Abstract
This article aims to provide an overview of the problems that exist in relation to malnutrition and the elderly population. The changes that occur in body composition during ageing are described and how this may affect disease risk. The possible metabolic processes behind weight loss are discussed and the numerous factors that affect nutritional status in the older age group are described. Prevention of malnutrition in this group is important and so the roles of nutrition screening and assessment are examined.
Keywords: aged, appetite, malnutrition, sarcopenia, body composition
The elderly population is extremely diverse, ranging from fit, active, and healthy octogenarians to extremely frail, totally dependent people with chronic disease and severe disabilities. The elderly population is increasing, currently about 16% of the population is over 65 years, and 2% are over 85 years. These figures are predicted to rise dramatically in the next 30 years.1 In 2002, 63% of 65–74 year olds and 72% of people aged over 75 years reported a longstanding illness. Almost two thirds of general and acute hospital beds are used by people aged over 65 years, and people over 75 years have longer hospital stays.2 The burden of disease in this population is clear, and this influences the nutritional status of this population. There are also changes in body composition that occur during ageing that also influence nutritional status. This paper describes these changes and the influence on health and focuses on the problem of malnutrition in this age group.
Body composition of the elderly population
Body composition changes during malnutrition, with the loss of both fat and muscle tissue, but body composition also changes with age. It is important to understand what changes occur because of ageing so malnutrition related alterations can be identified and interpreted correctly.
Body composition of elderly people
There is general agreement that body fat mass increases up to about 75 years of age and then decreases or remains stable.3,4,5 There is some evidence that central accumulation of fat increases with ageing, while appendicular fat mass decreases.3,6 It is known that this pattern of fat distribution is associated with an increased risk of stroke, diabetes, hyperlipidaemia, heart disease, and hypertension,7 all of which commonly afflict the elderly.
Fat free mass (FFM) includes muscle, organ tissue, skin, and bone, and has been shown to decrease with age, starting at an earlier age than fat mass loss, around 40–50 years.3,4,8,9 Most of this loss is attributable to a reduction in skeletal muscle, and bone mineral density in women. The studies cited here all used healthy subjects, thus these changes may be accentuated in a population of sick older adults.
The muscle content of the body is important because of the relation of muscle mass to physical function, strength, and morbidity. Even a 10% loss of lean tissue in previously healthy adults has been shown to impair immunity, increase infection risk, and be associated with increased mortality.10,11 Thus for older adults with a background of FFM loss the adverse consequences are likely to be greater.
Body composition changes seen with malnutrition and disease
The main body composition changes observed with starvation are a loss of FFM and fat mass. In an elderly population the size of these fat and lean tissue losses may be greater, as they are in addition to age related losses in these compartments. This has been clearly illustrated by Hébuterne et al12 in a study in which the body composition of old (78±7 years) and young (26±5 years) people with varying degrees of weight loss was compared. Anorexia was the main reason for weight loss, and sex and inflammatory status were similar in all groups. The two age groups were divided into three groups by their current BMI;
Group 1: 18.5–20 kg/m2
Group 2: 16–18.5 kg/m2
Group 3: less than 16 kg/m2
In the young group the per cent FFM was similar in all three BMI groups (81%, 84%, and 85% respectively) suggesting any loss was from the fat mass. In the old group the per cent FFM decreased with the BMI (70%, 67%, 61% respectively). Per cent body cell mass (BCM) was also compared and showed striking differences, 57%, 56%, and 49% in the young group and 41%, 34%, and 25% in the old group. BCM is the functionally important compartment of FFM, including muscle, viscera and the immune system, and excluding extracellular fluid, collagen, and bone. It determines energy expenditure, protein needs, and metabolic response to physiological stress. These data show a much greater loss of BCM in the old group, which could reflect previous age related losses, but could also suggest changes in metabolism, resulting in a fat sparing utilisation of energy stores in the elderly.
However, weight loss during disease is not solely due to reduced energy intake, as body composition changes do not always reflect those seen in starvation. There are different mechanisms at work during disease that change the response to an energy deficiency and this results in different patterns of body composition.
Aetiology of weight loss
It has been proposed by Roubenoff13 that weight loss in older adults can be divided into three distinct types:
Wasting, an involuntary loss of weight, which is primarily caused by inadequate dietary intake. This may be attributable to both disease and psychosocial factors, and may occur with a background of cachexia or sarcopenia or both.
Cachexia, an involuntary loss of FFM or BCM, which is caused by catabolism, and results in changes in body composition but in which weight loss may not be initially present. It is characterised by a raised metabolic rate and increased protein degradation.
Sarcopenia, an involuntary loss of muscle mass, which may be an intrinsic part of the ageing process rather than the effect of age associated disease.
Wasting
Wasting requires whole body negative energy balance and is mainly attributable to a decreased food intake. The control of appetite, and other factors that affect food intake are discussed later in this article. The mechanisms underlying the loss of appetite are central to the development of wasting.
Cachexia
Cachexia is distinguished by the presence of an acute immune response. This involves the production first of interleukin 1 (IL1), which then triggers other parts of the immune response, including the production of tumour necrosis factor α (TNFα) and then IL6. These cytokines are thought to be important in the metabolic response to injury or stress, because receptors for them are found on virtually every cell in the body. Thus, they have profound effects on hormone production, hormonal control of metabolism, and direct effects on tissues. The result is an increase in resting energy expenditure, a net export of amino acids from the muscle to the liver, an increase in gluconeogenesis, and a shift from the synthesis of albumin to the production of acute phase proteins, such as C reactive protein. Overall, the effect on body composition is that nitrogen balance becomes negative, so muscle mass is lost.
Cachexia is seen in many diseases such as rheumatoid arthritis, congestive heart failure, HIV infection, and cancer, and also in situations of metabolic stress such as trauma, infections, and pressure sores. The concentrations of the three cytokines are higher than normal in cachectic patients and weight gain is associated with a reduction in these levels.14
Sarcopenia
The exact aetiology is unknown and so it cannot be assumed that sarcopenia is a normal part of ageing, but as muscle loss is seen in even healthy people it would seem metabolic changes occurring during ageing make it a universal phenomenon.
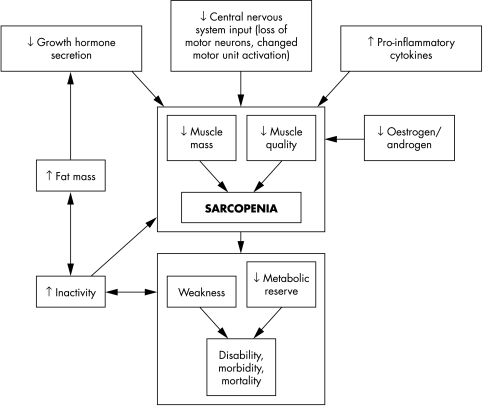
A lack of physical activity is definitely crucial to the development of sarcopenia. It has been clearly shown that muscle disuse leads to muscle loss and increased activity slows and reduces muscle loss.15 However, exercise does not completely prevent sarcopenia, so inactivity is not the sole cause. Hormonal, neural, and cytokine activity all seem to play a part (fig 1). Growth hormone, testosterone, and oestrogen have all been investigated as possible mediators of sarcopenia, however, the mechanism of their involvement remains unclear.
Figure 1 Potentially important factors in the aetiology of sarcopenia. Adapted from Roubenoff.15
Cytokines are also implicated in sarcopenia.14 The pro‐inflammatory cytokines such as TNFα, IL1, IL6, serotonin, and interferon gamma, are known to stimulate release of acute phase proteins, protein breakdown in muscle, and fat breakdown in adipose tissue and their role has been established in cachexia as described above. Ageing is characterised by progressively increased concentrations of glucocorticoids and catecholamines and decreasing production of growth and sex hormones, which in turn results in increased concentrations of pro‐inflammatory cytokines. There is substantial evidence for the involvement of a TNFα related systemic inflammation in the mechanism of muscle loss and anorexia in a number of diseases, such as chronic obstructive pulmonary disease,16 HIV, and rheumatoid arthritis. This may also be the case during the ageing process.
A recent paper has shown that inflammatory cytokines TNFα and IL1β interfere with myogenic differentiation—that is, the development of satellite stem cells into functional muscle fibres.17 Muscle fibres are constantly replaced as they become damaged or degenerate. If satellite cells are prevented from differentiating into functional fibres, muscle wasting will occur. This might explain the mechanism of muscle wasting in conditions where a systemic inflammatory response is present.
The current concept of the role of the central nervous system (CNS) in sarcopenia is that neurones are lost from the spinal cord, and that this in turn leads to the loss of muscle.15 In addition, the remaining neurones “adopt” muscle fibres and then control larger units of muscle cells. As a consequence of this the units become less efficient, which could lead to tremor and weakness. Strokes and neural diseases show that neurone death results in muscle atrophy, so it is possible that this could be one of the underlying mechanisms in the development of sarcopenia. Indeed Roubenoff15 believes the CNS role to be central to the development of sarcopenia and in particular differentiates this type of weight loss from wasting and cachexia.
Further research is required into all these possible mechanisms, but with a better understanding of the underlying processes it may be possible to develop treatments that counteract the various changes that occur during ageing.
Malnutrition in the older population
Malnutrition can be defined as the state of being poorly nourished. It may be caused by the lack of one or more nutrients (under‐nutrition), or an excess of nutrients (over‐nutrition). For the purpose of this paper, malnutrition will refer to the state of under‐nutrition as this remains the common use throughout the published literature.
In the ageing and sick population malnutrition is an important problem that has been seen in hospitals,18 residential care,19 and in the community.20 Prevalence rates have been estimated for the general hospital population to be between 11% to 44%, but this rises in elderly groups to 29%–61%.18 Malnutrition is not an inevitable side effect of ageing, but many changes associated with the process of ageing can promote malnutrition.21 For example, ageing is frequently associated with decreases in taste acuity and smell, deteriorating dental health, and decreases in physical activity, which may all affect nutrient intake.22 Any change in nutrient intake can lead to malnutrition with its potentially serious consequences. Many studies have found a direct relation between the degree of malnutrition and increased length of stay, treatment costs, return to usual life,23,24 and re‐admission to hospital rates.25 Therefore the treatment and prevention of malnutrition, which is most common in the older age group, is an important challenge for the health care system.
Risk factors for malnutrition
Medical factors
Poor appetite
Poor dentition, other oral problems and dysphagia
Loss of taste and smell
Respiratory disorders, for example, emphysema
Gastrointestinal disorders, for example, malabsorption
Endocrine disorders, for example, diabetes, thyrotoxicosis
Neurological disorders, for example, cerebrovascular accident, Parkinson's disease
Infections, for example, urinary tract infection, chest infection
Physical disability, for example, arthritis, poor mobility
Drug interactions, for example, digoxin, metformin, antibiotics, etc
Other disease states, for example, cancer
Lifestyle and social factors
Lack of knowledge about food, cooking, and nutrition
Isolation/loneliness
Poverty
Inability to shop or prepare food
Psychological
Confusion
Dementia
Depression
Bereavement
Anxiety
Additional risk factors in hospital
Food service—sole nutritional supply is hospital food, limited choice, presentation may be poor
Slow eating and limited time for meals
Missing dentures
Needs feeding/supervision
Inability to reach food, use cutlery, or open packages
Unpleasant sights, sounds, and smells
Increased nutrient requirement, for example, because od infections, catabolic state, wound healing, etc
Limited provision for religious or cultural dietary needs
Nil by mouth or miss meals while having tests
Causes of malnutrition
The causes of malnutrition are extremely varied, and they can be divided into three main types: medical, social, and psychological. Examples of each of these causes are shown in the box. This box also illustrates the additional problems faced by hospital patients, giving an indication of the reasons why rates of malnutrition have been shown to increase during hospitalisation.26,27,28
Appetite
Poor appetite or anorexia is probably the major cause of malnutrition and is mediated by a variety of factors. It is well known that energy intake decreases with age,29,30 and that micro‐nutrient deficiencies are more likely to occur with a reduced energy intake.31 The mechanisms behind this reduction have yet to be fully elucidated but a number of contributing theories have been developed. These are reviewed in detail by various authors32,33 but an overview is provided here.
Roberts et al has shown clearly that there are differences in the mechanisms for the control of food intake between old and young men.34 Their experiment, entailing underfeeding followed by a period of ad libitum feeding (as much food as is wanted), resulted in both young and old men losing weight, but the young men quickly regained the lost weight in the ad libitum period whereas the old men did not. They concluded that ageing seemed to be associated with an impaired ability to regulate food intake. Further studies by the same group suggest that this may be attributable to a failure of older people to reduce their energy expenditure during periods of negative energy balance.35 This has serious implications for older people suffering anorexia because of episodes of disease or enforced fasting resulting from major surgery. The weight lost will take longer to be re‐gained in older people, and they will be at greater risk of all the consequences related to malnutrition during this time.
Morley and colleagues have studied the gastrointestinal tract and satiation in detail. Morley's review36 provides a summary of this work, which essentially shows that satiety is determined by a process involving relaxation of the stomach wall and the hormone cholecystokinin (CCK). CCK levels increase with ageing and also slow gastric emptying, both of which would lead to earlier satiety.37 Morley also describes his preliminary work on stomach relaxation, which suggests that the stomach capacity is reduced during ageing and hence signals from the stretch of the stomach wall may be increased and occur earlier, thus increasing satiety.36 This work is also supported by other investigators who have shown that older people are less responsive than the young to stomach contents, in terms of hunger ratings.38
There are several peptide hormones released by the gut including; ghrelin, CCK, peptide‐YY, glucagon‐like peptide 1, oxyntomodulin, and pancreatic polypeptide that play a part in regulating appetite. Ghrelin has a stimulatory effect, the others are all inhibitory. Infusions of ghrelin have been shown to increase appetite in anorectic cancer patients resulting in increased food intake and thus may provide a method of appetite stimulation.39 It is possible that ghrelin secretion may reduce during ageing or during illness or trauma. The evidence for changes in the secretion of all these peptides during ageing is limited as the research in this field is in its infancy. It remains to be discovered whether dysregulation of these gut hormones is the underlying mechanism to the anorexia of ageing.
Cytokines are also thought to be involved in the regulation of appetite.36 As described previously, ageing is associated with an increase in glucocorticoids and catecholamines, and a decrease in growth hormone and sex hormones.14 This has the effect of increasing levels of TNFα, IL1, IL6, and serotonin, which are known to cause anorexia, and may also be involved in increased muscle breakdown and nitrogen loss. These cytokines are also increased during infections, injuries, and longstanding inflammation. The exact relation between appetite controlling peptides and the cytokine cascade is not known, and is an area for future research.
The CNS also plays a part and many neurotransmitters are involved in the regulation of food intake. To date, the research on age related changes in the nervous system's effect on appetite, is confined mainly to animal studies. However, it would appear that nitric oxide may play a central part in coordinating appetite regulation.36 The opioids are thought to increase food intake and opioid antagonists decrease food intake in young adults. Ageing is associated with a loss of opioid receptors and reduced brain concentrations of endogenous opioids,32 so theoretically older people may be less sensitive to their action.
The preceding discussion shows that multiple factors are involved in appetite regulation, and the manner in which they operate may change during ageing in itself, as well as during disease. Much remains to be explained to fully understand all these interacting processes.
Taste and smell
Taste and smell are also implicated in the loss of appetite through a perceived decline in the pleasantness of food.40 Taste is also an important part of the cephalic phase response that prepares the body for digestion. It helps modulate food choice and meal size by increasing satiety and the pleasure of eating.41 Loss of taste and smell is common in the elderly and can be exacerbated by disease and drugs.41,42 Studies show how important taste alterations can be; for example, an elderly person (with one or more medical conditions, and who takes an average of three medications) needs 11 times as much salt and almost three times as much sugar to detect these tastes in foods compared with younger people.43
The cause of taste loss is not fully understood but possible theories include a reduction in the number of taste buds, or a decrease in the functioning of receptors in cell membranes involved in the taste sensation.41 Many drugs can change taste and smell, including drugs in the following groups: lipid lowering drugs, antihistamines, antibiotics, anti‐inflammatories, bronchodilators and other asthma drugs, antihypertensives, Parkinson's disease treatments, and antidepressants. The mechanism by which these drugs affect taste or smell remains unknown.
A few studies have been conducted that show that improving the flavour of the foods, can improve nutritional intake and increase body weight in hospital and nursing home patients, as well as the healthy elderly.44,45
Oral health and dental status
Oral health and dentition have been shown to significantly affect food intake and generally deteriorate with ageing. The National Diet and Nutrition Survey for people aged 65 years and over, included a survey of oral health.46 This clearly showed that older people have less of their own teeth; 59% of people aged 65–74 years were dentate but only 35% of people aged 75 years or over. Furthermore, edentate people reported greater difficulty with eating a range of foods, more chewing problems occurred, and mouth dryness was more common. Chewing problems are associated with a greater likelihood of poor general health and decreased quality of life.47
The dietary data from the National Diet and Nutrition Survey,19 showed that energy intake was lower in edentate people, as were many of the micronutrients (calcium, iron, vitamins A, C and E, and some B vitamins), fibre, and protein. Again, natural teeth gave a distinct advantage; nutrient intake was better and BMI was higher in dentate people.
Dysphagia
Dysphagia can cause malnutrition by leading to a reduced food intake. This was shown clearly by Brynes et al48 who found that if dysphagia was a major problem, and tube feeding had not been instigated, daily unsupported intake may be as low as 275 kcal (14.5% of estimated energy requirements). Earlier work by Mowe et al49 showed that up to 64% of elderly in‐patients exhibit some degree of dysphagia. Keller50 also showed that dysphagia was associated with malnutrition. Gariballa et al51 showed that stroke patients with swallowing problems had a worse nutritional status compared with those with no swallowing problem.
Disease and disability
All the diseases listed in the box are associated with higher rates of malnutrition in the older population. The rates of hospital malnutrition, and worsening malnutrition during illness, both suggest that disease increases the risk of malnutrition. In addition, many drugs have side effects that can affect nutrient intake. This may be by changes in taste as discussed earlier, or through other effects, such as nausea and vomiting, delayed gastric emptying, anorexia, diarrhoea, and malabsorption. It is well known that the elderly have the highest incidence of polypharmacy and thus are at high risk of experiencing these adverse side effects.
Lifestyle and social factors
A number of studies have been undertaken that aim to identify the social and lifestyle factors that may predict malnutrition, these are summarised in Payette et al.52 Payette et al constructed a theoretical model of the determinants of nutritional intake in community living elders, incorporating findings from 11 other studies. These determinants included cooking knowledge, loneliness and isolation, food beliefs and attitudes, psychological factors such as depression, stress and bereavement, services and assistance available, dentition, food availability, food expenditure, functional disability, appetite, and disease status. They tried to establish which were the most important determinants in a group of high risk elders. They found stress, burden of disease, poor appetite, and vision to be significant independent determinants of intake in this group.52 These results show that although lifestyle factors may influence the development of malnutrition, they do so to a lesser extent than stress, disease, appetite, and poor vision.
The National Diet and Nutrition Survey for people aged 65 years and over includes information regarding isolation and income. Energy intake was less in those living alone when compared with those living with others. Energy, protein, fibre, and many micronutrients were consumed in significantly smaller amounts in lower income groups.19
Dementia and confusion
Many of the studies investigating the relation between cognition and nutrition focus on nutritional deficiencies as a cause of dementia or cognitive decline. There is far less information available about dementia or other cognitive dysfunction as a cause of malnutrition. Incalzi et al,27 when studying hospital in‐patients, found an inverse relation between energy intake and cognition on admission, which suggests that cognition may impair the ability or desire to eat. Weight loss and changed eating behaviour is a recognised characteristic of the progressive dementing process, and uncontrolled weight loss is almost inevitable in the latter stages.53 Fifty per cent of patients with Alzheimer's disease lose the ability to feed themselves eight years after diagnosis.54 However, work by Priefer and Robbins,55 suggests that eating problems, such as slower oral manipulation of food and slower swallow response, may appear even in the early stages of Alzheimer's disease.
A study conducted by Berkhout et al56 aimed to investigate the cause of unintentional weight loss in demented nursing home patients. They found a strong positive relation between weight loss and choosing food, bringing it to the mouth, and chewing. This relation was seen in both dementing patients and patients with other disorders, thus suggesting that it is these deficits in eating function that cause the weight loss, rather the factors relating to the dementia itself.
It is known that olfactory changes occur during Alzheimer's disease, and smell is an important component of the sense of taste. This may also affect eating behaviour and food intake in these patients.57
It is therefore clear from the small amount of data available that profound changes in feeding ability occur during dementia, and these difficulties may begin early in the dementing process, ultimately leading to weight loss and malnutrition.
Depression and other psychological factors
Changed food intake is a symptom of depression and several studies have provided evidence to suggest that this a comparatively common cause of weight loss and malnutrition in the elderly.58,59,60 Bereavement has also been shown to be associated with negative effects on eating behaviours and nutrient intake.61
Anxiety or stress is also thought to be associated with changes of food intake. For example, low mood may lead people to eat more and may result in their seeking “comfort foods” or foods that make them feel better. Much of the research seems to focus on this aspect of the relation, with little information about how anxiety may decrease food intake.62 There has been some suggestion however, that people vary in their response to stress, some eating and others fasting. There may also be differences between the effects of acute and chronic anxiety.63
Re‐nutrition
Studies show that nutritional support can reverse weight loss and produce weight gain.64,65,66 However, this effect is not universal as patients do not all respond in the same way. Given the multifactorial causes of malnutrition this is hardly surprising. What is interesting, is the idea that ageing changes in some way the metabolic response to nutritional support, so that it may take longer to reverse weight loss and achieve weight gain in older people compared with young people.
There is little research to investigate how ageing influences the process of re‐nutrition, and what has been done suggests that differences do exist. Hébuterne et al67 studied the differences in response to re‐feeding, between old and young patients, using overnight enteral feeding. Both groups received support for similar lengths of time, had similar tolerance to the intervention, and achieved similar level of energy intake. The results showed that weight, serum protein levels, and a global nutrition score all improved significantly more in the younger than older group. However, the young and old groups were not matched to reduce the confounding factors, such as disease state or sex. In addition, there is no information about the actual metabolic needs of the patients, to show how much the energy intake differed from the energy requirement. Nevertheless, the results suggest that differences in response between the young and old groups may exist. Animal data exist to support these findings.68
These studies all support the suggestion that ageing is accompanied with changes to the ability to restore FFM after periods of unintentional weight loss. However, the mechanisms and metabolic changes underlying this phenomenon are as yet unknown.
Summary
Body composition and therefore energy stores change during ageing, making malnutrition a greater risk. Many other factors contribute to increasing the risk of malnutrition. An understanding of these causes is essential to formulate appropriate treatment strategies. Although the provision of an adequate supply of energy and nutrients is obviously key to the treatment of malnutrition, used alone this intervention will not necessarily be successful. Other causative factors must also be considered and addressed.
The key to treatment is identifying the malnutrition risk and treating as early as possible. The data presented here show that energy and appetite regulation may well change during ageing, thus weight lost is not necessarily regained as in younger adults, so early identification and treatment is crucial. There are three areas to consider to screen patients for risk of malnutrition:
Is the patient already underweight? Assessed using BMI, where less than 20 kg/m2 shows an increased risk of malnutrition. Some researchers have argued that the ideal BMI range for older adults (specifically those over 75 years) should be shifted up to 23–25 kg/m2 to allow for body composition changes. Stevens has reviewed the evidence for this.69
Has the patient lost any weight unintentionally? A loss of greater than 5% in three months or 10% in six months is indicative of increased risk of malnutrition.
Has the patient's appetite or food intake changed? A reduction in food intake or appetite, including enforced periods of nil by mouth, increases nutritional risk
If the answer is yes to any of these questions further assessment is necessary.70 There are a wide variety of nutrition screening tools available, one of which is the “MUST” tool (http://www.bapen.org.uk/the‐must.htm ). This is a validated tool suitable for use in both the acute sector and the community, which is simple and quick to use in most cases. Further assessment should include the identification of the many possible influencing factors described in this paper.
It is without doubt that malnutrition is an important influencing factor in the outcome of clinical diseases, and so it is vital that effective treatments and preventative measures are developed.
Abbreviations
FFM - fat free mass
BMI - body mass index
BCM - body cell mass
IL - interleukin
TNFα - tumour necrosis factor α
CNS - central nervous system
CCK - cholecystokinin
Footnotes
This article is part of a series on ageing edited by Professor Chris Bulpitt.
Funding: none.
Conflicts of interest: none declared.
References
- 1.Office of National Statistics Population trends. PT 118, table 1.4 (population age and sex). London: ONS, 2004
- 2.Office for National Statistics Living in Britain: results from the 2002 General Household Survey. London: ONS, 2004
- 3.Kyle U G, Genton L, Hans D.et al Total body mass, fat mass, fat‐free mass, and skeletal muscle in older people: cross‐sectional differences in 60‐year‐old persons. J Am Geriatr Soc 2001491633–1640. [DOI] [PubMed] [Google Scholar]
- 4.Baumgartner R N, Stauber P M, McHugh D.et al Cross‐sectional age differences in body composition in persons 60+ years of age. J Gerontol A Biol Sci Med Sci 199550M307–M316. [DOI] [PubMed] [Google Scholar]
- 5.Silver A J, Guillen C P, Kahl M J.et al Effect of ageing on body fat. J Am Geriatr Soc 199341211–213. [DOI] [PubMed] [Google Scholar]
- 6.Enzi G, Gasparo M, Biondetti P R.et al Subcutaneous and visceral fat distribution according to sex, age, and overweight, evaluated by computed tomography. Am J Clin Nutr 198644739–746. [DOI] [PubMed] [Google Scholar]
- 7.Kuczmarski R J. Need for body composition information in elderly subjects. Am J Clin Nutr 198950(suppl 5)1150–1157. [DOI] [PubMed] [Google Scholar]
- 8.Forbes G B, Reina J C. Adult lean body mass declines with age: some longitudinal observations. Metabolism 197019653–663. [DOI] [PubMed] [Google Scholar]
- 9.Novak L P. Ageing, total body potassium, fat‐free mass, and cell mass in males and females between ages 18 and 85 years. J Gerontol 197227438–443. [DOI] [PubMed] [Google Scholar]
- 10.Broadwin J, Goodman‐Gruen D, Slymen D. Ability of fat and fat‐free mass percentages to predict functional disability in older men and women. J Am Geriatr Soc 2001491641–1645. [DOI] [PubMed] [Google Scholar]
- 11.Landers K A, Hunter G R, Wetzstein C J.et al The interrelationship among muscle mass, strength, and the ability to perform physical tasks of daily living in younger and older women. J Gerontol A Biol Sci Med Sci 200156B443–B448. [DOI] [PubMed] [Google Scholar]
- 12.Hebuterne X, Bermon S, Schneider S M. Ageing and muscle: the effects of malnutrition, re‐nutrition, and physical exercise. Curr Opin Clin Nutr Met Care 20014295–300. [DOI] [PubMed] [Google Scholar]
- 13.Roubenoff R. The pathophysiology of wasting in the elderly. J Nutr 1999129(uppl 1)256–9S. [DOI] [PubMed] [Google Scholar]
- 14.Yeh S S, Schuster M W. Geriatric cachexia: the role of cytokines. Am J Clin Nutr 199970183–197. [DOI] [PubMed] [Google Scholar]
- 15.Roubenoff R. Sarcopenia and its implications for the elderly. Eur J Clin Nutr 200054(suppl 3)S40–S47. [DOI] [PubMed] [Google Scholar]
- 16.de Godoy I, Donahoe M, Calhoun W J.et al Elevated TNF‐alpha production by peripheral blood monocytes of weight‐ losing COPD patients. Am J Respir Crit Care Med 1996153633–637. [DOI] [PubMed] [Google Scholar]
- 17.Langen R C, Schols A M, Kelders M C.et al Inflammatory cytokines inhibit myogenic differentiation through activation of nuclear factor‐kappaB. FASEB J 2001151169–1180. [DOI] [PubMed] [Google Scholar]
- 18.Corish C A, Kennedy N P. Protein‐energy undernutrition in hospital in‐patients. Br J Nutr 200083575–591. [DOI] [PubMed] [Google Scholar]
- 19.Finch S, Doyle W, Lowe C.et alNational Diet and Nutrition Survey: people aged 65 years and over. Vol 1. Report of the diet and nutrition survey. London: The Stationery Office, 1998
- 20.Edington J, Kon P, Martyn C N. Prevalence of malnutrition in patients in general practice. Clin Nutr 19961560–63. [DOI] [PubMed] [Google Scholar]
- 21.Landi F, Zuccala G, Gambassi G.et al Body mass index and mortality among older people living in the community. J Am Geriatr Soc 1999471072–1076. [DOI] [PubMed] [Google Scholar]
- 22.Gariballa S E, Sinclair A J. Nutrition, ageing and ill health. Br J Nutr 1998807–23. [DOI] [PubMed] [Google Scholar]
- 23.Delmi M, Rapin C H, Bengoa J M.et al Dietary supplementation in elderly patients with fractured neck of the femur. Lancet 19903351013–1016. [DOI] [PubMed] [Google Scholar]
- 24.Robinson G, Goldstein M, Levine G M. Impact of nutritional status on DRG length of stay. JPEN 19871149–51. [DOI] [PubMed] [Google Scholar]
- 25.Friedmann J M, Jensen G L, Smiciklas W H.et al Predicting early nonelective hospital readmission in nutritionally compromised older adults. Am J Clin Nutr 1997651714–1720. [DOI] [PubMed] [Google Scholar]
- 26.Mcwhirter J P, Pennington C R. Incidence and recognition of malnutrition in hospital. BMJ 1994308945–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Incalzi R A, Capparella O, Gemma A.et al Inadequate caloric intake: a risk factor for mortality of geriatric patients in the acute‐care hospital. Age Ageing 199827303–310. [Google Scholar]
- 28.Klipstein‐Grobusch K. Reilly JJ, Potter J, et al. Energy intake and expenditure in elderly patients admitted to hospital with acute illness. Br J Nutr 199573323–334. [DOI] [PubMed] [Google Scholar]
- 29.de Groot C P, van Staveren W A, de Graaf C. Determinants of macronutrient intake in elderly people. Eur J Clin Nutr 200054(suppl 3)S70–S76. [DOI] [PubMed] [Google Scholar]
- 30.Wakimoto P, Block G. Dietary intake, dietary patterns, and changes with age: an epidemiological perspective. J Gerontol A Biol Sci Med Sci 20015665–80. [DOI] [PubMed] [Google Scholar]
- 31.de Groot C P, van den B T, van Staveren W. Energy intake and micronutrient intake in elderly Europeans: seeking the minimum requirement in the SENECA study. Age Ageing 199928469–474. [DOI] [PubMed] [Google Scholar]
- 32.Chapman I M, I Endocrinology of anorexia of ageing. Baillieres Clin Endocrinol Metab 200418437–452. [DOI] [PubMed] [Google Scholar]
- 33.Donini L M. Eating habits and appetite control in the elderly: the anorexia of ageing. Int Psychogeriatr 20031573–87. [DOI] [PubMed] [Google Scholar]
- 34.Roberts S B, Fuss P, Heyman M B.et al Control of food intake in older men. JAMA 19942721601–1606. [DOI] [PubMed] [Google Scholar]
- 35.Das S K, Moriguti J C, McCrory M A.et al An underfeeding study in healthy men and women provides further evidence of impaired regulation of energy expenditure in old age. J Nutr 20011311833–1838. [DOI] [PubMed] [Google Scholar]
- 36.Morley J E. Decreased food intake with ageing. J Gerontol A Biol Sci Med Sci 20015681–88. [DOI] [PubMed] [Google Scholar]
- 37.Clarkston W K, Pantano M M, Morley J E.et al Evidence for the anorexia of ageing: gastrointestinal transit and hunger in healthy elderly vs. young adults. Am J Physiol 1997272R243–R248. [DOI] [PubMed] [Google Scholar]
- 38.De Castro J M. Age‐related changes in spontaneous food intake and hunger in humans. Appetite 199321255–272. [DOI] [PubMed] [Google Scholar]
- 39.Neary N M, Small C J, Wren A M.et al Ghrelin increases energy intake in cancer patients with impaired appetite: acute, randomized, placebo‐controlled trial. J Clin Endocrinol Metab 2004892832–2836. [DOI] [PubMed] [Google Scholar]
- 40.Hetherington M M. Taste and appetite regulation in the elderly. Proc Nutr Soc 199857625–631. [DOI] [PubMed] [Google Scholar]
- 41.Schiffman S S. Taste and smell losses in normal ageing and disease. JAMA 19972781357–1362. [PubMed] [Google Scholar]
- 42.Duffy V B, Backstrand J R, Ferris A M. Olfactory dysfunction and related nutritional risk in free‐living, elderly women. J Am Diet Assoc 199595879–884. [DOI] [PubMed] [Google Scholar]
- 43.Schiffman S S, Gatlin C A. Clinical physiology of taste and smell. Annu Rev Nutr 199313405–436. [DOI] [PubMed] [Google Scholar]
- 44.Schiffman S S. Intensification of sensory properties of foods for the elderly. J Nutr 2000130(suppl 4)927–30S. [DOI] [PubMed] [Google Scholar]
- 45.Mathey M F, Siebelink E, de Graaf C.et al Flavor enhancement of food improves dietary intake and nutritional status of elderly nursing home residents. J Gerontol A Biol Sci Med Sci 200156M200–M205. [DOI] [PubMed] [Google Scholar]
- 46.Steele J.National Diet and Nutrition Survey: people aged 65 years and over. Vol 2. Report of the oral health survey. London: The Stationery Office, 1998
- 47.Nakanishi N, Hino Y, Ida O.et al Associations between self‐assessed masticatory disability and health of community‐residing elderly people. Community Dent Oral Epidemiol 199927366–371. [DOI] [PubMed] [Google Scholar]
- 48.Brynes A E, Stratton R J, Wright L.et al Energy intakes fail to meet requirements on texture modified diets. Proc Nutr Soc 199857117A [Google Scholar]
- 49.Mowe M, Bohmer T, Kindt E. Reduced nutritional status in an elderly population (>70 y) is probable before disease and possibly contributes to the development of disease. Am J Clin Nutr 199459317–324. [DOI] [PubMed] [Google Scholar]
- 50.Keller H H. Malnutrition in institutionalized elderly: how and why? J Am Geriatr Soc 1993411212–1218. [DOI] [PubMed] [Google Scholar]
- 51.Gariballa S E, Parker S G, Taub N.et al Nutritional status of hospitalized acute stroke patients. Br J Nutr 199879481–487. [DOI] [PubMed] [Google Scholar]
- 52.Payette H, Gray‐Donald K, Cyr R.et al Predictors of dietary intake in a functionally dependent elderly population in the community. Am J Public Health 199585677–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Claggett M S. Nutritional factors relevant to Alzheimer's disease. J Am Diet Assoc 198989392–396. [PubMed] [Google Scholar]
- 54.Volicer L, Seltzer B, Rheaume Y.et al Progression of Alzheimer‐type dementia in institutionalised patients: a cross sectional study. J Appl Gerontol 1987683–94. [Google Scholar]
- 55.Priefer B A, Robbins J. Eating changes in mild‐stage Alzheimer's disease: a pilot study. Dysphagia 199712212–221. [DOI] [PubMed] [Google Scholar]
- 56.Berkhout A M M, Cools H J M, van Houwelingen H C. The relationship between difficulties in feeding oneself and loss of weight in nursing home patients with dementia. Age Ageing 199827637–641. [DOI] [PubMed] [Google Scholar]
- 57.Gilbert P E, Paul E, Barr P J. Differences in olfactory and visual memory in patients with pathologically confirmed Alzheimer's disease and the Lewy body variant of Alzheimer's disease. Journal of the International Neuropsychological Society 200410835–842. [DOI] [PubMed] [Google Scholar]
- 58.Blaum C S, Fries B E, Fiatarone M A. Factors associated with low body mass index and weight loss in nursing home residents. J Gerontol A Biol Sci Med Sci 199550M162–M168. [DOI] [PubMed] [Google Scholar]
- 59.Thompson M P, Morris L K. Unexplained weight loss in the ambulatory elderly. J Am Geriatr Soc 199139497–500. [DOI] [PubMed] [Google Scholar]
- 60.Westin T, Jansson A, Zenckert C.et al Mental depression is associated with malnutrition in patients with head and neck cancer. Arch Otolaryngol Head Neck Surg 19881141449–1453. [DOI] [PubMed] [Google Scholar]
- 61.Rosenbloom C A, Whittington F J. The effects of bereavement on eating behaviors and nutrient intakes in elderly widowed persons. J Gerontol 199348S223–S229. [DOI] [PubMed] [Google Scholar]
- 62.Baum A. Health psychology: mapping biobehavioral contributions to health and illness. Annu Rev Psychol 199950137–163. [DOI] [PubMed] [Google Scholar]
- 63.Bellisle F, Louis‐Sylvestre J, Linet N.et al Anxiety and food intake in men. Psychosom Med 199052452–457. [DOI] [PubMed] [Google Scholar]
- 64.Potter J, Langhorne P, Roberts M. Routine protein energy supplementation in adults: systematic review. BMJ 1998317495–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Green C J. Existence, causes and consequences of disease‐related malnutrition in the hospital and the community, and clinical and financial benefits of nutritional intervention. Clin Nutr 199918(suppl 2)3–28.10459070 [Google Scholar]
- 66.Akner G, Cederholm T. Treatment of protein‐energy malnutrition in chronic nonmalignant disorders. Am J Clin Nutr 2001746–24. [DOI] [PubMed] [Google Scholar]
- 67.Hebuterne X, Broussard J F, Rampal P. Acute renutrition by cyclic enteral nutrition in elderly and younger patients. JAMA 1995273638–643. [DOI] [PubMed] [Google Scholar]
- 68.Walrand S, Chambon‐Savanovitch C, Felgines C.et al Ageing: a barrier to renutrition? Nutritional and immunologic evidence in rats. Am J Clin Nutr 200072816–824. [DOI] [PubMed] [Google Scholar]
- 69.Stevens J. Impact of age on associations between weight and mortality. Nutr Rev 200058129–137. [DOI] [PubMed] [Google Scholar]
- 70.Working party of the Royal College of Physicians Nutrition and patients: a doctor's responsibility. London: Royal College of Physicians, 2002