Abstract
Swallowing musculature is asymmetrically represented in both motor cortices. Stroke affecting the hemisphere with the dominant swallowing projection results in dysphagia and clinical recovery has been correlated with compensatory changes in the previously non‐dominant, unaffected hemisphere. This asymmetric bilaterality may explain why up to half of stroke patients are dysphagic and why many will regain a safe swallow over a comparatively short period. Despite this propensity for recovery, dysphagia carries a sevenfold increased risk of aspiration pneumonia and is an independent predictor of mortality. The identification, clinical course, pathophysiology, and treatment of dysphagia after stroke are discussed in this review.
Keywords: aspiration pneumonia, pathophysiology, swallowing, treatment, videofluoroscopy
Before discussing the clinical aspects of dysphagia after stroke it is worth considering what exactly is meant by the term dysphagia. In the context of stroke, oropharyngeal dysphagia is probably best defined as a disruption of bolus flow through the mouth and pharynx. As the function of swallowing is the safe delivery of a food bolus into the stomach, then the immediate complication of dysphagia is food entering the airway. Dysphagia in this context is not a subjective symptom and it does not normally refer to any oesophageal abnormality.
A related but distinct term is aspiration, which is the incursion of food material into the airway and below the true vocal cords. Aspiration is thus one of the most important consequences of dysphagia along with malnutrition.
How common is dysphagia after stroke?
Numerous studies have tried to establish the incidence of dysphagia after stroke with figures ranging from 23% to 50%.1,2,3,4,5,6,7,8,9,10,11 At first glance, these figures seem to represent a wide range. The explanation for this lies in variations in study design and in the identification of dysphagia, both of which merit further discussion.
Study designs
Only a handful of studies have looked at sample sizes greater than 100 and differences in reported entry criteria for these studies have resulted in a variable case mix. Studies are started at different time points, which means a variable amount of recovery will have taken place at the time of assessment. Stroke severity may not be assessed and comorbidity such as chronic obstructive pulmonary disease may not be accounted for. All these factors make it difficult to pool or compare data. In addition, there are discrepancies between different authors' methods of identifying dysphagia.
Identification of dysphagia
Swallowing assessments are generally split into bedside clinical examinations or instrumental investigations. Because each approach provides differing data with variable accuracy, the incidence of dysphagia can fluctuate depending on which assessment is used.
Bedside examination remains the cornerstone of clinical practice in most hospitals. Clinicians, nurses, and speech and language therapists are taught to present small volumes of food or water to patients and to watch for signs of dysphagia and aspiration. Among other signs, clinicians will look for loss of liquid from the mouth, dyspraxia or poor coordination of muscles, facial weakness, delayed pharyngeal/laryngeal elevation, coughing or throat clearing, breathlessness, and changes in voice quality after swallow.12 It should be noted that the interpretation of an intact gag reflex as an indicator of safe swallowing has been largely discredited.13 There are other factors taken into account including some that are not directly related to the stroke such as background respiratory function. Patients with advanced chronic lung diseases may aspirate not because of a neuromuscular problem but because they cannot maintain a sufficient period of apnoea while swallowing. Despite the broad assessments undertaken at the bedside, the problem with this method is that it relies on findings that are subjective and clinician dependent.
Several investigators have tried to create objective and reliable scoring systems for the bedside assessment. Inevitably, as with most screening systems, if the sensitivity of the scale is improved, its specificity declines when compared with the current gold standard; videofluoroscopy (VFS) (see table 1). An important factor in the low sensitivity of the bedside examination is patients who aspirate without clinically overt signs, known as silent aspirators. As many as half of all patients aspirating on VFS will do so silently.14,15,16,17 In addition, patients will commonly show varying responses to aspiration during a single VFS assessment.
Table 1 Sensitivity and specificity for aspiration assessed with bedside swallow tests compared with videofluoroscopy.
In the context of stroke, oropharyngeal dysphagia is probably best defined as a disruption of bolus flow through the mouth and pharynx.
Aspiration is the incursion of food material into the airway and beyond the true vocal cords.
Videofluoroscopy
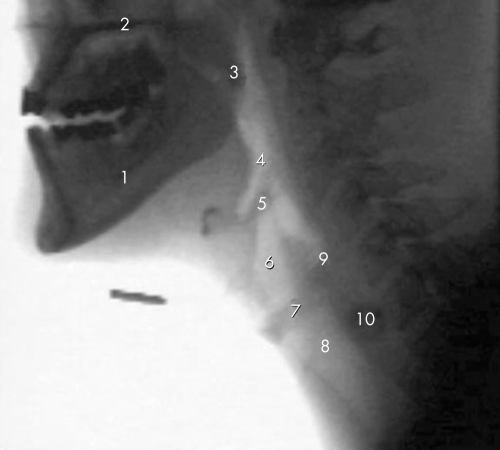
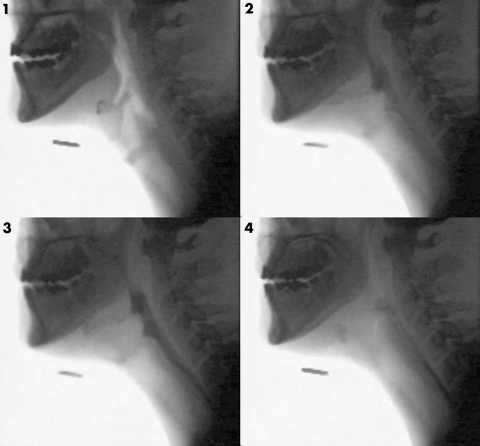
Also known as a modified barium swallow, VFS has traditionally been the gold standard for swallowing assessments.25 It entails the administration of radio‐opaque barium liquid with moving images captured in the lateral view. Figure 1 shows a VFS image outlining the anatomy of the oropharynx and figure 2 illustrates the flow of barium through the oropharynx. Occasionally anterior‐posterior views are also obtained. The barium can be mixed with water to varying consistencies or added to other foods.
Figure 1 VFS image illustrating the anatomy of the oropharynx. (1) Ramus of mandible; (2) hard palate; (3) soft palate; (4) pharynx; (5) epiglottis; (6) laryngeal vestibule; (7) vocal cords; (8) trachea; (9) region of the upper oesophageal sphincter; (10) region of the oesophagus.
Figure 2 Serial VFS images showing the normal passage of a barium bolus through the pharynx. Image (1) is the resting state. Barium appears black as it passes through the pharynx (2), the upper oesophageal sphincter (3), and the proximal oesophagus (4).
VFS has the advantages of visualisation and quantification of barium through the oral cavity as well as the pharynx and oesophagus. It can be recorded and played back in slow motion and differentiates between abnormal physiology, penetration of barium into the airway, and true aspiration (barium entering the airway below the true vocal cords). Most hospitals will have fluoroscopy services on site and an assessment often takes just 10 or 15 minutes. Most examinations are undertaken with the aim of establishing which consistencies would be safe for a patient to consume and which posture or manoeuvre might aid safe swallowing.
Disadvantages of VFS include the exposure to radiation albeit low dose. The procedure is carried out under “ideal” conditions that may not reflect events on the ward. Barium's density is significantly different to normal food and therefore its passage may not indicate the aspiration risk with other foods. There is no standard protocol for the volumes tested or the consistencies delivered and it only judges a person's performance on one day. As with the bedside examination, it is remarkably open to interpretation with an interrater reliability (κ values) for aspiration of between 0.4 and 0.8.6,18,26,27 However, this correlation is improved with training and experience.2,28
Fibreoptic endoscopic evaluation of swallowing
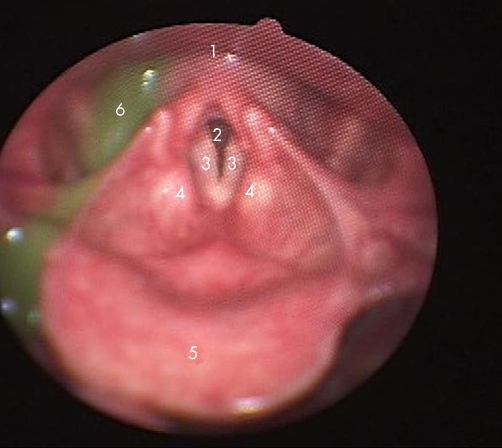
In the past 20 years an alternative to VFS has been developed. Fibreoptic endoscopic evaluation of swallowing (FEES) was first reported in 198829 and entails the placement of a nasendoscope to the level of the uvula or soft palate to give a view of the hypopharynx and larynx (see fig 3). It has an excellent safety record with epistaxis being seen in less than 1 in 1000 patients.30 Various foods can be tested and it does not entail radiation. It permits anatomical assessment as well as sensory testing and crucially, it is performed at the bedside with normal meals and can be repeated as often as necessary.
Figure 3 View with fibreoptic endoscopic evaluation of swallowing. (1) Route to oesophagus; (2) trachea; (3) vocal cords; (4) aryepiglottic folds; (5) epiglottis; (6) fluid with green dye. Image courtesy of KayPENTAX, Lincoln Park, NJ, USA.
Bedside clinical assessment tests a patient with varying volumes and consistencies while observing for tell tale signs of aspiration. As the sensitivity of screening tests improve, the specificity declines.
On the other hand FEES does require a skilled operator and technical equipment that is not widely available. No information is gained about the oral phase of swallowing and there is a white out as the bolus passes through the pharynx and the pharyngeal constrictors contract around the lens. There also remains some controversy as to whether local anaesthetic sprayed into the nostril affects swallowing physiology or not. Table 2 shows the advantages and disadvantages of VFS compared with FEES.
Table 2 Advantages and disadvantages of videofluoroscopy and fibreoptic endoscopic evaluation of swallowing.
| Videofluoroscopy | |
| Advantages | Widely available, rapid and safe |
| Assessment of all stages of swallowing | |
| Variety of foods can be tested | |
| Allows the assessment of therapeutic manoeuvres | |
| Disadvantages | Radiation exposure |
| Findings may not reflect ward behaviour | |
| Density of barium means aspiration may not reflect risk with other foods | |
| Training required for interpretation | |
| Fibreoptic endoscopic evaluation of swallowing | |
| Advantages | Performed at the bedside with normal meals |
| Gives better anatomical data of the pharynx/larynx | |
| Can be repeated regularly | |
| Sensory testing can be undertaken | |
| Disadvantages | Not widely available |
| Requires skilled operators | |
| White out often obscures the period of aspiration | |
| No information is gathered on oral control | |
Direct comparisons of VFS and FEES have been undertaken and have shown that there is unlikely to be a difference between clinical management guided by either of these two techniques.31
Other assessment tools
Bedside assessments may be improved by the simple measure of following oxygen saturations with pulse oximetry during a swallowing task.21 Zaidi et al first reported that a decrease of 2% in oxygen saturation after drinking 10 ml of water was predictive of aspiration on a videofluoroscopy assessment.32 One other institute has been able to replicate these findings33 whereas two investigators have found contradictory results.34,35 Further work is required to establish which patients might benefit from this additional assessment and also to define whether serial measurements can be used to track recovery.
Other screening tools have been advocated but remain research tools only at present with limited data in stroke patients. These include scintigraphy, ultrasound, and impedance tomography.36,37,38
Overview of identification of dysphagia
Ideally a clinician should have access to bedside screening tests, VFS and FEES. It is important to recognise the strengths and weaknesses of each assessment and build a risk profile rather than categorising patients into dysphagic or non‐dysphagic. The current best practice should be bedside clinical testing with a low threshold for instrumental examination both to identify silent aspirators and to guide management.
Videofluoroscopy is a commonly available investigation for the assessment of swallowing. Data are available on anatomy, all stages of swallowing physiology, the presence of aspiration, and the response to therapeutic manoeuvres. It does however, entail ionising radiation and is performed in somewhat artificial settings.
Clinical course
Despite the problems associated with diagnosis of dysphagia the clinical impression is that “recovery” is comparatively common and takes place over days to weeks.5,7,39 Two large studies used the objective tool of VFS to plot the recovery. The first was Smithard et al who found an aspiration incidence of 22% at a median of two days after stroke and 15% at one month.40,41
Fibreoptic evaluation of swallowing can be performed at the bedside with a variety of food substances and has an excellent safety profile. It can be repeated regularly and permits sensory testing. In contrast it is not widely available and requires both specialised equipment and trained operators.
Mann et al prospectively studied 128 stroke patients with a VFS at a median time of 10 days after symptom onset.7 They found dysphagia in 64% and aspiration in 22%. Their definition of dysphagia on VFS was the delay, disorder, and/or weakness of any component of swallowing that adversely affected bolus delivery and increased the risk of aspiration. Six months later 11 patients had been lost to follow up and five had died. The remainder were reassessed and those who were initially dysphagic underwent a repeat VFS examination. VFS identified swallowing abnormalities in 80% of the 67 repeat examinations and 25% were aspirating. Despite this only 15 patients had not returned to their pre‐stroke diet. No further follow up beyond the initial six months was reported.
Logemann reported a persistent delay in temporal measures of swallowing after recovery from dysphagia after stroke.12 There was also a slight increase in pharyngeal residue and her suggestion was that swallowing may recover functionally but may remain impaired at a more intricate level. This may also account for the increased incidence of dysphagia after a second or third stroke.
For those patients who fail to recover a safe swallow in the short term, the alternative is enteral feeding via a percutaneous endoscopic gastrostomy (PEG) tube. However, PEG feeding should not be seen as an end point in dysphagia rehabilitation. James et al addressed the long term outcome of this group in a retrospective manner.42 They reviewed 126 patients who had a PEG in situ after an acute stroke. Median length of follow up was 31 months (range 4–71). Over half (57%) had died, almost a third (29%) had been able to remove their PEG after improvements in their swallowing, the remainder still had a PEG in situ. Others have also reported recovery from dysphagia months or years after a stroke but the rate of recovery remains slow.43,44,45
Does dysphagia matter?
With such a clear propensity for recovery, various authors have tried to evaluate the impact of dysphagia on a wide variety of clinical outcomes.15 Several authors have correlated clinical dysphagia with increased risk of chest infections.5,7,14,41 Of the studies using VFS to confirm aspiration, Holas et al46 and Kidd et al47 found an increased risk of chest infection. In a review of over 14 000 patients in the USA, Katzan et al identified a threefold increased risk of death among stroke patients if they developed pneumonia.48 This risk was calculated after adjustment for stroke severity. Mortality for dysphagic stroke patients therefore stands at between 27% and 37%.41,48 Unpublished data from our department suggest a poorer prognosis for patients aspirating on VFS compared with those that demonstrated safe swallowing and perhaps unsurprisingly, the difference in mortality is over the first three to six months only.
An absolute correlation between aspiration and chest infection would not be inevitable as numerous other factors such as mental state, posture, dentition, immune status, age, and respiratory comorbidity may also play a part.49,50,51 Overall around 12%–30% of all stroke patients will suffer a chest infection during their inpatient stay.3,7,47,52
Poor nutritional state has certainly been correlated with increased mortality after admission with acute stroke.53 Although this reflects pre‐stroke status rather than post‐stroke dysphagia, one might infer that continued decline in nutritional status may still be important in this group of patients.
Dysphagia does therefore have prognostic implications and should be assessed in all patients presenting with symptoms of a stroke.
Why do half of patients develop dysphagia? Central nervous system control of swallowing
A series of experiments from Hamdy et al have used transcranial magnetic stimulation (TMS) to probe the role of the motor cortex in health and after stroke. TMS is a safe and non‐invasive technique that can stimulate focal areas of the cerebral cortex and thereby map connections from motor cortex to target muscles. The strength of the projections from motor cortex is denoted by the amplitude of electromyographic (EMG) traces at the target muscle. Initial studies in healthy volunteers described how midline swallowing muscles are represented bilaterally in the motor cortex but in an asymmetric manner.54 This has led to the hypothesis that some subjects have a “dominant” swallowing hemisphere.
It was subsequently postulated that stroke affecting the dominant hemisphere was more likely to result in dysphagia.55 Twenty patients were recruited after their first stroke confirmed on computed tomography of the brain. TMS was delivered to sites over both hemispheres in turn and any resulting EMG response at the pharyngeus muscle was recorded. Eight of the patients were dysphagic. Stimulation of the affected hemisphere produced similarly small EMG responses in both dysphagic and non‐dysphagic patients. In contrast, stimulation of the unaffected hemisphere produced significantly smaller responses in the dysphagic patients. Although studied retrospectively, this did indeed suggest that lesions of the dominant hemisphere were more likely to result in dysphagia.
Although up to half of acute stroke patients will be dysphagic, most will have recovered a safe swallow by one month.
Mechanisms for swallowing recovery in dysphagic stroke
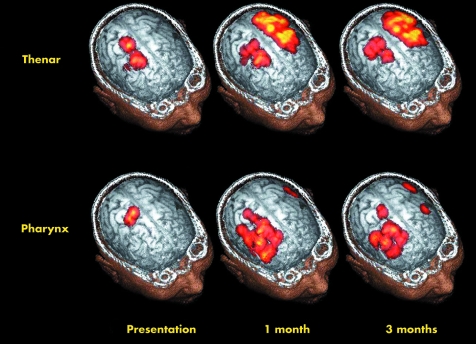
TMS was also used to scrutinise cortical connections over time.56 Twenty eight patients were recruited and their cortical maps in response to TMS of both hemispheres were plotted at one week, one month, and three months after stroke. VFS assessment of their swallow was also undertaken and EMG responses of the thenar muscle were used as a control. The key finding was that dysphagic patients who recovered over time showed an increase in their cortical maps over the unaffected hemisphere at one month and three months. The patients who remained dysphagic did not show this change in their pharyngeal cortical maps. Cortical representation of the thenar muscle reappeared in the affected hemisphere (see fig 4) The conclusion drawn was that recovery from dysphagia after stroke might follow reorganisation of the unaffected motor cortex.
Figure 4 Graphical representation of the changes in cortical maps after a left hemisphere stroke over a period of three months. Reprinted from Hamdy S, Aziz Q, Rothwell JC, et al. Recovery of swallowing after dysphagic stroke relates to functional reorganization in the intact motor cortex. Gastroenterology 1998;115:1104–1112 with permission from American Gastroenterological Association.
Management
Clearly the goals in dysphagia therapy are to reduce the morbidity and mortality associated with chest infections, improve nutritional status, and return patients to a normal diet with resultant improvement of their quality of life.
Unfortunately, there is a paucity of evidence for dysphagia therapy, which has been highlighted in a Cochrane review57 as well as an American Gastroenterology Association technical review.58 Broadly speaking, therapy can be differentiated into compensatory and rehabilitative strategies. The former aims to keep patients safe when eating whereas the latter aims to speed the recovery process. Because few active measures have proved efficacy, the general aim is often to prevent chest infections while spontaneous recovery takes place.
Dysphagia after stroke carries a threefold increased mortality risk and a sixfold to sevenfold fold increased risk of aspiration pneumonia. It should be screened for on every patient presenting with symptoms of a stroke.
Compensatory strategies
Standard practice is to change the consistency of food and fluids given to dysphagic patients with the most severely affected patients left nil by mouth. The rationale for changed consistencies comes from findings at the bedside and during VFS. Although numerous studies have described the changes in swallowing physiology with thickened fluids59,60,61,62,63,64 none have shown clinical efficacy in stroke patients. VFS regularly identifies individual patients who endure reduced aspiration with particular consistencies of food. It is therefore unlikely that ethical approval would ever be gained to randomise patients to normal diet and fluids when they have been noted to aspirate on such consistencies, making an objective study of food consistencies difficult.
In a similar manner, only expert consensus supports the use of manoeuvres such as a chin tuck when swallowing, head turn or the Mendelsohn manoeuvre. Chin tuck entails asking patients to lower their chin towards their chest before swallowing.65 This brings the epiglottis and the aryepiglottic folds closer to together and it is the apposition of these structures that will close the airway during swallowing. Head turn is a simple rotation of the head to the paretic side and may increase bolus flow.66 The Mendelsohn's manoeuvre requires a little more training and entails the sustained contraction of the suprahyoid muscles in an effort to maintain laryngeal elevation and thus upper oesophageal sphincter opening and airway closure.67 Swallowing assessments are thus viewed as individual treatment trials and any of these techniques can be advocated if an individual patient is noted to swallow safely when exercising any particular method. It should be noted however, that their efficacy remains controversial.
There is more substantive literature on the use of nasogastric (NG) and PEG feeding. The first question to raise is do they improve outcome? Unfortunately, the answer is probably “no.” Although the studies were not exclusively in stroke patients, there are a handful of trials suggesting that NG and PEG feeding do not reduce the incidence of pneumonia or death.68,69,70,71 One study in stroke patients did suggest a pronounced benefit from NG feeding but these results have not been reproduced elsewhere in the literature.72 Dziewas et al followed up 100 stoke patients fed by NG tube and found a 44% pneumonia rate. Although there was no control group for comparison it is clear that a large proportion of patients fed by NG tube will still develop respiratory infection.73
Why a person without any oral intake should develop pneumonia is best explained by the fact that the oral cavity is rich in bacterial flora and that this is the source of pathogenic organisms. Further evidence for this premise comes from regression analyses identifying poor dentition (which is a proxy for greater oral bacterial colonisation) as an independent predictor of chest infection.74
Although there may not be a reduced infection rate with PEG feeding, there is certainly an improved nutritional status because of more consistent delivery of feed.75,76 There seems little doubt that if patients are receiving a restricted oral diet, then they will at least benefit from nutrition and hydration albeit with questionable protection from pneumonia.
If we accept that NG and PEG feeding have a role, the next question is when to start such a regimen and which of the two to select. Fortunately, clinical decisions are at least supported by a large multicentre randomised controlled trial. The feed or ordinary diet (FOOD) trial recruited dysphagic stroke patients to two studies relevant to this discussion.77,78 The first trial randomised stroke patients within seven days of admission to either early NG feeding or to no tube feeding for more than seven days. An intention to treat analysis of the 859 enrolled patients showed an absolute reduction in death of 5.8% (95%CI −0.8 to 12.5%, p = 0.09) without reducing the incidence of pneumonia. The absolute reduction in the combined outcome of death or poor outcome was just 1.2% (−4.2 to 6.6%, p = 0.7). Although statistical significance at the 5% level was not reached, the authors suggested that early enteral feeding may keep patients alive who would otherwise have died but those patients remain severely impaired and dependent. The reduced mortality could not be attributed to reduced aspiration.
The second trial randomised dysphagic patients to either NG or PEG feeding. The latter was associated with an increased risk of death or poor outcome of 7.8% (0% to 15%, p = 0.05). It might be concluded from these two studies that early NG feeding is likely to be beneficial but PEG feeding can be reasonably delayed for a period of weeks. In the longer term PEG feeding does improve nutritional status and should be considered when artificial nutrition and hydration is required beyond two weeks.
There are few clinical trials guiding therapy. The mainstay of management is to keep patients safe while spontaneous recovery takes place. This is achieved through compensatory strategies such as changing food consistencies, regulating bolus size, head rotation before swallowing, and the chin tuck manoeuvre.
Parenteral nutrition has never been tested in the stroke setting but is likely to be associated with significant complications and is rarely fruitful.
Nasogastric and percutaneous gastrostomy feeding have not been shown to reduce the rate of chest infections but will improve nutritional status.
Rehabilitative strategies
In terms of head and neck exercises, the best evidence supports an exercise aimed at opening the upper oesophageal sphincter. In chronic dysphagic subjects including stroke patients, Shaker et al described an exercise that improved sphincter opening and thereby reduced post‐swallow pharyngeal residue.79 Briefly, it comprises the patient lying supine on a bed and raising their head off the bed for a period of seconds and repeating this 20 times. It was felt this would reinforce the action of the suprahyoid muscles that are critical to upper oesophageal sphincter opening.
Key references
Shaker R, Easterling C, Kern M, et al. Rehabilitation of swallowing by exercise in tube‐fed patients with pharyngeal dysphagia secondary to abnormal UES opening. Gastroenterology 2002;122:1314–21.
Hamdy S, Aziz Q, Rothwell JC, et al. Explaining oropharyngeal dysphagia after unilateral hemispheric stroke. Lancet 1997;350:686–92.
Mann G, Hankey GJ, Cameron D. Swallowing function after stroke: prognosis and prognostic factors at 6 months. Stroke 1999;30:744–8.
Dennis MS, Lewis SC, Warlow C. Effect of timing and method of enteral tube feeding for dysphagic stroke patients (FOOD): a multicentre randomised controlled trial. Lancet 2005;365:764–72.
Fraser C, Power M, Hamdy S, et al. Driving plasticity in human adult motor cortex is associated with improved motor function after brain injury. Neuron 2002;34:831–40.
Specific exercises will improve opening of the upper oesophageal sphincter in stroke patients with sphincter dysfunction.
Pharyngeal electrical stimulation has shown promising results in preliminary studies and further trials are underway.
There are insufficient data to support biofeedback or drug therapy for post‐stroke dysphagia.
A variety of oral stimulation techniques have been used without clear evidence of efficacy. These include tactile and thermal stimulation.80 In contrast, pharyngeal electrical stimulation may have a therapeutic role.81 Fraser et al initially investigated the effects of pharyngeal electrical stimulation in healthy volunteers with TMS. They established that at specific parameters, a sustained increase in swallowing motor cortex excitability could be achieved. They then applied the specified stimulation parameters to 10 of 16 dysphagic stroke patients while six patients received sham stimulation. Standardised VFS was used before and one hour after the stimulation/sham to assess any changes. Whereas the sham group showed no change in aspiration, the intervention group showed a 30% reduction in aspiration. Although too early to make any strong recommendations, these data show promise and a larger trial is underway to establish for how long the effect might last and how often this therapy needs to be repeated.
Limited numbers of drugs have been advocated to reduce aspiration including nifedipine82 and ACE inhibitors.83 However, the trial data remain too limited to support their use at present. Biofeedback for post‐stroke dysphagia rehabilitation has been pioneered over recent years84,85 with commercial systems becoming more widely available. As yet no randomised controlled trial has been reported. Table 3 shows the levels of evidence for various treatment regimens.
Table 3 Grading of evidence base for rehabilitation strategies in dysphagic stroke patients. National Institute of Health and Clinical Excellence criteria are used for grading.
| Intervention | Level of evidence | Comments |
|---|---|---|
| Head raising exercise | Ib | Specific exercises will improve opening of the upper oesophageal sphincter in stroke patients with sphincter dysfunction. |
| Pharyngeal stimulation | Ib | Pharyngeal electrical stimulation has shown promising results in preliminary studies and further trials are under way. |
| ACE inhibitors | III | No RCT |
| Biofeedback | IV | Two studies totalling 55 patients. No RCT |
| Oral exercises | None | No published evidence |
| Oral stimulation | None | Recent publications suggest no efficacy |
RCT, randomised controlled trial.
Summary
Dysphagia affects up to half of acute stroke patients and carries a threefold to sevenfold increased risk of aspiration pneumonia. With the subsequent mortality associated with pneumonia, dysphagia has been recognised as an independent predictor of mortality after stroke. Fortunately, most patients will make a functional recovery over a period of days to weeks.
This incidence and remarkable recovery rate may be accounted for by the bilateral distribution of control of swallowing musculature in the motor cortex. After hemispheric stroke, neuroplastic adaptation permits the control of swallowing musculature to be reorganised to the unaffected hemisphere.
Identifying dysphagia can be done by bedside testing or instrumental examination such as VFS and FEES but best practice would combine both along with a global assessment of the stroke patient.
Management should be aimed at facilitating safe swallowing while spontaneous recovery takes place. Enteral feeding tubes are commonly used when oral intake is not deemed sufficient for nutrition and hydration purposes. However, this approach has not been shown to reduce the incidence of aspiration pneumonia in most of the studies published to date although it does improve nutritional status. Manoeuvres to aid safe swallowing can only be offered on an individual basis by trained practitioners and are not universally applicable to all dysphagic patients. Few of these techniques have been tested in randomised controlled trials. Much more work is required to investigate the true impact of current dysphagia therapy and to work towards developing new therapies of the future.
Self assessment questions (answers after the references)
-
Regarding the incidence of dysphagia after an acute stroke?
all patients with a clinical diagnosis of stroke should be screened for dysphagia
only 10% will be dysphagic on admission
up to 50% will be dysphagic on admission
up to 10% will be dysphagic at discharge
-
What is meant by the term “silent aspirator”?
The patient is not aware of any problems with swallowing
The patient does not cough when swallowing
There are no bedside signs of aspiration despite aspiration seen on instrumental examination
The patient has a normal instrumental swallowing assessment but is still at risk of chest infections
-
Which of the following is an advantage of fibreoptic endoscopic evaluation of swallowing?
Can be undertaken at the bedside with normal food
Can be performed by any ward staff
Can detect anatomical as well as physiological abnormalities
Has a very low complication rate
-
Which exercises or manoeuvres have been shown to improve upper oesophageal sphincter opening?
Head raising exercises
Tongue protrusion
Chin tuck
Mendelsohn manoeuvre
-
Which of the following statements about artificial enteral feeding in dysphagic stroke patients is true?
Enteral feeding reduces the risk of chest infection
Enteral feeding reduces the risk of malnutrition
Recovery of swallowing is unlikely after gastrostomy tube placement
Gastrostomy feeding is superior to nasogastric feeding even in the first few weeks after stroke
-
Which factors other than oropharyngeal motor function should be taken into account during a swallowing assessment?
Cognitive function
Patient posture
Dentition
Respiratory status
Abbreviations
VFS - videofluoroscopy
FEES - fibreoptic endoscopic evaluation of swallowing
PEG - precutaneous endoscopic gastrostomy
TMS - transcranial magnetic stimulation
EMG - electromyography
NG - nasogastric
Answers
1. (A), (C), (D) are true. Royal College of Physician guidelines reiterate that all stroke patients need screening for dysphagia. Studies based on more objective instrumental assessments of dysphagia have suggested an incidence of up to 50% after stroke. 2. (C) is true. Most dysphagic patients will not volunteer problems with swallowing but “silent aspirator” shows that the clinician cannot detect signs of aspiration at the bedside. (B) and (D) would be true of non‐dysphagic patients. 3. (A), (C), and (D) are true. FEES is likely to become more widely available as an alternative to videofluoroscopy. Training is required and commonly it is the nurses, doctors, and speech and language therapists that take on the role. 4. (A) Described by Shaker et al and (D) act on upper oesophageal sphincter opening to facilitate bolus flow through the pharynx. 5. (B) is the only true answer. There is insufficient evidence for enteral feeding reducing pneumonia and recovery of swallowing can take place months after a stroke. The recent FOOD trial suggested no clear benefit of gastrostomy ahead of nasogastric feeding in the short term. 6. All of the answers are true.
Footnotes
Competing interests: SS is a clinical research fellow employed by Manchester University through grants from The Health Foundation and the Medical Research Council. SH is a lecturer at Manchester University and honorary consultant Gastroenterologist at Hope Hospital, Salford. Neither author has any competing interests to declare.
References
- 1.Daniels S K, Ballo L A, Mahoney M C.et al Clinical predictors of dysphagia and aspiration risk: outcome measures in acute stroke patients. Arch Phys Med Rehabil 2000811030–1033. [DOI] [PubMed] [Google Scholar]
- 2.Daniels S K, Brailey K, Priestly D H.et al Aspiration in patients with acute stroke. Arch Phys Med Rehabil 19987914–19. [DOI] [PubMed] [Google Scholar]
- 3.Johnson E R, McKenzie S W, Sievers A. Aspiration pneumonia in stroke. Arch Phys Med Rehabil 199374973–976. [PubMed] [Google Scholar]
- 4.Kidd D, Lawson J, Nesbitt R.et al Aspiration in acute stroke: a clinical study with videofluoroscopy. Q J Med 199386825–829. [PubMed] [Google Scholar]
- 5.Gordon C, Hewer R L, Wade D T. Dysphagia in acute stroke. BMJ (Clin Res Ed) 1987295411–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mann G, Hankey G J, Cameron D. Swallowing disorders following acute stroke: prevalence and diagnostic accuracy. Cerebrovasc Dis 200010380–386. [DOI] [PubMed] [Google Scholar]
- 7.Mann G, Hankey G J, Cameron D. Swallowing function after stroke: prognosis and prognostic factors at 6 months. Stroke 199930744–748. [DOI] [PubMed] [Google Scholar]
- 8.Robbins J, Levin R L. Swallowing after unilateral stroke of the cerebral cortex: preliminary experience. Dysphagia 1988311–17. [DOI] [PubMed] [Google Scholar]
- 9.Sellars C, Campbell A M, Stott D J.et al Swallowing abnormalities after acute stroke: a case control study. Dysphagia 199914212–218. [DOI] [PubMed] [Google Scholar]
- 10.Odderson I R, Keaton J C, McKenna B S. Swallow management in patients on an acute stroke pathway: quality is cost effective. Arch Phys Med Rehabil 1995761130–1133. [DOI] [PubMed] [Google Scholar]
- 11.Wolfe C D, Taub N A, Woodrow J.et al Does the incidence, severity, or case fatality of stroke vary in southern England? J Epidemiol Community Health 199347139–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Logemann J A.Evaluation and treatment of swallowing disorders. 2nd ed. Austin, TX: Pro, ed, 1983406
- 13.Leder S B. Videofluoroscopic evaluation of aspiration with visual examination of the gag reflex and velar movement. Dysphagia 19971221–23. [PubMed] [Google Scholar]
- 14.DePippo K, Holas M, Reding M.et al Dysphagia therapy following stroke: a controlled trial. Neurology 1994441655–1660. [DOI] [PubMed] [Google Scholar]
- 15.Perry L, Love C P. Screening for dysphagia and aspiration in acute stroke: a systematic review. Dysphagia 2001167–18. [DOI] [PubMed] [Google Scholar]
- 16.Lundy D S, Smith C, Colangelo L.et al Aspiration: cause and implications. Otolaryngol Head Neck Surg 1999120474–478. [DOI] [PubMed] [Google Scholar]
- 17.Smith C H, Logemann J A, Colangelo L A.et al Incidence and patient characteristics associated with silent aspiration in the acute care setting. Dysphagia 1999141–7. [DOI] [PubMed] [Google Scholar]
- 18.Smithard D, O'Neill, Park C, et al Can bedside assessment reliably exclude aspiration following acute stroke? Age Ageing 19982799–106. [DOI] [PubMed] [Google Scholar]
- 19.DePippo K L, Holas M A, Reding M J. Validation of the 3‐oz water swallow test for aspiration following stroke. Arch Neurol 1992491259–1261. [DOI] [PubMed] [Google Scholar]
- 20.Splaingard M L, Hutchins B, Sulton L D.et al Aspiration in rehabilitation patients: videofluoroscopy vs bedside clinical assessment. Arch Phys Med Rehabil 198869637–640. [PubMed] [Google Scholar]
- 21.Smith H A, Lee S H, O'Neill P A.et al The combination of bedside swallowing assessment and oxygen saturation monitoring of swallowing in acute stroke: a safe and humane screening tool. Age Ageing 200029495–499. [DOI] [PubMed] [Google Scholar]
- 22.Daniels S K, McAdam C P, Brailey K.et al Clinical assessment of swallowing and prediction of dysphagia severity. Am J Speech Lang Pathol 1997617–24. [Google Scholar]
- 23.McCullough G H, Wertz R T, Rosenbek J C. Sensitivity and specificity of clinical/bedside examination signs for detecting aspiration in adults subsequent to stroke. J Commun Disord 20013455–72. [DOI] [PubMed] [Google Scholar]
- 24.Nishiwaki K, Tsuji T, Liu M.et al Identification of a simple screening tool for dysphagia in patients with stroke using factor analysis of multiple dysphagia variables. J Rehabil Med 200537247–251. [DOI] [PubMed] [Google Scholar]
- 25.Horner J, Massey E W. Silent aspiration following stroke. Neurology 198838317–319. [DOI] [PubMed] [Google Scholar]
- 26.Kuhlemeier K V, Yates, Palmer JB Intra‐ and interrater variation in the evaluation of videofluorographic swallowing studies. Dysphagia 199813142–147. [DOI] [PubMed] [Google Scholar]
- 27.Ekberg O, Nylander G, Fork F T.et al Interobserver variability in cineradiographic assessment of pharyngeal function during swallow. Dysphagia 1988346–48. [DOI] [PubMed] [Google Scholar]
- 28.Daniels S K, Brailey K, Foundas A L. Lingual discoordination and dysphagia following acute stroke: analyses of lesion localization. Dysphagia 19991485–92. [DOI] [PubMed] [Google Scholar]
- 29.Langmore S E, Schatz K, Olsen N. Fiberoptic endoscopic examination of swallowing safety: a new procedure. Dysphagia 19882216–219. [DOI] [PubMed] [Google Scholar]
- 30.Aviv J E, Murry T, Zschommler A.et al Flexible endoscopic evaluation of swallowing with sensory testing: patient characteristics and analysis of safety in 1,340 consecutive examinations. Ann Otol Rhinol Laryngol 2005114173–176. [DOI] [PubMed] [Google Scholar]
- 31.Doggett D L, Turkelson C M, Coates V. Recent developments in diagnosis and intervention for aspiration and dysphagia in stroke and other neuromuscular disorders. Curr Atheroscler Rep 20024311–318. [DOI] [PubMed] [Google Scholar]
- 32.Zaidi N H, Smith H A, King S C.et al Oxygen desaturation on swallowing as a potential marker of aspiration in acute stroke. Age Ageing 199524267–270. [DOI] [PubMed] [Google Scholar]
- 33.Collins M J, Bakheit A M. Does pulse oximetry reliably detect aspiration in dysphagic stroke patients? Stroke 1997281773–1775. [DOI] [PubMed] [Google Scholar]
- 34.Sellars C, Dunnet C, Carter R. A preliminary comparison of videofluoroscopy of swallow and pulse oximetry in the identification of aspiration in dysphagic patients. Dysphagia 19981382–86. [DOI] [PubMed] [Google Scholar]
- 35.Colodny N. Comparison of dysphagics and nondysphagics on pulse oximetry during oral feeding. Dysphagia 20001568–73. [DOI] [PubMed] [Google Scholar]
- 36.Miller J L, Watkin K L. Lateral pharyngeal wall motion during swallowing using real time ultrasound. Dysphagia 199712125–132. [DOI] [PubMed] [Google Scholar]
- 37.Hamlet S, Choi J, Zormeier M.et al Normal adult swallowing of liquid and viscous material: scintigraphic data on bolus transit and oropharyngeal residues. Dysphagia 19961141–47. [DOI] [PubMed] [Google Scholar]
- 38.Hughes T A, Liu, Griffiths H, et al The repeatability and variability of electrical impedance tomography indices of pharyngeal transit time in normal adults. Physiol Meas 199516(suppl A)A79–A86. [DOI] [PubMed] [Google Scholar]
- 39.Barer D H. The natural history and functional consequences of dysphagia after hemispheric stroke. J Neurol Neurosurg Psychiatry 198952236–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Smithard D G, O'Neill P A, England R E.et al The natural history of dysphagia following a stroke. Dysphagia 199712188–193. [DOI] [PubMed] [Google Scholar]
- 41.Smithard D G, O'Neill P A, Park C.et al Complications and outcome after acute stroke: Does dysphagia matter? Stroke 1996271200–1204. [DOI] [PubMed] [Google Scholar]
- 42.James A, Kapur K, Hawthorne A B. Long‐term outcome of percutaneous endoscopic gastrostomy feeding in patients with dysphagic stroke. Age Ageing 199827671–676. [DOI] [PubMed] [Google Scholar]
- 43.Rehman H U, Knox J. There is a need for a regular review of swallowing ability in patients after PEG insertion to identify patients with delayed recovery of swallowing. Dysphagia 20001548. [DOI] [PubMed] [Google Scholar]
- 44.Hull M A, Rawlings J, Murray F E.et al Audit of outcome of long‐term enteral nutrition by percutaneous endoscopic gastrostomy. Lancet 1993341869–872. [DOI] [PubMed] [Google Scholar]
- 45.Wanklyn P, Cox N, Belfield Outcome in patients who require a gastrostomy after stroke. Age Ageing 199524510–514. [DOI] [PubMed] [Google Scholar]
- 46.Holas M A, DePippo K L, Reding M J. Aspiration and relative risk of medical complications following stroke. Arch Neurol 1994511051–1053. [DOI] [PubMed] [Google Scholar]
- 47.Kidd D, Lawson J, Nesbitt R.et al The natural history and clinical consequences of aspiration in acute stroke. QJM 199588409–413. [PubMed] [Google Scholar]
- 48.Katzan I L, Cebul R D, Husak S H.et al The effect of pneumonia on mortality among patients hospitalized for acute stroke. Neurology 200360620–625. [DOI] [PubMed] [Google Scholar]
- 49.Parker C, Power M, Hamdy S.et al Awareness of dysphagia by patients following stroke predicts swallowing performance. Dysphagia 20041928–35. [DOI] [PubMed] [Google Scholar]
- 50.Finegold S M. Aspiration pneumonia. Rev Infect Dis 199113(suppl 9)S737–S742. [DOI] [PubMed] [Google Scholar]
- 51.Langmore S E, Skarupski K A, Park P S.et al Predictors of aspiration pneumonia in nursing home residents. Dysphagia 200217298–307. [DOI] [PubMed] [Google Scholar]
- 52.Davenport R J, Dennis M S, Wellwood I.et al Complications after acute stroke. Stroke 199627415–420. [DOI] [PubMed] [Google Scholar]
- 53.Poor nutritional status on admission predicts poor outcomes after stroke: observational data from the FOOD trial Stroke. 2003;34:1450–1456. doi: 10.1161/01.STR.0000074037.49197.8C. [DOI] [PubMed] [Google Scholar]
- 54.Hamdy S, Aziz Q, Rothwell J C.et al The cortical topography of human swallowing musculature in health and disease. Nat Med 199621217–1224. [DOI] [PubMed] [Google Scholar]
- 55.Hamdy S, Aziz Q, Rothwell J C.et al Explaining oropharyngeal dysphagia after unilateral hemispheric stroke. Lancet 1997350686–692. [DOI] [PubMed] [Google Scholar]
- 56.Hamdy S, Aziz Q, Rothwell J C.et al Recovery of swallowing after dysphagic stroke relates to functional reorganization in the intact motor cortex. Gastroenterology 19981151104–1112. [DOI] [PubMed] [Google Scholar]
- 57.Bath P M, Bath F J, Smithard D G. Interventions for dysphagia in acute stroke. Cochrane Library. Issue 2. Oxford: Update Software, 2000 [DOI] [PubMed]
- 58.Cook I J, Kahrilas P J. AGA technical review on management of oropharyngeal dysphagia. Gastroenterology 1999116455–478. [DOI] [PubMed] [Google Scholar]
- 59.Bisch E M, Logemann J A, Rademaker A W.et al Pharyngeal effects of bolus volume, viscosity, and temperature in patients with dysphagia resulting from neurologic impairment and in normal subjects. J Speech Hear Res 1994371041–1059. [DOI] [PubMed] [Google Scholar]
- 60.Butler S G, Postma G N, Fischer E. Effects of viscosity, taste, and bolus volume on swallowing apnea duration of normal adults. Otolaryngol Head Neck Surg 2004131860–863. [DOI] [PubMed] [Google Scholar]
- 61.Steele C M, Van Lieshout P H. Influence of bolus consistency on lingual behaviors in sequential swallowing. Dysphagia 200419192–206. [DOI] [PubMed] [Google Scholar]
- 62.Hiss S G, Strauss M, Treole K.et al Effects of age, gender, bolus volume, bolus viscosity, and gustation on swallowing apnea onset relative to lingual bolus propulsion onset in normal adults. J Speech Lang Hear Res 200447572–583. [DOI] [PubMed] [Google Scholar]
- 63.Kendall K A, Leonard R J, McKenzie S W. Accommodation to changes in bolus viscosity in normal deglutition: a videofluoroscopic study. Ann Otol Rhinol Laryngol 20011101059–1065. [DOI] [PubMed] [Google Scholar]
- 64.Raut V V, McKee G J, Johnston B T. Effect of bolus consistency on swallowing–does altering consistency help? Eur Arch Otorhinolaryngol 200125849–53. [DOI] [PubMed] [Google Scholar]
- 65.Shanahan T K, Logemann J A, Rademaker A W.et al Chin‐down posture effect on aspiration in dysphagic patients. Arch Phys Med Rehabil 199374736–739. [DOI] [PubMed] [Google Scholar]
- 66.Logemann J A, Kahrilas P J, Kobara M.et al The benefit of head rotation on pharyngoesophageal dysphagia. Arch Phys Med Rehabil 198970767–771. [PubMed] [Google Scholar]
- 67.Kahrilas P J, Logemann J A, Krugler C.et al Volitional augmentation of upper esophageal sphincter opening during swallowing. Am J Physiol 1991260)G450–G456. [DOI] [PubMed] [Google Scholar]
- 68.Cogen R, Weinryb J. Aspiration pneumonia in nursing home patients fed via gastrostomy tubes. Am J Gastroenterol 1989841509–1512. [PubMed] [Google Scholar]
- 69.Hassett J M, Sunby C, Flint L M, No elimination of aspiration pneumonia in neurologically disabled patients with feeding gastrostomy Surg Gynecol Obstet. 1988;167:383–388. [PubMed] [Google Scholar]
- 70.Finucane T E, Bynum J P. Use of tube feeding to prevent aspiration pneumonia. Lancet 19963481421–1424. [DOI] [PubMed] [Google Scholar]
- 71.Ciocon J O, Silverstone F A, Graver L M.et al Tube feedings in elderly patients. Indications, benefits, and complications. Arch Intern Med 1988148429–433. [PubMed] [Google Scholar]
- 72.Nakajoh K, Nakagawa T, Sekizawa K.et al Relation between incidence of pneumonia and protective reflexes in post‐stroke patients with oral or tube feeding. J Intern Med 200024739–42. [DOI] [PubMed] [Google Scholar]
- 73.Dziewas R, Ritter M, Schilling M.et al Pneumonia in acute stroke patients fed by nasogastric tube. J Neurol Neurosurg Psychiatry 200475852–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Langmore S E, Terpenning M S, Schork A.et al Predictors of aspiration pneumonia: how important is dysphagia? Dysphagia 19981369–81. [DOI] [PubMed] [Google Scholar]
- 75.Norton B, Homer‐Ward M, Donnelly M T.et al A randomised prospective comparison of percutaneous endoscopic gastrostomy and nasogastric tube feeding after acute dysphagic stroke. BMJ 199631213–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Park R H, Allison M C, Lang J.et al Randomised comparison of percutaneous endoscopic gastrostomy and nasogastric tube feeding in patients with persisting neurological dysphagia. BMJ 19923041406–1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dennis M S, Lewis S C, Warlow C. Effect of timing and method of enteral tube feeding for dysphagic stroke patients (FOOD): a multicentre randomised controlled trial. Lancet 2005365764–772. [DOI] [PubMed] [Google Scholar]
- 78.Dennis M S, Lewis S C, Warlow C. Routine oral nutritional supplementation for stroke patients in hospital (FOOD): a multicentre randomised controlled trial. Lancet 2005365755–763. [DOI] [PubMed] [Google Scholar]
- 79.Shaker R, Easterling C, Kern M.et al Rehabilitation of swallowing by exercise in tube‐fed patients with pharyngeal dysphagia secondary to abnormal UES opening. Gastroenterology 20021221314–1321. [DOI] [PubMed] [Google Scholar]
- 80.Lazarra G, Lazarus C, Logemann J A. Impact of thermal stimulation on the triggering of the swallow reflex. Dysphagia 1986173–77. [Google Scholar]
- 81.Fraser C, Power M, Hamdy S.et al Driving plasticity in human adult motor cortex is associated with improved motor function after brain injury. Neuron 200234831–840. [DOI] [PubMed] [Google Scholar]
- 82.Perez I, Smithard D G, Davies H.et al Pharmacological treatment of dysphagia in stroke. Dysphagia 19981312–16. [DOI] [PubMed] [Google Scholar]
- 83.Arai T, Yasuda Y, Takaya T.et al ACE inhibitors and symptomless dysphagia. Lancet 1998352115–116. [DOI] [PubMed] [Google Scholar]
- 84.Crary M A, Carnaby G D M, Groher M E.et al Functional benefits of dysphagia therapy using adjunctive sEMG biofeedback. Dysphagia 200419160–164. [DOI] [PubMed] [Google Scholar]
- 85.Huckabee M L, Cannito M P. Outcomes of swallowing rehabilitation in chronic brainstem dysphagia: a retrospective evaluation. Dysphagia 19991493–109. [DOI] [PubMed] [Google Scholar]