Abstract
Growth hormone (GH) is synthesised and secreted by the somatotroph cells of the anterior lobe of the pituitary gland. Its actions involve multiple organs and systems, affecting postnatal longitudinal growth as well as protein, lipid, and carbohydrate metabolism. GH hypersecretion results in gigantism or acromegaly, a condition associated with significant morbidity and mortality, while GH deficiency results in growth retardation in children and the GH deficiency syndrome in adults. This article, aimed at non‐paediatric physicians, examines the clinical features, diagnosis, and current concepts in the management of these conditions.
Keywords: growth hormone, acromegaly, growth hormone deficiency, hypopituitarism
Growth hormone (GH) is an anabolic hormone that is synthesised and secreted by the somatotroph cells of the anterior lobe of the pituitary gland. It is a member of the GH gene family, which includes prolactin and the placental lactogens. Using x ray crystallography, the three dimensional structure of human GH has been shown to consist of two disulfide bridges, four α‐helices arranged in an “up‐up‐down‐down” topology, and three shorter connective helices.1
GH exerts its biological effects by binding to the extracellular domain of the GH receptor, a single pass protein that also contains transmembrane and intracellular regions. A single GH molecule binds to two GH receptor molecules, resulting in dimerisation of the receptor.2 This GH induced GH receptor dimerisation is thought to be the first step in the signal pathway that ultimately results in the various biological effects associated with GH.3
GH actions involve multiple organs and systems. Postnatal longitudinal growth and development, but not intrauterine growth, are dependent on normal pulsatile GH secretion.4 GH is also responsible for changes in protein, lipid, and carbohydrate metabolism.5
The somatomedin hypothesis postulated that the observed effects of GH are mediated via a growth factor, initially labelled “somatomedin”6,7 and subsequently identified as insulin‐like growth factor (IGF) 1.8 However, recent evidence suggests that not all actions of GH are mediated by IGF1 and many factors other than GH contribute to the expression of serum IGF1 including nutritional state, liver function, serum protease activity, IGF1 binding proteins, and sex hormones.5
GH secretion is regulated by the hypothalamus and the mediators of GH actions. Regulatory factors include GH releasing hormone (GHRH), somatostatin, GH releasing peptide (ghrelin), and IGF1. Disorders of the GH/IGF1 system result either from GH hypersecretion (gigantism, acromegaly) or GH deficiency. This article, aimed at non‐paediatric physicians, examines the clinical features, diagnosis, and current concepts in the management of these conditions.
Acromegaly
The term acromegaly is derived from the Greek words akron, meaning extremity, and megas meaning great. Acromegaly is a chronic endocrine disease first described by the French neurologist Pierre Marie in 1886. It is caused almost invariably by a GH secreting pituitary adenoma, although rarely it may be attributable to a hypothalamic tumour secreting GHRH or ectopic GHRH secretion from a carcinoid tumour (predominantly of the pancreas or bronchus). It is a rare condition, with an estimated prevalence of around 60 per million and an annual incidence of 3–4 per million,9 but active acromegaly is associated with significant morbidity and an increase in mortality compared with the general population.10,11,12,13,14
Molecular pathogenesis
Pituitary adenomas generally result from dysregulated monoclonal expansion of a mutated cell, pointing to an intrinsic defect as the primary neoplastic event in pituitary tumourigenesis.15 Tumour formation is most probably the ultimate result of a series of genetic changes involving tumour suppressor gene inactivation and oncogene activation. Stimulatory G protein (Gs) is involved in the mediation of GHRH action and contains an α‐subunit; an activating mutation of the α‐subunit gene (gsp) leads to persistently activated stimulatory G protein and high intracellular levels of cyclic AMP. This defect mimics stimulation of adenylyl cyclase by GHRH receptor activation, resulting in autonomous GH secretion.15 The gsp mutation has been found in 40% of human GH secreting pituitary adenomas, and is comparatively specific for somatotroph tumourigenesis.
Clinical features
The clinical features of acromegaly are attributable to the somatic and metabolic effects of prolonged excess GH exposure or to local effects of an expanding pituitary mass.16 They often develop insidiously over many years, resulting in delayed diagnosis.17 Most patients experience headaches and sweating. The most typical clinical signs are the coarse facial features, large, spade shaped hands and enlarged feet resulting from soft tissue swelling and bony enlargement. The facial features include deep nasolabial furrows, prominent supraorbital ridges, and enlargement of the lips and nose. Growth of the mandible results in prognathism, malocclusion, and widened inter‐dental spaces. Other common features include enlargement of the tongue (macroglossia), swelling of the nasopharyngeal tissue, sleep apnoea, lethargy, skin tags, goitre, and colonic polyps. The expanding pituitary mass may cause hypopituitarism, reproductive disorders, and visual symptoms. GH hypersecretion occurring before the epiphyses have fused results in excess linear bone growth and gigantism.
Growth hormone (GH)
An anabolic hormone
Synthesised in the anterior lobe of the pituitary gland
Acts by binding to two GH receptor molecules
Responsible for longitudinal growth
Modulates protein, lipid, and carbohydrate metabolism
Secretion regulated by the hypothalamus
Complications
Musculoskeletal complications
The most significant cause of functional disability in acromegaly is arthropathy. Acromegalic arthropathy affects up to 70% of patients and involves both the axial and peripheral skeleton.18,19 The underlying pathophysiology is not entirely understood, but it has been hypothesised that GH excess stimulates local production of IGF1 in cartilage, resulting in thickening of the cartilage, change of the normal joint geometry, and joint hypermobility.18 Radiological findings include narrowing of the joint spaces, osteophytes, and other features seen in osteoarthritis. Symptomatic carpal tunnel syndrome is also a frequent finding, affecting up to 60% of patients, and is thought to be attributable to oedema of the median nerve in the carpal tunnel, rather than extrinsic nerve compression.18,20
Cardiovascular complications
Acromegaly is characterised by a high incidence of cardiovascular disease, which contributes significantly to morbidity and mortality. Hypertension occurs in around a third of all patients, ranging in some series up to 60%.21 The mechanisms underlying the development of hypertension in acromegaly remain unclear although experimental and clinical studies suggest possible roles for the anti‐natriuretic action of GH, changed sympathetic tone, and direct effects of vascular growth factors.21 Acromegalic cardiomyopathy is caused by the effects of chronic GH excess on the heart. It is characterised by biventricular concentric hypertrophy, with thickened ventricle walls but normal sized chambers.18 The cardiac hypertrophy is associated with functional changes (decreased ejection fraction in response to exercise), valve abnormalities, and a high incidence of arrhythmias.22,23,24,25 The incidence and severity of the abnormalities increase in elderly patients with long disease duration.26,27
Metabolic complications
GH counteracts the effects of insulin on glucose and lipid metabolism, resulting in metabolic complications in patients with acromegaly. The most frequent of these is changed glucose metabolism. The prevalence of overt diabetes mellitus ranges from 19% to 56% in patients with active acromegaly, with impaired glucose tolerance (IGT) occurring in up to 46%.18,28 Studies investigating the pathogenesis of changed glucose metabolism in acromegaly suggest GH excess induces insulin resistance by impairing the ability of insulin to suppress gluconeogenesis, decreasing peripheral glucose utilisation, and reducing insulin receptor numbers and binding affinity.18
Acromegaly
Attributable to GH excess
Annual incidence 3–4 per million
Prevalence 60 per million
Associated with significant morbidity and mortality
Treatment modalities; surgery, radiotherapy, medical therapy
Clinical features of acromegaly
Coarse facial features
Large, spade shaped hands
Large feet
Prognathism and macroglossia
Sleep apnoea
Goitre
Colonic polyps
Neoplastic complications
The association between the GH/IGF1 axis and neoplasia such as breast, prostate, and colon cancer has been the subject of basic and clinical research for many years. Data linking IGFs to tumourigenesis and neoplastic growth have been extensively reviewed by Khandwala et al.29 However, epidemiological studies exploring the link between acromegaly, cancer incidence, and mortality have given rise to conflicting data, leading to significant debate. Early studies suggested an increased incidence of neoplasia overall, particularly of the breast17 and colon,30 in patients with acromegaly. Evidence from more recent studies, however, has failed to confirm these findings, and suggests overall cancer incidence is not increased in acromegaly.31,32 What has emerged is that patients with acromegaly have an increased risk of developing colorectal cancer, although the exact magnitude of this risk and the role of screening programmes remain the subject of much debate.33,34,35,36 In addition, recent epidemiological studies found cancer death rates in cohorts of patients with acromegaly were similar to those in the general population, suggesting malignancy is not a significant cause of mortality in patients with acromegaly.14,37,38
Diagnostic investigations
Biochemical diagnosis
The clinical features of acromegaly are outlined above, but as it often develops insidiously, few patients present immediately at the time of onset of symptoms and the most common mode of presentation is as an incidental finding by a healthcare professional.39 Biochemical confirmation relies on the demonstration of excessive GH secretion. Baseline biochemical assessment includes a fasting or random GH and IGF1 measurement, with a random GH level less than 0.4 μg/l (0.8 mU/l) and a normal age and sex matched IGF1 excluding the diagnosis of acromegaly.40 However, as GH is secreted in a pulsatile manner, single GH measurements provide inadequate information regarding GH increase and therefore if either of these levels is not achieved or index of suspicion is high, an oral glucose tolerance test (OGTT) should be performed with 75 g oral glucose and subsequent measurements of glucose and GH every 30 minutes over two hours. Failure of GH suppression to less than 1 μg/l (2 mU/l) during an OGTT suggests the diagnosis of acromegaly, but the results should always be considered in conjunction with an IGF1 measurement as other conditions (for example, diabetes mellitus, renal disease) can cause discordantly increased GH levels.40 Twenty four hour mean GH levels can also be used in the diagnosis of acromegaly (mean 24 hour GH less than 2.5 μg/l (5 mU/l) excludes acromegaly), but 24 hour sampling is time consuming and less cost effective than an OGTT, and is now usually reserved for research purposes or for patients with equivocal OGTT or IGF1 results.39 Thyrotropin releasing hormone (TRH), GHRH, or gonadotrophin releasing hormone (GnRH) stimulation tests and measurement of IGF binding protein 3 offer no additional information for the diagnosis of acromegaly.
Complications of acromegaly
Musculoskeletal; arthropathy, carpal tunnel syndrome
Cardiovascular; hypertension, cardiomyopathy, arrhythmias
Metabolic; impaired glucose and lipid metabolism
Neoplastic; colorectal cancer
Radiological investigation
Magnetic resonance imaging (MRI) is now considered the gold standard for investigating pituitary adenomas, providing invaluable information about tumour size and extension, and permitting accurate delineation of parasellar structures.41,42 The information obtained is improved if this is performed at a centre specialised in examination of the pituitary fossa.43 For patients in whom it is not possible to perform MRI, computed tomography (CT) offers a reasonable alternative.
Other investigations
Visual field defects occur in up to 20% of patients with acromegaly, predominantly in those with larger tumours, suprasellar extension, and higher GH levels.44 A bitemporal hemianopia is the most common defect but other forms may also be seen. Visual fields should be tested by confrontation during initial assessment, and if necessary this should be followed by formal documentation using Goldman perimetry.
Over 50% of patients with acromegaly have pituitary dysfunction affecting the secretion of at least one other anterior pituitary hormone,39 therefore basal pituitary function should be assessed by measuring serum prolactin, gonadotrophins, testosterone/oestradiol, thyroid stimulating hormone (TSH), free T3, free T4, and serum cortisol, although dynamic testing of the hypothalamo‐pituitary‐adrenal axis may be necessary.
In rare cases where a GHRH secreting tumour is suspected, serum GHRH levels can be measured.
Management
Several retrospective studies have shown a twofold to threefold increased mortality in acromegalic patients compared with age and sex matched controls, with death predominantly attributable to vascular disease, respiratory disease and, in the earlier studies, malignancy.10,11,12,14,17,31,37,45,46 However, results from the more recent studies also showed that the increased mortality associated with acromegaly can be diminished if treatment is successful in reducing GH hypersecretion to less than 2–2.5 μg/l (4–5 mU/l), whether this is measured as the mean of a growth hormone day profile or as a random growth hormone level.11,12,14,31,37,38 This has resulted in the publication of a consensus statement defining the criteria for cure of acromegaly as a normal age matched serum IGF1 level and a GH nadir of less than 1 μg/l (2 mU/l) during an oral glucose tolerance test.40
Diagnostic tests
Biochemical; oral glucose tolerance test, serum IGF1
Radiological; pituitary MRI (CT if MRI not possible)
Visual field assessment
Assessment of other anterior pituitary hormones
The main aims of treatment of acromegaly are reversing the symptoms and signs of the disease, treating the underlying cause, preventing disease recurrence, and improving long term survival. This entails the use of surgery, radiotherapy, and/or medical therapy.
Surgery
Transsphenoidal surgery (TSS) is still considered first line treatment for acromegaly in most patients. Based on strict biochemical criteria (mean GH less than 2.5 μg/l (5 mU/l), suppressed GH during an OGTT and/or normal IGF1), the overall remission rate after TSS ranges from 55% to 70%.13,46,47,48,49,50 A number of factors have emerged as crucial in determining outcome after TSS, including tumour size, extrasellar extension, dural invasion, and pre‐treatment GH levels. Remission rates for microadenomas are around 80% to 90%, while those for macroadenomas are around 50%.13,46,47,48 The expertise of the pituitary surgeon also plays a key part in determining outcome, as has been illustrated by data from our centre in Birmingham; surgical outcome improved significantly when surgery was performed by a single dedicated pituitary surgeon rather than eight different surgeons, as had been the case previously.48
Surgical complications such as cerebrospinal fluid (CSF) leaks, diabetes insipidus, and development of new hypopituitarism are infrequent, occurring in less than 7% of cases, while recurrence after initial surgical remission occurs in around 6%.13,47,50
Radiotherapy
Radiotherapy has been used in the management of acromegaly for nearly a century, with conventional fractionated radiotherapy lowering GH levels over 20 years to less than 5 μg/l (10 mU/l) in 70% to 90% of patients.51 Radiotherapy is also effective at controlling tumour growth as shown by Brada et al, with progression free survival of 94% at 10 years and 88% at 20 years in patients with pituitary adenomas treated with external beam radiotherapy.52 However, a number of factors have led to a re‐evaluation of the role of radiotherapy in the management of acromegaly. Apart from the long lag time to clinical effect, during which the patients continue to suffer the effects of GH hypersecretion, a number of studies have shown that IGF1 levels remain raised in a significant proportion of patients whose GH levels are reduced to 2–2.5 μg/l (4–5 mU/l) after treatment with radiotherapy.51,53 Hypopituitarism, which itself has been linked to premature mortality,54 is a significant problem, and at 10 years, around half of all patients treated with radiotherapy will have developed new anterior hormone deficiencies.55 In addition, it is now well recognised that patients treated with radiotherapy have a significantly increased risk of cerebrovascular events and cerebrovascular mortality compared with the general population.14,56,57 Radiation induced visual deterioration and the development of second brain tumours occur in a small proportion of patients,52 and although cognitive dysfunction is listed as a side effect of radiotherapy, recent evidence suggests it occurs in patients with pituitary tumours regardless of treatment modality.58
Gamma knife radiosurgery, which permits a highly precise circumscribed delivery of radiation to a target in a single session, has also been used to treat acromegaly.59 In a recent study, around 25% of patients treated with gamma knife radiosurgery achieved GH less than 2.5 μg/l (5 mU/l) and IGF1 normalisation within five years, with significant tumour shrinkage.60 However, more long term follow up data are required to fully assess its role in the management of acromegaly.
At present, radiotherapy should be reserved for patients in whom satisfactory control of tumour growth, GH, and IGF1 has not been achieved by surgery and/or medical therapy.
Medical therapy
Traditionally, TSS and/or radiotherapy have been considered first line treatment for acromegaly, but despite recent advances in both these forms of treatment, the overall surgical cure rate remains around 60% and radiotherapy is characterised by delayed effect and a high incidence of hypopituitarism, as discussed above. This means medical therapy is necessary in a significant proportion of patients, and current options include dopamine agonists, somatostatin analogues, and more recently GH receptor antagonists.
Dopamine agonists
The dopamine agonist bromocriptine was the first effective medical treatment for acromegaly, but it lowers GH secretion to the desired levels in less than 20% of cases and is associated with a number of side effects including nausea, dizziness, and headaches.61 Newer dopamine agonists like cabergoline are better tolerated and more efficacious, lowering GH levels to less than 2 μg/l (4 mU/l) in up to 46% of cases, with greater responses in patients with tumours co‐secreting GH and prolactin.62 This is not uncommon, as up to one third of these adenomas are plurihormonal acidophil cell derived tumours and co‐secrete both hormones.63
Somatostatin analogues
The use of dopamine agonists has largely been superseded by the introduction of somatostatin analogues, which exert their biological effects by activating somatostatin receptors (predominantly sub‐receptor types 2 and 5) in the pituitary.64 Octreotide, a long acting synthetic somatostatin analogue, has been used to treat acromegaly for two decades, and since the mid‐1990s, three slow release depot preparations have been introduced; Sandostatin LAR (Novartis), Lanreotide LA (Ipsen), and Lanreotide Autogel (Ipsen). These have been shown to be both effective and safe, suppressing GH levels to less than 2–2.5 μg/l (4–5 mU/l) and normalising serum IGF1 levels in 50%–70% of cases.64,65,66,67 In addition, tumour shrinkage by 20%–50% has been reported in around 30% of patients pre‐selected for octreotide responsiveness.64,68,69,70
Although somatostatin analogues have traditionally been used as an adjunct to surgery and radiotherapy, they are increasingly being used as first line therapy in the treatment of acromegaly. Several studies in which somatostatin analogues were given to previously untreated (de novo) patients showed suppression of GH and IGF1 to a similar extent to that seen in patients who received the drugs after surgery.69,71,72,73,74 These findings have led the authors to conclude that if the possibility of surgical cure is low, and if there is no visual compromise, then medical treatment with somatostatin analogues alone is as effective biochemically and clinically as the combination of surgery followed by medical therapy, and offers a reasonable primary therapeutic modality.
Given the recognised efficacy of somatostatin analogues in improving biochemical parameters and reducing tumour size, a number of studies have investigated the impact of pre‐treatment with these drugs on surgical outcome. While some have shown no clear benefits, others have found improvements in terms of remission rates and clinical conditions, including preoperative blood pressure, cardiac function, glucose metabolism, and shorter hospital stays.75,76
There are a number of side effects associated with somatostatin analogue therapy, but these are rarely severe and in general do not limit treatment. Around 50% of patients experience gastrointestinal symptoms including diarrhoea, nausea, and abdominal discomfort, but these are usually transient.64 New gallstone formation (usually asymptomatic) occurs in 10%–20% of patients and a small number develop impaired glucose metabolism.73
GH receptor antagonists
Pegvisomant is a novel, genetically engineered GH receptor antagonist that, in contrast with dopamine agonists and somatostatin analogues, inhibits GH action rather than secretion. It exerts its biological actions by preventing functional dimerisation of the GH receptor.77 Clinical studies have shown that pegvisomant is remarkably effective, improving clinical symptoms and signs and resulting in IGF1 normalisation in over 90% of patients.78,79,80 The drug seems to be safe and well tolerated. Because pegvisomant works by blocking the actions of GH, efficacy is independent of tumour characteristics, such as the density of somatostatin receptors. Despite normalised IGF1 levels, GH levels remain raised in these patients, albeit with minimal or neutralised bioactivity because of receptor block. Concerns of the raised GH concentrations being reflected in tumour growth need to be clarified by additional long term monitoring studies.
There is now overwhelming evidence that the clinical outcome in patients with acromegaly can be improved substantially by means of earlier diagnosis, better surgical expertise, a multidisciplinary approach to management, and effective medical therapies used in a flexible manner. The factors that are important in choosing an optimal strategy now increasingly include patient preference, coincidental comorbidity, and cost‐benefit analyses.
Growth hormone deficiency
Growth hormone deficiency in adulthood can result from onset during childhood or later in life. Idiopathic GH deficiency is the most common cause of GH deficiency in children.81 It is a poorly defined and often reversible condition that presents with short stature and low growth velocity for age. In contrast, adult onset GH deficiency generally presents as part of a combined pituitary hormone deficiency phenotype (hypopituitarism) and is commonly attributable to a pituitary adenoma and/or treatment with surgery or radiotherapy.82 GH is usually the first anterior pituitary hormone to be affected in patients with large pituitary adenomas or after pituitary surgery and radiotherapy. Other causes of GH deficiency are listed in the box.
Growth hormone deficiency syndrome
There is no single symptom or sign that is pathognomonic of GH deficiency in adult life, but a well defined constellation of symptoms and signs has been identified in adults with GH deficiency, leading to the recognition of a “GH deficiency syndrome”. In adult life, GH deficiency is associated with changed body composition, changed metabolism, reduced exercise capacity, and impaired quality of life.82,83 Adults with GH deficiency have reduced skeletal muscle, reduced lean body mass and increased fat mass, particularly distributed in the truncal region, mostly in visceral tissue. In addition, these patients have reduced bone mass compared with matched healthy controls, and are at increased risk of sustaining osteoporotic fractures.84
Adult GH deficiency and hypopituitarism have been associated with a number of risk factors for cardiovascular disease, including vascular endothelial dysfunction, dyslipidaemia, and insulin resistance.85 Reduced left ventricular mass, impaired cardiac systolic function, and impaired response to peak exercise have also been reported.86 These findings, along with the reduced lean body mass, result in reduced muscle strength and exercise capacity.
Using validated questionnaires, several studies have reported reduced self perceived psychological wellbeing and quality of life in adults with GH deficiency.83 Characteristic features include depressed mood, anxiety, lack of energy, social isolation, and impaired wellbeing.
Diagnosing GH deficiency in children is straightforward, as it is associated with growth retardation. However, diagnosis of adult onset GH deficiency is much more challenging, as none of the features associated with the condition are pathognomonic. Although GH response to a number of pharmacological stimuli including clonidine, glucagon, arginine, and GHRH have been used to diagnose GH deficiency,82 the insulin tolerance test is currently considered the diagnostic test of choice,87 with a peak GH response of less than 3 μg/l confirming the diagnosis. This test can, however, be unpleasant for patients and is contraindicated in those with epilepsy and ischaemic heart disease, resulting in an ongoing search for newer and safer tests to diagnose GH deficiency. Recently developed strategies include the combined arginine and GHRH stimulation test and the combined GHRH and GH releasing peptide 6 stimulation test.88
Causes of GH deficiency
Pituitary tumours
Genetic causes; Laron syndrome, pituitary transcription factor 1 (Pit1), and prophet of Pit1 (PROP1) mutations
Septo‐optic dysplasia
Parapituitary tumours; craniopharyngiomas, meningiomas, metastases
Radiotherapy; pituitary, cranial
Traumatic brain injury
Pituitary infarction; apoplexy, Sheehan's syndrome
Pituitary infiltration; sarcoidosis, lymphocytic hypophysitis
Infection; tuberculosis
Chemotherapy
Growth hormone deficiency and mortality
A number of studies have examined mortality in patients with hypopituitarism and all have confirmed increased mortality compared with age matched controls, predominantly attributable to respiratory, cardiovascular, and cerebrovascular disease.54,89,90,91 The extent to which GH deficiency influences this increased mortality remains unclear and has been the subject of much debate. Although adult GH deficiency has been implicated as having a vital role in mediating increased vascular mortality in hypopituitarism,92 the largest study examining mortality in hypopituitarism reported that the only hormone deficiency that was conclusively implicated in the excess mortality was untreated gonadotrophin deficiency.54 Furthermore, evidence is lacking that GH replacement in GH deficiency patients leads to improved survival. In fact, untreated patients with isolated GH deficiency due to GH gene deletion, patients with multiple pituitary hormone deficiency due to PROP1 gene mutation, and patients with isolated IGF1 deficiency due to GH receptor gene mutation (Laron syndrome) can survive to an advanced age, reaching ages of 80–90 years.93 By contrast, a recent study of 11 subjects living in a Swiss valley with isolated GH deficiency due to deletion of the GH1 gene reported a significantly reduced life span in comparison with siblings, as well as with the normal population living in the valley.94
GH replacement
Replacement therapy with GH has consistently been shown to have beneficial effects on body composition, bone turnover, cardiovascular risk factors, and quality of life.83 In studies investigating the effects of GH replacement on body composition, lean body mass increased by 2 to 5.5 kg, while (predominantly abdominal) fat mass was reduced by 4 to 6 kg after six months. Longer term treatment (12 months or more) led to increases in bone mineral density and biochemical evidence of bone remodelling.
The effects of GH replacement on cardiac morphology and function are well described, with a number of studies showing a significant increase in left ventricular mass and stroke volume.86 This results in improved cardiac performance.
GH therapy leads to an improvement in vascular risk due to a number of factors including changed nitric oxide availability and changes in lipid fractions and inflammation mediators. However, most studies to date do not show a beneficial effect on insulin resistance.85
Growth hormone deficiency syndrome
Reduced lean body mass, increased fat mass
Dyslipidaemia, insulin resistance, vascular endothelial dysfunction
Reduced left ventricular mass, impaired systolic function
Impaired quality of life
Several studies assessing the impact of GH replacement on psychological wellbeing in patients with GH deficiency have shown significant improvements in mood and quality of life, especially in those patients with greatest psychological morbidity.83
Despite the beneficial effects of GH therapy outlined above, to date no data exist on the effects of GH replacement on mortality.
GH replacement is associated with few side effects, the most common being fluid retention, arthralgia, and myalgia. Reassuringly, data from long term studies suggest there is no significant risk of tumour development or recurrence.83
Conclusion
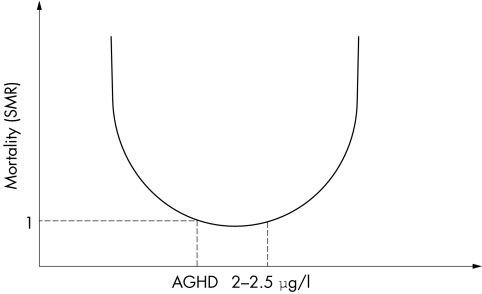
GH is essential for growth, but also modulates protein, lipid, and carbohydrate metabolism. There is clear evidence that GH excess is associated with reduced survival, and suggestions that GH deficiency may play a vital part in the increased mortality seen in patients with hypopituitarism. These clinical findings suggest that there is probably an optimal level of circulating GH required to maintain normal health, represented in figure 1 as a U shaped curve; in disorders of GH excess or deficiency, restoration of GH levels to normal reduces mortality risk to that of the normal population.
Figure 1 U shaped curve showing the optimal level of circulating GH required to maintain normal health. Both GH excess and GH deficiency seem to be associated with an increase in mortality. AGHD, adult growth hormone deficiency; SMR, standardised mortality ratio.
Questions (true (T)/false (F); answers at end of references)
-
Growth hormone
Is synthesised in the posterior lobe of the pituitary gland
Is responsible for intrauterine and postnatal growth
Modulates protein, lipid, and carbohydrate metabolism
Effects are mediated predominantly via IGF1
Binds to two GH receptor molecules
-
Acromegaly
Is most commonly caused by a GH secreting pituitary adenoma
Is diagnosed by showing increased GH and reduced IGF1 levels
Is always associated with tall stature
Is associated with reduced survival
Is associated with a reduction in GH levels when treated with pegvisomant
-
Adult onset growth hormone deficiency
Results in short stature
Is associated with increased mortality that can be reversed by GH replacement
Leads to impaired quality of life
Is often associated with deficiencies in other anterior pituitary hormones
Can occur after a head injury
Abbreviations
GH - growth hormone
IGF - insulin‐like growth factor
OGTT - oral glucose tolerance test
IGT - impaired glucose intolerance
GHRH - GH releasing hormone
TRH - thyrotropin releasing hormone
MRI - magnetic resonance imaging
CT - computed tomography
TSS - transsphenoidal surgery
Answers
1. (A) F, (B) F, (C) T, (D) T, (E) T; 2. (A) T, (B) F, (C) F, (D) T, (E) F; 3. (A) F, (B) F, (C) T, (D) T, (E) T.
Footnotes
Funding: John Ayuk is supported by an Ipsen Fund Clinical Research Fellowship.
Conflicts of interest: none.
References
- 1.de Vos A M, Ultsch M, Kossiakoff A A. Human growth hormone and extracellular domain of its receptor: crystal structure of the complex. Science 1992255306–312. [DOI] [PubMed] [Google Scholar]
- 2.Cunningham B C, Ultsch M, de Vos A M.et al Dimerization of the extracellular domain of the human growth hormone receptor by a single hormone molecule. Science 1991254821–825. [DOI] [PubMed] [Google Scholar]
- 3.Argetsinger L S, Carter‐Su C. Mechanism of signaling by growth hormone receptor. Physiol Rev 1996761089–1107. [DOI] [PubMed] [Google Scholar]
- 4.Gluckman P D, Grumbach M M, Kaplan S L. The neuroendocrine regulation and function of growth hormone and prolactin in the mammalian fetus. Endocr Rev 19812363–395. [DOI] [PubMed] [Google Scholar]
- 5.Le Roith D, Bondy C, Yakar S.et al The somatomedin hypothesis: 2001. Endocr Rev 20012253–74. [DOI] [PubMed] [Google Scholar]
- 6.Daughaday W H, Hall K, Raben M S.et al Somatomedin: proposed designation for sulphation factor. Nature 1972235107. [DOI] [PubMed] [Google Scholar]
- 7.Salmon W D, Jr, Daughaday W H. A hormonally controlled serum factor which stimulates sulfate incorporation by cartilage in vitro. 1956. J Lab Clin Med 1990116408–419. [PubMed] [Google Scholar]
- 8.Klapper D G, Svoboda M E, van Wyk J J. Sequence analysis of somatomedin‐C: confirmation of identity with insulin‐like growth factor I. Endocrinology 19831122215–2217. [DOI] [PubMed] [Google Scholar]
- 9.Holdaway I M, Rajasoorya C. Epidemiology of acromegaly. Pituitary 1999229–41. [DOI] [PubMed] [Google Scholar]
- 10.Wright A D, Hill D M, Lowy C.et al Mortality in acromegaly. Q J Med 1970391–16. [PubMed] [Google Scholar]
- 11.Bates A S, Van't Hoff W.et al An audit of outcome of treatment in acromegaly. Q J Med 199386293–299. [PubMed] [Google Scholar]
- 12.Rajasoorya C, Holdaway I M, Wrightson P.et al Determinants of clinical outcome and survival in acromegaly. Clin Endocrinol (Oxf) 19944195–102. [DOI] [PubMed] [Google Scholar]
- 13.Swearingen B, Barker F G, Katznelson L.et al Long‐term mortality after transsphenoidal surgery and adjunctive therapy for acromegaly. J Clin Endocrinol Metab 1998833419–3426. [DOI] [PubMed] [Google Scholar]
- 14.Ayuk J, Clayton R N, Holder G.et al Growth hormone and pituitary radiotherapy, but not serum insulin‐like growth factor‐I concentrations, predict excess mortality in patients with acromegaly. J Clin Endocrinol Metab 2004891613–1617. [DOI] [PubMed] [Google Scholar]
- 15.Drange M R, Melmed S. Molecular pathogenesis of acromegaly. Pituitary 1999243–50. [DOI] [PubMed] [Google Scholar]
- 16.Melmed S. Acromegaly. N Engl J Med 1990322966–977. [DOI] [PubMed] [Google Scholar]
- 17.Nabarro J D. Acromegaly. Clin Endocrinol (Oxf) 198726481–512. [DOI] [PubMed] [Google Scholar]
- 18.Colao A, Ferone D, Marzullo P.et al Systemic complications of acromegaly: epidemiology, pathogenesis, and management. Endocr Rev 200425102–152. [DOI] [PubMed] [Google Scholar]
- 19.Scarpa R, De Brasi D, Pivonello R.et al Acromegalic axial arthropathy: a clinical case‐control study. J Clin Endocrinol Metab 200489598–603. [DOI] [PubMed] [Google Scholar]
- 20.Jenkins P J, Sohaib S A, Akker S.et al The pathology of median neuropathy in acromegaly. Ann Intern Med 2000133197–201. [DOI] [PubMed] [Google Scholar]
- 21.Bondanelli M, Ambrosio M R, degli Uberti E C. Pathogenesis and prevalence of hypertension in acromegaly. Pituitary 20014239–249. [DOI] [PubMed] [Google Scholar]
- 22.Kahaly G, Olshausen K V, Mohr‐Kahaly S.et al Arrhythmia profile in acromegaly. Eur Heart J 19921351–56. [DOI] [PubMed] [Google Scholar]
- 23.Herrmann B L, Bruch C, Saller B.et al Occurrence of ventricular late potentials in patients with active acromegaly. Clin Endocrinol (Oxf) 200155201–207. [DOI] [PubMed] [Google Scholar]
- 24.Clayton R N. Cardiovascular function in acromegaly. Endocr Rev 200324272–277. [DOI] [PubMed] [Google Scholar]
- 25.Colao A, Spinelli L, Marzullo P.et al High prevalence of cardiac valve disease in acromegaly: an observational, analytical, case‐control study. J Clin Endocrinol Metab 2003883196–3201. [DOI] [PubMed] [Google Scholar]
- 26.Colao A, Cuocolo A, Marzullo P.et al Impact of patient's age and disease duration on cardiac performance in acromegaly: a radionuclide angiography study. J Clin Endocrinol Metab 1999841518–1523. [DOI] [PubMed] [Google Scholar]
- 27.Matta M P, Caron P. Acromegalic cardiomyopathy: a review of the literature. Pituitary 20036203–207. [DOI] [PubMed] [Google Scholar]
- 28.Kreze A, Kreze‐Spirova E, Mikulecky M. Risk factors for glucose intolerance in active acromegaly. Braz J Med Biol Res 2001341429–1433. [DOI] [PubMed] [Google Scholar]
- 29.Khandwala H M, McCutcheon I E, Flyvbjerg A.et al The effects of insulin‐like growth factors on tumorigenesis and neoplastic growth. Endocr Rev 200021215–244. [DOI] [PubMed] [Google Scholar]
- 30.Ron E, Gridley G, Hrubec Z.et al Acromegaly and gastrointestinal cancer. Cancer 1991681673–1677. [DOI] [PubMed] [Google Scholar]
- 31.Orme S M, McNally R J, Cartwright R A.et al Mortality and cancer incidence in acromegaly: a retrospective cohort study. United Kingdom Acromegaly Study Group. J Clin Endocrinol Metab 1998832730–2734. [DOI] [PubMed] [Google Scholar]
- 32.Melmed S. Acromegaly and cancer: not a problem? J Clin Endocrinol Metab 2001862929–2934. [DOI] [PubMed] [Google Scholar]
- 33.Jenkins P J, Fairclough P D. Colorectal neoplasia in acromegaly. Clin Endocrinol (Oxf) 200155727–729. [DOI] [PubMed] [Google Scholar]
- 34.Renehan A G, Odwyer S T, Shalet S M. Screening colonoscopy for acromegaly in perspective. Clin Endocrinol (Oxf) 200155731–733. [DOI] [PubMed] [Google Scholar]
- 35.Perry I, Stewart P M, Kane K. Colorectal screening guidelines in acromegaly. (Letter). Gut 2003521387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Renehan A G, O'Connell J, O'Halloran D.et al Acromegaly and colorectal cancer: a comprehensive review of epidemiology, biological mechanisms, and clinical implications. Horm Metab Res 200335712–725. [DOI] [PubMed] [Google Scholar]
- 37.Holdaway I M, Rajasoorya R C, Gamble G D. Factors influencing mortality in acromegaly. J Clin Endocrinol Metab 200489667–674. [DOI] [PubMed] [Google Scholar]
- 38.Mestron A, Webb S M, Astorga R.et al Epidemiology, clinical characteristics, outcome, morbidity and mortality in acromegaly based on the Spanish Acromegaly Registry (Registro Espanol de Acromegalia, REA). Eur J Endocrinol 2004151439–446. [DOI] [PubMed] [Google Scholar]
- 39.Duncan E, Wass J A. Investigation protocol: acromegaly and its investigation. Clin Endocrinol (Oxf) 199950285–293. [DOI] [PubMed] [Google Scholar]
- 40.Giustina A, Barkan A, Casanueva F F.et al Criteria for cure of acromegaly: a consensus statement. J Clin Endocrinol Metab 200085526–529. [DOI] [PubMed] [Google Scholar]
- 41.Marro B, Zouaoui A, Sahel M.et al MRI of pituitary adenomas in acromegaly. Neuroradiology 199739394–399. [DOI] [PubMed] [Google Scholar]
- 42.Bourdelot A, Coste J, Hazebroucq V.et al Clinical, hormonal and magnetic resonance imaging (MRI) predictors of transsphenoidal surgery outcome in acromegaly. Eur J Endocrinol 2004150763–771. [DOI] [PubMed] [Google Scholar]
- 43.Lissett C A, Shalet S M. Management of pituitary tumours: strategy for investigation and follow‐up. Horm Res 200053(suppl 3)65–70. [DOI] [PubMed] [Google Scholar]
- 44.Rivoal O, Brezin A P, Feldman‐Billard S.et al Goldmann perimetry in acromegaly: a survey of 307 cases from 1951 through 1996. Ophthalmology 2000107991–997. [DOI] [PubMed] [Google Scholar]
- 45.Alexander L, Appleton D, Hall R.et al Epidemiology of acromegaly in the Newcastle region. Clin Endocrinol (Oxf) 19801271–79. [DOI] [PubMed] [Google Scholar]
- 46.Beauregard C, Truong U, Hardy J.et al Long‐term outcome and mortality after transsphenoidal adenomectomy for acromegaly. Clin Endocrinol (Oxf) 20035886–91. [DOI] [PubMed] [Google Scholar]
- 47.Freda P U, Wardlaw S L, Post K D. Long‐term endocrinological follow‐up evaluation in 115 patients who underwent transsphenoidal surgery for acromegaly. J Neurosurg 199889353–358. [DOI] [PubMed] [Google Scholar]
- 48.Gittoes N J, Sheppard M C, Johnson A P.et al Outcome of surgery for acromegaly—the experience of a dedicated pituitary surgeon. QJM 199992741–745. [DOI] [PubMed] [Google Scholar]
- 49.Biermasz N R, van Dulken H, Roelfsema F. Ten‐year follow‐up results of transsphenoidal microsurgery in acromegaly. J Clin Endocrinol Metab 2000854596–4602. [DOI] [PubMed] [Google Scholar]
- 50.Kreutzer J, Vance M L, Lopes M B.et al Surgical management of GH‐secreting pituitary adenomas: an outcome study using modern remission criteria. J Clin Endocrinol Metab 2001864072–4077. [DOI] [PubMed] [Google Scholar]
- 51.Barkan A L. Radiotherapy in acromegaly: the argument against. Clin Endocrinol (Oxf) 200358132–135. [DOI] [PubMed] [Google Scholar]
- 52.Brada M, Rajan B, Traish D.et al The long‐term efficacy of conservative surgery and radiotherapy in the control of pituitary adenomas. Clin Endocrinol (Oxf) 199338571–578. [DOI] [PubMed] [Google Scholar]
- 53.Peacey S R, Toogood A A, Veldhuis J D.et al The relationship between 24‐hour growth hormone secretion and insulin‐like growth factor I in patients with successfully treated acromegaly: impact of surgery or radiotherapy. J Clin Endocrinol Metab 200186259–266. [DOI] [PubMed] [Google Scholar]
- 54.Tomlinson J W, Holden N, Hills R K.et al Association between premature mortality and hypopituitarism. West Midlands Prospective Hypopituitary Study Group. Lancet 2001357425–431. [DOI] [PubMed] [Google Scholar]
- 55.Wass J A. Radiotherapy in acromegaly: a protagonists viewpoint. Clin Endocrinol (Oxf) 200358128–131. [DOI] [PubMed] [Google Scholar]
- 56.Brada M, Burchell L, Ashley S.et al The incidence of cerebrovascular accidents in patients with pituitary adenoma. Int J Radiat Oncol Biol Phys 199945693–698. [DOI] [PubMed] [Google Scholar]
- 57.Brada M, Ashley S, Ford D.et al Cerebrovascular mortality in patients with pituitary adenoma. Clin Endocrinol (Oxf) 200257713–717. [DOI] [PubMed] [Google Scholar]
- 58.Noad R, Narayanan K R, Howlett T.et al Evaluation of the effect of radiotherapy for pituitary tumours on cognitive function and quality of life. Clin Oncol (R Coll Radiol) 200416233–237. [DOI] [PubMed] [Google Scholar]
- 59.Mahmoud‐Ahmed A S, Suh J H, Mayberg M R. Gamma knife radiosurgery in the management of patients with acromegaly: a review. Pituitary 20014223–230. [DOI] [PubMed] [Google Scholar]
- 60.Attanasio R, Epaminonda P, Motti E.et al Gamma‐knife radiosurgery in acromegaly: a 4‐year follow‐up study. J Clin Endocrinol Metab 2003883105–3112. [DOI] [PubMed] [Google Scholar]
- 61.Jaffe C A, Barkan A L. Treatment of acromegaly with dopamine agonists. Endocrinol Metab Clin North Am 199221713–735. [PubMed] [Google Scholar]
- 62.Abs R, Verhelst J, Maiter D.et al Cabergoline in the treatment of acromegaly: a study in 64 patients. J Clin Endocrinol Metab 199883374–378. [DOI] [PubMed] [Google Scholar]
- 63.Kovacs K, Horvath E. Pathology of pituitary tumors. Endocrinol Metab Clin North Am 198716529–551. [PubMed] [Google Scholar]
- 64.Freda P U. Somatostatin analogs in acromegaly. J Clin Endocrinol Metab 2002873013–3018. [DOI] [PubMed] [Google Scholar]
- 65.Stewart P M, Kane K F, Stewart S E.et al Depot long‐acting somatostatin analog (Sandostatin‐LAR) is an effective treatment for acromegaly. J Clin Endocrinol Metab 1995803267–3272. [DOI] [PubMed] [Google Scholar]
- 66.al Maskari M, Gebbie J, Kendall‐Taylor P. The effect of a new slow‐release, long‐acting somatostatin analogue, lanreotide, in acromegaly. Clin Endocrinol (Oxf) 199645415–421. [DOI] [PubMed] [Google Scholar]
- 67.Lancranjan I, Atkinson A B. Results of a European multicentre study with Sandostatin LAR in acromegalic patients. Sandostatin LAR Group. Pituitary 19991105–114. [DOI] [PubMed] [Google Scholar]
- 68.Cozzi R, Barausse M, Sberna M.et al Lanreotide 60 mg, a longer‐acting somatostatin analog: tumor shrinkage and hormonal normalization in acromegaly. Pituitary 20003231–238. [DOI] [PubMed] [Google Scholar]
- 69.Colao A, Ferone D, Marzullo P.et al Long‐term effects of depot long‐acting somatostatin analog octreotide on hormone levels and tumor mass in acromegaly. J Clin Endocrinol Metab 2001862779–2786. [DOI] [PubMed] [Google Scholar]
- 70.Bevan J S, Atkin S L, Atkinson A B.et al Primary medical therapy for acromegaly: an open, prospective, multicenter study of the effects of subcutaneous and intramuscular slow‐ release octreotide on growth hormone, insulin‐like growth factor‐I, and tumor size. J Clin Endocrinol Metab 2002874554–4563. [DOI] [PubMed] [Google Scholar]
- 71.Newman C B, Melmed S, George A.et al Octreotide as primary therapy for acromegaly. J Clin Endocrinol Metab 1998833034–3040. [DOI] [PubMed] [Google Scholar]
- 72.Attanasio R, Baldelli R, Pivonello R.et al Lanreotide 60 mg, a new long‐acting formulation: effectiveness in the chronic treatment of acromegaly. J Clin Endocrinol Metab 2003885258–5265. [DOI] [PubMed] [Google Scholar]
- 73.Sheppard M C. Primary medical therapy for acromegaly. Clin Endocrinol (Oxf) 200358387–399. [DOI] [PubMed] [Google Scholar]
- 74.Ayuk J, Stewart S E, Stewart P M.et al Efficacy of Sandostatin LAR (long‐acting somatostatin analogue) is similar in patients with untreated acromegaly and in those previously treated with surgery and/or radiotherapy. Clin Endocrinol (Oxf) 200460375–381. [DOI] [PubMed] [Google Scholar]
- 75.Colao A, Ferone D, Cappabianca P.et al Effect of octreotide pretreatment on surgical outcome in acromegaly. J Clin Endocrinol Metab 1997823308–3314. [DOI] [PubMed] [Google Scholar]
- 76.Ben Shlomo A, Melmed S. Clinical review 154: the role of pharmacotherapy in perioperative management of patients with acromegaly. J Clin Endocrinol Metab 200388963–968. [DOI] [PubMed] [Google Scholar]
- 77.Kopchick J J, Parkinson C, Stevens E C.et al Growth hormone receptor antagonists: discovery, development, and use in patients with acromegaly. Endocr Rev 200223623–646. [DOI] [PubMed] [Google Scholar]
- 78.Trainer P J, Drake W M, Katznelson L.et al Treatment of acromegaly with the growth hormone‐receptor antagonist pegvisomant. N Engl J Med 20003421171–1177. [DOI] [PubMed] [Google Scholar]
- 79.van der Lely A J, Hutson R K, Trainer P J.et al Long‐term treatment of acromegaly with pegvisomant, a growth hormone receptor antagonist. Lancet 20013581754–1759. [DOI] [PubMed] [Google Scholar]
- 80.Ayuk J, Sheppard M C. The role of growth hormone‐receptor antagonism in relation to acromegaly. Expert Opin Pharmacother 200452279–2285. [DOI] [PubMed] [Google Scholar]
- 81.Dattani M, Preece M. Growth hormone deficiency and related disorders: insights into causation, diagnosis, and treatment. Lancet 20043631977–1987. [DOI] [PubMed] [Google Scholar]
- 82.Shalet S M, Toogood A, Rahim A.et al The diagnosis of growth hormone deficiency in children and adults. Endocr Rev 199819203–223. [DOI] [PubMed] [Google Scholar]
- 83.Carroll P V, Christ E R, Sonksen P H. Growth hormone replacement in adults with growth hormone deficiency: assessment of current knowledge. Trends Endocrinol Metab 200011231–238. [DOI] [PubMed] [Google Scholar]
- 84.Rosen T, Wilhelmsen L, Landin‐Wilhelmsen K.et al Increased fracture frequency in adult patients with hypopituitarism and GH deficiency. Eur J Endocrinol 1997137240–245. [DOI] [PubMed] [Google Scholar]
- 85.McCallum R W, Petrie J R, Dominiczak A F.et al Growth hormone deficiency and vascular risk. Clin Endocrinol (Oxf) 20025711–24. [DOI] [PubMed] [Google Scholar]
- 86.Colao A, Vitale G, Pivonello R.et al The heart: an end‐organ of GH action. Eur J Endocrinol 2004151(suppl 1)S93–101. [DOI] [PubMed] [Google Scholar]
- 87.Growth Hormone Research Society Workshop on Adult Growth Hormone Deficiency Consensus guidelines for the diagnosis, treatment of adults with growth hormone deficiency: summary statement of the Growth Hormone Research Society Workshop on Adult Growth Hormone Deficiency. J Clin Endocrinol Metab 199883379–381. [DOI] [PubMed] [Google Scholar]
- 88.Abs R. Update on the diagnosis of GH deficiency in adults. Eur J Endocrinol 2003148(suppl 2)S3–S8. [DOI] [PubMed] [Google Scholar]
- 89.Rosen T, Bengtsson B A. Premature mortality due to cardiovascular disease in hypopituitarism. Lancet 1990336285–288. [DOI] [PubMed] [Google Scholar]
- 90.Bates A S, Van't Hoff W, Jones P J.et al The effect of hypopituitarism on life expectancy. J Clin Endocrinol Metab 1996811169–1172. [DOI] [PubMed] [Google Scholar]
- 91.Bulow B, Hagmar L, Mikoczy Z.et al Increased cerebrovascular mortality in patients with hypopituitarism. Clin Endocrinol (Oxf) 19974675–81. [DOI] [PubMed] [Google Scholar]
- 92.Erfurth E M, Bulow B, Eskilsson J.et al High incidence of cardiovascular disease and increased prevalence of cardiovascular risk factors in women with hypopituitarism not receiving growth hormone treatment: preliminary results. Growth Horm IGF Res 19999(suppl A)21–24. [DOI] [PubMed] [Google Scholar]
- 93.Laron Z. Do deficiencies in growth hormone and insulin‐like growth factor‐1 (IGF‐1) shorten or prolong longevity? Mech Ageing Dev 2005126305–307. [DOI] [PubMed] [Google Scholar]
- 94.Besson A, Salemi S, Gallati S.et al Reduced longevity in untreated patients with isolated growth hormone deficiency. J Clin Endocrinol Metab 2003883664–3667. [DOI] [PubMed] [Google Scholar]