W.et al Laboratory diagnosis and genetic analysis of an echovirus 30‐associated outbreak of aseptic meningitis in Taiwan in 2001. J Clin Microbiol 2002404439–4444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ramers C, Billman G, Hartin M.et al Impact of a diagnostic cerebrospinal fluid enterovirus polymerase chain reaction test on patient management. JAMA 20002832680–2685. [DOI] [PubMed] [Google Scholar]
- 35.Makwana N, Nye K, Riordan F A. Meningitis without a petechial rash in children in the Hib vaccine era. J Infect 200449297–301. [DOI] [PubMed] [Google Scholar]
- 36.McKinlay M A, Pevear D C, Rossmann M G. Treatment of the picornavirus common cold by inhibitors of viral uncoating and attachment. Annu Rev Microbiol 199246635–654. [DOI] [PubMed] [Google Scholar]
- 37.Abzug M J, Cloud G, Bradley J.et al Double blind placebo‐controlled trial of pleconaril in infants with enterovirus meningitis. Pediatr Infect Dis J 200322335–341. [DOI] [PubMed] [Google Scholar]
- 38.Hayden F G, Herrington D T, Coats T L.et al Efficacy and safety of oral pleconaril for treatment of colds due to picornaviruses in adults: results of 2 double‐blind, randomized, placebo‐controlled trials. Clin Infect Dis 2003361523–1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rotbart H A, Webster A D. Treatment of potentially life‐threatening enterovirus infections with pleconaril. Clin Infect Dis 200132228–235. [DOI] [PubMed] [Google Scholar]
- 40.Shafran S D, Halota W, Gilbert D.et al Pleconaril is effective for enteroviral meningitis in adolescents and adults: a randomzed placebo controlled multicentrer trial. 39th Interscience conference on antimicrobial agents and chemotherapy, San Francisco, 26–29 Sep 1999; No 1904