Abstract
Background
Based on the preclinical and phase 1 studies, prasugrel, a novel platelet ADP P2Y12 receptor blocker, may be a more potent platelet inhibitor than clopidogrel. This study compared the antiplatelet properties of prasugrel in a small subset of patients enrolled in the JUMBO trial, and compared with historic clopidogrel treated controls.
Methods and results
Nine patients undergoing coronary stenting were randomised to one of three arms of prasugrel (40 mg loading, and 7.5 mg maintenance, n = 1; 60/10 mg, n = 4; or 60/15 mg, n = 2), or clopidogrel (300/75 mg, n = 2). Aspirin and GP IIb/IIIa inhibitors were permitted. Platelet activity was assessed at baseline, at 4, and 24 hours, and at 30 days after stent implantation in substudy participants, and compared with 124 historic controls who received clopidogrel. Independent of the loading, or maintenance dose, patients treated with prasugrel exhibited significantly more potent platelet inhibition as determined by ADP, and collagen induced aggregation, Ultegra Analyser, and surface expression of PECAM‐1, GPIIb/IIIa antigen, and activity with PAC‐1 antibody, GPIb, P‐selectin, CD40‐ligand, GP37, and thrombospondin receptor expression when compared with those treated with clopidogrel. There were no differences between antiplatelet agents with regard to vitronectin, LAMP‐1, PAR‐1 (intact and cleaved epitopes) thrombin receptor expression, or formation of platelet‐monocyte microparticles. Expression of GPIIb antigen, vitronectin, and LAMP‐3 receptor were not affected by both agents. Two patients treated with prasugrel 10 mg/daily exhibited complete inhibition of collagen induced aggregation at 30 days.
Conclusion
At the dosing regimens chosen in the JUMBO trial, it seems that prasugrel is a more potent antiplatelet agent than clopidogrel. Two episodes of profound platelet inhibition, which are not seen with clopidogrel, raise the possibility of higher bleeding risks especially during long term prasugrel use. Whether stronger platelet inhibition will yield better clinical outcomes and/or increased bleeding remains to be determined in an ongoing comparative phase 3 superiority trial (TRITON).
Keywords: platelets, prasugrel, clopidogrel, clinical trial
Clopidogrel, a thienopyridine, is a platelet ADP receptor blocker with proved clinical benefits during and after coronary stenting.1,2,3,4 Several large randomised clinical trials have shown the benefits of clopidogrel either as an alternative2 or an adjunct3,4 to aspirin in high risk patients. Furthermore, in a recent randomised trial of almost 46 000 patients with acute myocardial infarction, maintenance (75 mg) clopidogrel with no loading, in combination with thrombolytic agent and aspirin yielded a significant mortality reduction.5 However, despite proved benefits, clopidogrel has several shortcomings such as delayed platelet inhibition,6 response variability,7 occurrence of secondary thrombotic events,8 or/and stent thrombosis.9 Thus, the ongoing search for more efficient and safe antiplatelet agents is well justified.
Prasugrel, chemically known as ethanone, 2‐[2‐(acetyloxy)‐6,7‐dihydrothieno [3,2‐c] pyridin‐5 (4H)‐yl]‐1‐cyclopropyl‐2‐ (2‐fluorophenyl)‐, hydrochloride (CS‐747, LY640315), a novel potent thienopyridine P2Y12 receptor antagonist, has the potential to achieve higher levels of inhibition of ADP induced platelet aggregation than currently approved doses of clopidogrel.10 Prasugrel has been compared with clopidogrel in a dose finding phase 2 clinical trial joint utilisation of medications to block platelets optimally (JUMBO)‐TIMI 26. We conducted a platelet substudy in the frame of JUMBO by serial measurements of platelet characteristics using conventional aggregometry, rapid cartridge based rapid analyser and whole blood flow cytometry.
Methods
Patients
The study was approved by the Western Investigational Review Board (Olympia, WA, Protocol Number 20030599 WIRB), and by the JUMBO trial steering committee. The substudy was performed at St Joseph Medical Center (Towson, MD), and Union Memorial Hospital (Baltimore, MD). Satellite platelet laboratories were established at both facilities. Written informed consent was obtained from all patients. The study population consisted of nine patients undergoing coronary stenting who were randomised to two loading regimens (40 mg or 60 mg), and then three maintenance daily doses (7.5 mg, 10 mg, and 15 mg) prasugrel, or clopidogrel (300 mg/75 mg). Detailed design, inclusion and exclusion criteria are published elsewhere.11 Intravenous GP IIb/IIIa inhibitors were permitted. All patients received 325 mg aspirin daily for at least one week, and during the entire follow up phase of the study. The JUMBO data were also compared with those of 124 historic clopidogrel controls. All of these patients received 300 mg clopidogrel loading followed with 75 mg maintenance dose at the exact time points as required by the JUMBO. Thirty day follow up data were available for 34 historic patients. All platelet tests were identical and were performed during the same time frame as the index substudy except for the novel P2Y12 analyser cartridge for which control clopidogrel data (n = 26) were obtained prospectively.
Samples
Blood samples were obtained with a 19 gauge needle by direct venipuncture and drawn into two 7 ml vacutainer tubes at room temperature containing 3.8% trisodium citrate. The vacutainer tube was filled to capacity and gently inverted three to five times to ensure complete mixing of the anticoagulant. The first 4–5 ml of blood were used for other analysis, or discharged. All samples labelled with coded number and analysed by blinded technicians. Platelet studies were performed at baseline as well as 4 and 24 hours after stent implantation. The follow up platelet assays were performed 30 days after coronary intervention.
Platelet aggregation
The blood‐citrate mixture was centrifuged at 1200 g for 2.5 minutes. The resulting platelet rich plasma (PRP) was kept at room temperature for use within one hour. The platelet count was determined in the PRP sample and adjusted to 3.5×10 8/ml with homologous platelet‐poor plasma. Platelets were stimulated with 5 μmol ADP, 20 μmol ADP, and 4 μg/ml collagen (Chronolog, Havertown, PA) and aggregation was assessed as previously described using a Chronolog Lumi‐Aggregometer (model 560‐Ca) with the AggroLink software package. Aggregation was expressed as the maximal percentage change in light transmittance from baseline, using platelet‐poor plasma as a reference. Curves were analysed according to international standards.12
VerifyNow‐P2Y12 cartridge based analyser
The Ultegra device (Accumetrics, San Diego, CA) with adenosine diphosphate as agonist is an optical detection system, which measures platelet induced aggregation as an increase in light transmittance. This particular test cartridge has been designed to specifically monitor the antiplatelet effects of ADP receptor antagonists. The VerifyNow‐P2Y12 cartridge uses prostaglandin E1 in addition to ADP to increase intraplatelet cAMP, making the assay more sensitive and specific for the effects of ADP mediated by the P2Y12 receptor. When the activated platelets are exposed to the fibrinogen coated microparticles, agglutination occurs in proportion to the number of available platelet receptors. The whole blood citrate mixture is being added to the cartridge, and agglutination between platelets and coated beads is being recorded.13 Ultegra RPFA‐ADP assay results are reported as platelet activation units (PAU). The data mirror turbidometric platelet aggregation and reflect the degree of platelet ADP block. The assays were performed in duplicate. An electronic quality control test was performed on each instrument every day before performing any patient samples.
Whole blood flow cytometry
The surface expression of platelet receptors was determined by flow cytometry using the following monoclonal antibodies: CD 41 antigen (GP IIb/IIIa, (αII β(3) CD 42b (GP Ib), CD 62p (P‐selectin) (DAKO Corporation, Carpenteria, CA); PAC‐1 (GP IIb/IIIa activity) CD 31 (platelet/endothelial cell adhesion molecule (PECAM)‐1), CD 51/CD 61 ((αv β3, or vitronectin receptor), CD 63 (LIMP or LAMP‐3), CD 107a (LAMP‐1), CD 151 (PETA‐3), CD159 (CD40‐ligand), CD 165 (GP37) (PharMingen, San Diego, CA); CD36 (thrombospondin, GPIV), WEDE15, and SPAN12 (Beckman Coulter, Brea, CA). Formation of platelet‐leucocyte aggregates was assessed by dual labelling with pan‐platelet marker (CD151), and then with CD14, the macrophage receptor for endotoxin lipopolysaccharides. The blood‐citrate mixture (50 µl) was diluted with 450 µl TRIS buffered saline (TBS) (10 mmol/l TRIS, 0.15 mol/l sodium chloride) and mixed by inverting an Eppendorf tube gently two times. The appropriate primary antibody was then added (5 µl) and incubated at 37°C for 30 minutes, and then a secondary antibody was applied if needed. After incubation, 400 µl of 2% buffered paraformaldehyde was added for fixation. The samples were analysed on a Becton Dickinson FACScan flow cytometer (San Diego, CA) set up to measure fluorescent light scatter as previously described.14 All parameters were collected using four decade logarithmic amplification. The data were collected in list mode files and then analysed. P‐selectin was expressed as per cent positive cells.15 Other antigens were expressed as log mean fluorescence intensity.
Statistical analysis
Comparisons between and within treatment arms were made by two tailed analysis of variance with χ2 and Fisher's exact tests for discrete variables, and Wilcoxon rank sum for continuous variables. Data were expressed as mean (SD), and p<0.05 was considered significant. Differences between individual flow cytometric histograms were assessed using the Smirnov‐Kolmogorov test incorporated in the CELLQuest' (San Diego, CA) software. Statistical analyses were performed using SPSS/E11.5 (SPSS, Chicago, IL).
Results
Demographics and clinical characteristics
Of the 176 screened patients, nine were enrolled. The primary reasons for such a high screen failure rate includes: pre‐existing clopidogrel (n = 103); therapy with proton pump inhibitors (n = 44); enoxaparin (n = 19); and age over 75 years (n = 23). All patients completed the 30 day study phase. There were no deaths or serious adverse events. Table 1 shows demographic and clinical characteristics.
Table 1 Demographics, risk factors, clinical characteristics, and concomitant drugs in the JUMBO subset and historic controls.
| Variables | Praugel Total (n = 7) | Prasugel Dose 1 (n = 1) | Prasugel Dose 2 (n = 4) | Prasugrel Dose 3 (n = 2) | Clopidogrel JUMBO (n = 2) | Clopidogrel historic (n = 124) |
|---|---|---|---|---|---|---|
| Age, y (SD) | 64.3 (8.8) | 61 | 60.7 (10.6) | 70.0 (1.4) | 54.0 (9.9) | 63.8 (10.5) |
| Male | 7 (100%) | 1 | 4 | 2 | 1 | 79 (63%) |
| Ethnic origin | ||||||
| White | 6 (85%) | 1 | 3 | 2 | 2 | 91 (73%) |
| African‐American | 1 (14%) | 1 | – | – | 15 (10%) | |
| History and risk factors | ||||||
| CAD | 3 (43%) | – | 1 | 2 | 1 | 71 (57%) |
| PTCA | 3 (43%) | – | 1 | 2 | 1 | 48 (39%) |
| CABG | 1 (14%) | 1 | – | – | 21 (17%) | |
| Previous MI | 1 (14%) | – | – | 1 | – | 36 (29%) |
| Stroke or TIA | – | – | – | – | – | 3 (2%) |
| CHF | – | – | – | – | – | 14 (11%) |
| Hypertension | 6 (86%) | 1 | 3 | 2 | 2 | 97 (78%) |
| PVD | – | – | – | – | – | 8 (6.5%) |
| Diabetes | – | – | – | – | 1 | 48 (39%) |
| Smoking | 4 (57%) | 1 | 1 | 2 | 77 (62%) | |
| Dyslipidaemia | 5 (71%) | 1 | 2 | 2 | 1 | 86 (69%) |
| Drugs | ||||||
| GPIIb/IIIa inhibitors | 4 (56%) | – | 3 | 1 | – | – |
| Aspirin | 7 (100%) | 1 | 4 | 2 | 2 | 124 (100%) |
| β blockers | 5 (71%) | 1 | 2 | 2 | 1 | 79 (64%) |
| ACE inhibitors | 2 (28%) | – | 1 | 1 | 1 | 43 (35%) |
| Statins | 4 (57%) | 1 | 1 | 2 | 1 | 85 (68%) |
| Ca channel blockers | 2 (28%) | 2 | – | – | 19 (15%) | |
| SSRIs | 1 (14%) | 1 | – | – | 17 (14%) |
Dose 1: prasugrel loading dose 40 mg+7.5 mg/day; dose 2: prasugrel loading dose 60 mg+10 mg/day; dose 3: prasugrel loading dose 60 mg+15 mg/day; clopidogrel: loading dose 300 mg +75 mg/day.
Age, sex, and race were fairly evenly distributed. However, there were some differences among groups, as expected, based on the small sample size and multiple treatment arms in the study. The distributions of risk factors as well as concomitant drugs were also similar, especially when comparing total prasugrel group with the historic clopidogrel cohort. There were no strokes, transient ischaemic attacks, heart failure, or diabetes in the JUMBO cohort. GP IIb/IIIa inhibitors were not permitted in the historic clopidogrel platelet studies.
Platelet data
Table 2 shows the platelet data dependent on the treatment assignments.
Table 2 Platelet characteristics in the JUMBO substudy and historic clopidogrel controls.
| Parameter | Study group | Baseline | 4 hours | 24 hours | 30 days |
|---|---|---|---|---|---|
| Conventional optical aggregometry | |||||
| ADP 5 µM (%) | CS‐747‐I (1) | 57 | 18 | 21 | 30 |
| CS‐747‐II (4) | 82.0 (11.3) | 8.5 (4.7)* | 9.8 (8.3)* | 40.0 (17.8)* | |
| CS‐747‐III (2) | 31.5 (37.5) | 7.0 (2.8)* | 11.5 (9.2)* | 19.0 (7.1)* | |
| CS‐747 (total) | 56.8 (31.9) | 9.4 (5.2)*† | 11.9 (8.1)*† | 32.6 (16.3)*† | |
| Clopidogrel (2) | 67.0 (8.5) | 40.0 (2.8)* | 66.0 (22.6) | 41.0 (14.1)* | |
| Clopidogrel (historic) | 53.1 (12.8) | 26.8 (10.8)* | 32.9 (14.5)* | 21.4 (6.0)* | |
| ADP 20 µM ** (%) | CS‐747‐I (1) | 59 | 26 | 28 | 57 |
| CS‐747‐II (4) | 67.0 (0.0) | 7.3 (7.9)* | 8.5 (12.4)* | 57.3 (16.0) | |
| CS‐747‐III (2) | 41.0 (56.6) | 14.0 (17.0)* | 21.5 (26.2)* | 29.0 (2.8)* | |
| CS‐747 (total) | 55.0 (31.2) | 11.9 (11.3)* | 15.0 (16.2)* | 49.1 (17.8) | |
| Clopidogrel (2) | 56.0 (9.9) | 47.5 (13.4) | 82.0 (21.2) | 56.0 (14.1) | |
| Collagen 4 µg/ml (%) | CS‐747‐I (1) | 51 | 13 | 18 | 30 |
| CS‐747‐II (4) | 52.5 (3.5) | 2.8 (2.9) | 5.5 (8.3) | 22.5 (30.2) | |
| CS‐747‐III (2) | 30.0 (39.6) | 4.0 (4.2)* | 13.5 (17.7)* | 19.5 (3.5)* | |
| CS‐747 (total) | 43.2 (23.3) | 4.6 (4.6)*† | 9.6 (10.7)*† | 22.7 (21.7)*† | |
| Clopidogrel (2) | 57.5 (12.0) | 25.0 (11.3)* | 60.0 (50.9) | 29.5 (23.3)* | |
| Collagen 5 µg/ml (%) | Clopidogrel (historic) | 56.8 (14.6) | 20.3 (9.9)* | 29.4 (15.8)* | 38.3 (5.2)* |
| Verify Now P2Y12 analyser | |||||
| ULTEGRA* (PAU) | CS‐747‐I (1) | 691 | 405 | 394 | 525 |
| CS‐747‐II (4) | 643 (40) | 26 (10)* | 145 (189)* | 545 (172) | |
| CS‐747‐III (2) | 425 (45) | 198 (147)* | 211 (179)* | 257 (42)* | |
| CS‐747 (total) | 565 (261)† | 129 (158)*† | 199 (177)*† | 431 (186)† | |
| Clopidogrel (2) | 360 (99) | 283 (139) | 499 (224) | 367 (249) | |
| Clopidogrel(12) (prospective) | 338 (54) | 280 (72) | 285 (69) | 168 (72)* | |
| Flow cytometry | |||||
| CD31 (PECAM‐1) (log MFI) | CS‐747‐I (1) | 68.07 | 24.89 | 26.09 | 43.21 |
| CS‐747‐II (4) | 55.5 (5.6) | 63.4 (31.2) | 63.5 (32.5) | 38.3 (31.2)* | |
| CS‐747‐III (2) | 85.3 (23.9) | 44.5 (29.2)* | 44.8 (21.8)* | 18.1 (0.7)* | |
| CS‐747 (total) | 69.9 (19.3) | 52.5 (29.3)† | 52.8 (28.7)† | 33.2 (24.4)*† | |
| Clopidogrel (2) | 68.5 (8.8) | 42.8 (1.3)* | 50.5 (15.6)* | 68.8 (35.2) | |
| Clopidogrel (historic) | 62.9 (12.2) | 41.6 (17.3)* | 38.0 (18.9)* | 58.6 (8.4) | |
| CD41 (GP‐IIb antigen) (log MFI) | CS‐747‐I (1) | 344 | 183 | 135 | 261 |
| CS‐747‐II (4) | 266 (25) | 194 (61) | 179 (50)* | 283 (22) | |
| CS‐747‐III (2) | 306 (260) | 203 (137)* | 198 (97)* | 309 (31) | |
| CS‐747 (total) | 258 (83) | 195 (71)† | 178 (57)*† | 330 (99)† | |
| Clopidogrel (2) | 350 (34) | 325 (48) | 416 (28) | 409 (83) | |
| Clopidogrel (historic) | 357 (51) | 325 (49) | 347 (58) | 251 (42)* | |
| PAC‐1 (GPIIb/IIIa activity) (log MFI) | CS‐747‐I (1) | 8.81 | 4.84 | 4.12 | 5.02 |
| CS‐747‐II (4) | 11.6 (3.8) | 5.2 (2.4)* | 4.9 (2.1)* | 7.1 (3.0)* | |
| CS‐747‐III (2) | 7.2 (5.6) | 5.9 (1.9) | 4.5 (1.0)* | 6.4 (0.2) | |
| CS‐747 (total) | 9.3 (4.1) | 5.3 (1.9)*† | 4.7 (1.6)*† | 6.6 (2.3)*† | |
| Clopidogrel (2) | 8.9 (0.6) | 5.6 (0.7)* | 8.2 (0.8) | 11.2 (1.0) | |
| Clopidogrel (historic) | 10.0 (2.1) | 6.7 (1.5)* | 9.6 (2.5) | 9.3 (2.3) | |
| CD42 (GP‐Ib) (log MFI) | CS‐747‐I (1) | 124.5 | 90.8 | 131.8 | 102.6 |
| CS‐747‐II (4) | 136.4 (29.8) | 161.9 (33.2) | 162.0 (47.9) | 146.6 (56.0) | |
| CS‐747‐III (2) | 166.2 (51.8) | 116.2 (6.9) | 149.3 (41.9) | 96.1 (13.7)* | |
| CS‐747 (total) | 145.9 (35.5) | 138.7 (38.3) | 154.0 (39.6)† | 125.9 (47.7)† | |
| Clopidogrel (2) | 120.3 (4.4) | 143.2 (20.6) | 133.3 (23.3) | 163.2 (48.7) | |
| Clopidogrel (historic) | 135.2 (35.1) | 134.2 (37.3) | 129.8 (37.6) | 168.0 (48.0) | |
| CD51/61 (vitronectin) (log MFI) | CS‐747‐I (1) | 7.74 | 8.91 | 8.32 | 10.18 |
| CS‐747‐II (4) | 10.1 (0.8) | 9.1 (1.6) | 8.8 (1.8) | 8.9 (2.5) | |
| CS‐747‐III (2) | 6.4 (0.2) | 5.8 (0.2) | 6.6 (0.4) | 7.0 (0.9) | |
| CS‐747 (total) | 8.2 (1.9) | 8.1 (2.0)† | 8.1 (1.7) | 8.5 (2.2) | |
| Clopidogrel (2) | 8.1 (0.9) | 7.6 (0.7) | 7.6 (1.8) | 9.6 (0.9) | |
| Clopidogrel (historic) | 7.9 (1.7) | 6.9 (1.6) | 7.4 (1.9) | 9.2 (2.0) | |
| CD62p (P‐selectin) (%+) | CS‐747‐I (1) | 6.06 | 6.36 | 5.99 | 7.68 |
| CS‐747‐II (4) | 10.4 (0.5) | 8.9 (1.2) | 8.4 (1.5) | 6.0 (3.0)* | |
| CS‐747‐III (2) | 13.4 (5.4) | 10.2 (7.1) | 7.9 (0.5)* | 4.9 (0.6)* | |
| CS‐747 | 10.8 (4.1) | 8.9 (3.3) | 7.9 (1.4)*† | 5.9 (2.3)*† | |
| Clopidogrel (2) | 7.8 (1.6) | 6.7 (1.2) | 8.4 (0.0) | 10.5 (1.0) | |
| Clopidogrel (historic) | 10.4 (3.4) | 10.0 (3.5) | 9.8 (3.6) | 9.6 (1.4) | |
| CD63 (LAMP‐3) (log MFI) | CS‐747‐I (1) | 7.41 | 6.23 | 8.69 | 7.34 |
| CS‐747‐II (4) | 7.6 (0.7) | 6.4 (0.8) | 6.1 (0.7) | 7.2 (1.6) | |
| CS‐747‐III (2) | 4.4 (1.1) | 5.7 (2.1) | 4.8 (0.7) | 3.8 (0.4) | |
| CS‐747 (total) | 6.3 (1.8) | 6.2 (1.1) | 6.1 (1.4) | 6.3 (2.0) | |
| Clopidogrel (2) | 4.3 (1.6) | 4.5 (1.3) | 4.5 (2.1) | 6.0 (2.7) | |
| Clopidogrel (historic) | 6.5 (1.5) | 6.1 (1.7) | 6.6 (1.5) | 6.5 (2.5) | |
| CD107a (LAMP‐1) (log MFI) | CS‐747‐I (1) | 3.41 | 2.27 | 2.49 | 3.04 |
| CS‐747‐II (4) | 6.1 (0.8) | 5.4 (2.4) | 4.4 (2.2)* | 5.6 (3.0) | |
| CS‐747‐III (2) | 4.2 (2.0) | 1.5 (0.1)* | 2.2 (1.0)* | 3.9 (2.0) | |
| CS‐747 (total) | 4.8 (1.6) | 3.8 (2.6) | 3.5 (1.9) | 4.7 (2.5) | |
| Clopidogrel (2) | 4.3 (1.6) | 4.6 (1.2) | 4.5 (1.9) | 6.0 (1.6) | |
| Clopidogrel (historic) | 4.8 (1.6) | 2.9 (1.1)* | 2.7 (1.2)* | 5.8 (2.1) | |
| CD151+CD14 (log MFI) | CS‐747‐I (1) | 83.52 | 49.85 | 53.53 | 64.32 |
| CS‐747‐II (4) | 63.5 (1.6) | 64.4 (25.4) | 64.0 (40.7) | 68.2 (28.0) | |
| CS‐747‐III (2) | 77.3 (12.7) | 56.5 (0.3)* | 64.7 (6.5) | 51.8 (21.5)* | |
| CS‐747 (total) | 73.0 (11.1) | 60.1 (18.9) | 62.7 (29.2) | 62.9 (23.0) | |
| Clopidogrel (2) | 54.0 (44.2) | 49.6 (30.6) | 59.4 (34.8) | 55.1 (1.1) | |
| Clopidogrel (historic) | 67.1 (14.5) | 62.6 (14.4) | 69.5 (22.4) | 66.0 (9.5) | |
| CD40‐ligand (log MFI) | CS‐747‐I (1) | 7.91 | 5.76 | 2.93 | 3.56 |
| CS‐747‐II (4) | 6.7 (1.0) | 5.3 (1.1) | 5.2 (0.4) | 4.6 (1.7)* | |
| CS‐747‐III (2) | 6.7 (0.6) | 3.6 (1.7)* | 4.1 (1.2)* | 3.5 (0.1)* | |
| CS‐747 (total) | 6.9 (0.8) | 4.8 (1.4)* | 4.6 (1.1)* | 4.1 (1.3)*† | |
| Clopidogrel (2) | 4.5 (0.2) | 6.2 (2.2) | 4.8 (0.6) | 6.9 (0.6) | |
| Clopidogrel (historic) | 5.5 (1.3) | 4.1 (1.3)* | 4.2 (1.3)* | 6.5 (1.6) | |
| CD165 (log MFI) | CS‐747‐I (1) | 27.4 | 26.3 | 25.35 | 7.28 |
| CS‐747‐II (4) | 21.7 (0.1) | 13.9 (3.3)* | 12.2 (3.3)* | 17.4 (6.9) | |
| CS‐747‐III (2) | 26.4 (1.1) | 21.1 (1.8) | 18.3 (2.9) | 11.8 (5.5)* | |
| CS‐747 (total) | 24.7 (2.8) | 17.8 (5.6)* | 15.8 (5.7)* | 14.3 (6.7)*† | |
| Clopidogrel (2) | 17.0 (7.7) | 13.7 (2.1) | 15.0 (1.7) | 19.5 (11.5) | |
| Clopidogrel (historic) | 23.9 (5.2) | 17.8 (5.8) | 18.3 (4.2) | 19.7 (3.0) | |
| PAR‐1 activated WEDE15 (log MFI) | CS‐747‐I (1) | 34.6 | 16.64 | 25.4 | 25.4 |
| CS‐747‐II (4) | 8.2 (2.0) | 16.1 (8.4)* | 15.8 (9.6)* | 20.3 (14.0)* | |
| CS‐747‐III (2) | 36.0 (19.1) | 19.0 (3.5)* | 24.4 (2.9)* | 16.5 (2.8)* | |
| CS‐747 (total) | 37.0 (9.8) | 17.0 (6.2)*† | 19.6 (8.4)*† | 18.8 (10.7)* | |
| Clopidogrel (2) | 32.8 (5.0) | 21.3 (1.4)* | 28.9 (6.6) | 35.1 (7.1) | |
| Clopidogrel (historic) | 30.2 (10.9) | 20.9 (9.3) | 25.7 (9.6) | 16.5 (3.2)* | |
| PAR‐1 intact SPAN12 (log MFI) | CS‐747‐I (1) | 9.8 | 16.4 | 18.87 | 27.5 |
| CS‐747‐II (4) | 11.5 (2.8) | 18.3 (7.6) | 12.1 (3.0) | 23.2 (12.2) | |
| CS‐747‐III (2) | 22.4 (7.9) | 12.6 (4.2)* | 12.1 (4.0)* | 15.7 (2.1)* | |
| CS‐747 (total) | 19.5 (5.4) | 13.3 (2.5)† | 12.0 (2.7)*† | 9.6 (8.1)* | |
| Clopidogrel (2) | 20.0 (2.1) | 13.3 (2.9)* | 19.5 (0.0) | 23.2 (5.2) | |
| Clopidogrel (historic) | 18.0 (4.8) | 16.2 (5.0) | 17.1 (5.3) | 6.2 (0.9)* | |
| Thrombospondin (log MFI) | CS‐747‐I (1) | 7.45 | 6.4 | 5.3 | 2.88 |
| CS‐747‐II (4) | 8.7 (3.0) | 6.4 (1.6)* | 6.7 (1.3)* | 5.6 (2.6)* | |
| CS‐747‐III (2) | 5.7 (0.7) | 6.3 (0.1) | 7.5 (3.1) | 4.0 (0.2) | |
| CS‐747 (total) | 7.3 (2.2) | 6.4 (1.1) | 6.7 (1.7) | 4.8 (2.1)*† | |
| Clopidogrel (2) | 4.5 (0.5) | 5.0 (0.5) | 7.5 (1.9)* | 9.2 (2.7)* | |
| Clopidogrel (historic) | 7.1 (2.0) | 6.7 (2.0) | 7.8 (2.8) | 8.1 (1.3) | |
*p Value compared with own baseline; p<0.05; **no historic data available; †p value CS‐747 total compared with. clopidogrel historic <0.05. PAU, platelet activation units; MFI, mean fluorescence intensity; %+, percentage of positive cells.
Baseline platelet characteristics differed among groups. These differences were seen for each platelet biomarker measured confirming pronounced interindividual variability of platelet activity in patients at presentation to the cardiac catheterisation laboratory. One of the patients in the prasugrel 10 mg/daily group has been removed from the 30 days analysis for the admitted non‐compliance. Treatment with prasugrel and clopidogrel resulted in significant inhibition of platelet activity as reflected by conventional aggregometry, rapid cartridge based platelet analyser, and some activation dependent surface receptors.
Prasugrel
Analysis of GPIIb/IIIa free patients suggested that loading with 40 mg (n = 1 of 1) or 60 mg (n = 3 of 6) prasugrel resulted in a rapid significant dose dependent 75%–80% platelet inhibition at four hours after coronary intervention. Importantly, there were no rebounds of platelet activation at 24 hours in the prasugrel treated patients. Loading with prasugrel resulted in a sustained platelet inhibition independently from the platelet assessment test and was significant for each biomarker with the exception of GPIb, vitronectin receptor, LAMP‐1, LAMP‐3, thrombospondin expression, and formation of the platelet‐monocyte microparticles. Treatment with prasugrel maintenance doses from 7.5 mg (n = 1); 10 mg (n = 3), and 15 mg (n = 2) shows significant mostly dose dependent inhibition of platelet activity at 30 days after stent placement. Two patients from the 10 mg/daily prasugrel group exhibited complete (IPA = 100%) inhibition of collagen induced aggregation at 30 days. Platelet inhibition at 30 days after prasugrel was highly significant for all platelet characteristics except GPIIb antigen, vitronectin, and LAMP‐3 receptor expression when compared with their own baseline.
Clopidogrel
There were only two patients enrolled in the clopidogrel arm in our substudy. Differences in the baseline platelet characteristics, and suspected non‐compliance in one patient make it impossible to analyse the platelet data. As a consequence, we used a large historic dataset involving a comparable patient population and blood sampling time points (for example, Serebruany et al16 and Gurbel et al17). Despite response variability, loading with 300 mg clopidogrel resulted in sustained inhibition at four hours, with rebound platelet activation occurring 24 hours after the intervention. Platelets were moderately inhibited (IPA‐50–70%) in all patients treated with the maintenance daily dose (75 mg) of clopidogrel for 30 days. We did not see residual platelet aggregation of less than 10% for any agonist used.
Prasugrel compared with clopidogrel
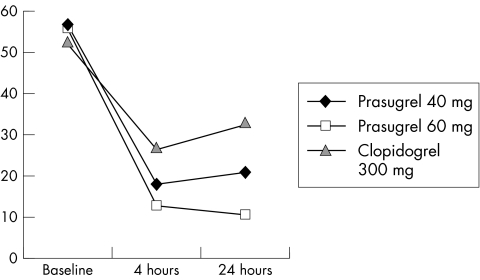
There were multiple significant differences in the degree of platelet inhibition between prasugrel and clopidogrel treated patients (see table 2 for details). All of these changes clearly suggest that for the doses used in the JUMBO trial, prasugrel is a much more potent antiplatelet agent than clopidogrel. Both loading regimens of prasugrel (40 mg and 60 mg) resulted in a dose dependent stronger platelet inhibition than those loaded with 300 mg clopidogrel. Most importantly, patients loaded with prasugrel uniformly exhibited lack of the peak rebound platelet activation at 24 hours, which is common for the 300 mg clopidogrel treated patients (fig 1). Long term treatment with all three maintenance doses of prasugrel (7.5 mg–10 mg–15 mg) for 30 days resulted in less interindividual response variability, and higher degree of platelet inhibition than similar long term regimen with 75 mg/daily clopidogrel. Moreover, two patients from the 10 mg/daily prasugrel group developed complete inhibition of collagen induced aggregation, which was not seen among the clopidogrel treated patients.
Figure 1 Graph illustrates changes of the 5 µM ADP induced platelet aggregation after loading with 40 mg and 60 mg prasugrel and 300 mg clopidogrel during coronary stenting. Higher degree of platelet inhibition, and lack of rebound at 24 hours after intervention distinguish prasugrel from clopidogrel.
Discussion
This small substudy provides the first prospective evidence that reports the platelet related effects of treatment with prasugrel compared with clopidogrel in patients with acute coronary syndromes who have undergone stent placement. Indeed, while the pattern of platelet inhibition for both agents was similar across all groups, prasugrel showed greater platelet inhibition than clopidogrel. Despite the small sample size, the randomised design increased the likelihood that the antiplatelet properties of prasugrel are more potent than those of clopidogrel, because the index data are consistent regardless of the method used for assessing platelet function. Applying a wide panel of techniques minimises the error by measuring different parameters indicative of various platelet activation dependent characteristics. In this study, the antiplatelet activity has been serially assessed by conventional optical aggregometry induced by several agonists, and by the Ultegra rapid analyser with the VerifyNow cartridge designed specifically for monitoring of P2Y12 platelet receptor activity. In addition, we used the whole blood flow cytometry determining the expression of multiple receptors located on the platelet surface. Considering the pronounced heterogeneity of platelet activity among and within groups, it was critical to apply multiple tests to comprehensively study platelet function to ensure adequate evaluation of platelet biomarkers.
Clopidogrel, an ADP receptor blocker, emerged more than a decade ago as a safer alternative to ticlopidine, another thienopyridine with a similar efficacy profile. Presently clopidogrel dominates the antiplatelet market with over 10 million people treated for secondary vascular event prevention in the USA, and over 100 000 patients enrolled in controlled studies worldwide.18 Ticlopidine and clopidogrel are irreversible P2Y12 antagonists and have been repeatedly proved as clinically beneficial antithrombotic agents. Considering that P2Y12 receptor is expressed nearly exclusively in platelets and brain (similar to the serotonin receptor) it represents an attractive target for the development of new antiplatelet agents. Novel antagonists for the P2Y12 receptor have been developed that either require metabolic activation to covalently inhibit P2Y12 and are irreversible (prasugrel), or simply are competitive in nature and thus reversible (AZD1640, cangrelor).
Prasugrel (CS‐747, LY640325) generates an active metabolite, R‐99224 in vivo, while being itself totally inactive in vitro.19,20 Animal data show that orally administered CS‐747 neutralised ADP induced decreases of cyclic AMP concentrations induced by prostaglandin E,21 suggesting that metabolites of CS‐747 interferes with G (i)‐linked P2T receptor.22 Prasugrel considerably inhibited ex vivo washed and conventional platelet aggregation in response to ADP, but not to thrombin, in a concentration related manner and prevented thrombus formation greater than clopidogrel and ticlopidine.19,20,22 There are few findings in healthy volunteers and patients with coronary artery disease suggesting that prasugrel at a dose 10 mg/daily for 10 days has been associated with significant cumulative inhibition of platelet aggregation from two days after the first dose until at least two days after the final dose.10,23,24
The index data are in full agreement with the available evidence suggesting that loading with prasugrel (40–60 mg), and maintenance with any of three tested doses (7.5–10.0–15.0 mg/daily) provides more potent platelet inhibition than clopidogrel 300 mg loading/75 mg/daily long term dosing. On the other hand our small but methodologically comprehensive study provides no proof or even a hint that the antiplatelet profile of prasugrel is unique and/or superior to platelet inhibition with clopidogrel. In fact, serial platelet characteristics follow similar, if not identical patterns, and may match very closely, if comparable, in terms of antiplatelet potency doses are chosen for both agents. Indeed, almost all randomised data with proved clinical benefit are available for no loading, or 300 mg clopidogrel dose, with the loading/maintenance clopidogrel ratio of 300/75 mg, or 4:1. The same ratios in JUMBO were 5.33:1 for the lowest dose, 6:1 for the medium dose, and equally to clopidogrel 4:1 only for the highest dose prasugrel. Inflated loading in addition to more potent maintenance dosing may contribute to the 30% higher bleeding events in the prasugrel treated patients.11 There is a 6:1 prasugrel ratio compared with 4:1 clopidogrel in the ongoing TRITON trial favouring prasugrel over conventional clopidogrel regimens. Both loading and maintenance doses of clopidogrel should be at least doubled in order not to compare apples and oranges. In short, despite obvious under dose clopidogrel “disadvantage”, more potent platelet inhibition may not necessarily result in better clinical outcomes. Quite opposite, stronger platelet inhibition with oral GP IIb/IIIa inhibitors has been associated with the highest among the antiplatelet agents bleeding rates,25 and most importantly increased cardiovascular mortality.26 While a higher loading dose may benefit prasugrel treated patients by reducing immediate post‐stent ischaemic events, a higher daily maintenance regimen may increase the risks of bleeding, and non‐compliance.
Two cases of complete inhibition of collagen induced aggregation at day 30 with 10 mg/daily prasugrel occurred in different satellite laboratory locations and were confirmed with positive control tests. Importantly, the same prasugrel dose is currently being evaluated in the TRITON trial. Such profound inhibition has not been seen after long term treatment with clopidogrel.27,28,29 In fact, when platelet aggregation is induced by collagen, treatment with 75 mg/daily of clopidogrel is associated with a residual platelet activity of 20%–40% when compared against baseline activity. The index observation confirms the previous report that responses to collagen and thrombin are impaired in platelets in a person with a bleeding disorder that has been linked to a defect in the P2Y12 receptor.30 How meaningful clinically such phenomenon may be remains to be seen. However, considering that oral GPIIb/IIIa inhibitors do not block completely the collagen induced aggregation,31 such evidence with prasugrel is alarming, and more dose finding studies are needed.
Among various receptors assessed in our study, changes in the expression of protease activated receptor‐1 (PAR‐1) deserve attention. PAR‐1 is a member of a novel gene family of G‐protein couples receptors and is responsible for attracting α thrombin to the platelet surface.32,33 We found significant reductions in PAR‐1 platelet expression after administration of both agents. This effect was strongest in prasugrel treated patients at 30 days, suggesting that long term treatment with P2Y12 antagonists may also act as a direct antithrombin agent shredding thrombin from the platelet surface. The importance of this finding is presently uncertain, but may represent a novel mechanism of antithrombotic properties of ADP receptor antagonists. In fact, the link between P2Y12 receptor and thrombin is not new. Several studies have suggested that P2Y12 serves as a cornerstone in modulation of interactions between ADP and thrombin.30,34,35,36 It has been reported that activation of phosphoinositide 3‐kinase35 and phospholipase D36 in human platelets by low concentrations of thrombin requires ADP, and these effects are blocked by ARL 66096, a selective inhibitor of P2Y12.
Another important practical issue is non‐compliance, which was suspected by the platelet tests at 30 days and then confirmed by one of the prasugrel treated patients. The index patient also stopped taking aspirin, which resulted in rebound platelet activation.37 As shown by the antiplatelet stroke prevention meta‐analysis, a significant portion of non‐compliant patients stop treatment because of adverse effects that are a real and/or perceived result of drug treatment.38 This was the case with the index non‐compliant patient, and seems particularly true for antiplatelet and anticoagulant therapies, where routine tasks like shaving or tooth brushing can produce annoying minor bleeding.39 Obviously, the risks for non‐compliance are higher for more potent antiplatelet regimens, and probably contributed substantially to the higher rates of mortality due to thrombotic events reported earlier after oral GPIIb/IIIa inhibitors.26
Finally, there is an increasing body of evidence that a delicate strategy with modest inhibition of platelet activity, especially in a long term setting may represent a substantial advantage over aggressive antiplatelet regimens. While there is no doubt that the concept of inhibiting platelets is vital for the treatment of vascular ischaemic disease, the optimal degree of such inhibition remains an unsolved mystery. It seems that the concepts “the more, the better”, and “one size fits all” may no longer be valid for ideal antiplatelet protection. Apparently, without routine individual laboratory assessment of platelet function, mild regimens will have an advantage of being more suitable for most patients, and contributed substantially to the success of clopidogrel. On the other hand, if we can determine baseline platelet status and intelligently apply treatment based on platelet activity in each particular patient, clinical outcomes may be better. Avoiding excessive bleeding risks after aggressive strategies in patients with normal or already decreased platelet function,40 but targeting those who indeed exhibit activated platelets may improve risk stratification and save lives.
Study limitations
The small sample size is an important limitation of this study. The results of this trial may have occurred by chance, however, the individual platelet characteristics after long term with prasugrel raises the most safety concern. Despite enrolling in two high volume catheterisation laboratories, broad exclusions such as pretreatment with clopidogrel, use of proton pump inhibitors, and age<75 years preclude higher entry numbers. A similar multicentre platelet substudy for another P2Y12 antagonist was not able to enrol a single patient because of the same reasons. Differences in the baseline clinical and platelet characteristics also limit the validation of our data. High frequency of concomitant drugs in general and use of GPIIb/IIIa inhibitors in particular may have affected the platelet characteristics beyond ADP receptor block, although making it a “real life” study. In addition, the expression of multiple activation dependent platelet receptors was studied but their individual roles in patients after stent implantation are unknown. It will be important to conduct larger prospective studies with prasugrel and determine how certain clinical subgroups (diabetic patients) may benefit from more aggressive antiplatelet regimens. It will also be critical to conduct longer (up to one year) serial assessments of platelet function to better define the durability of platelet inhibition and monitor compliance.
We conclude that for the higher loading and maintenance dosing regimens chosen in the JUMBO trial, prasugrel is a more potent antiplatelet agent than clopidogrel. Two episodes of profound platelet inhibition, which are not seen with clopidogrel, raise concerns with regard to higher bleeding risks, especially during long term prasugrel use. Whether stronger platelet inhibition will yield better clinical outcomes remains to be determined in the ongoing phase 3 superiority trial (TRITON).
Acknowledgements
The authors thank all the nurses and laboratory personnel for their technical excellence and outstanding effort of this trial.
Footnotes
Funding: the study was supported by Eli Lilly (Indianapolis, IN).
Conflicts of interest: VLS is listed as an inventor in the US patent application “Method for treating vascular diseases with prasugrel” assigned to Eli Lilly.
References
- 1.Pereillo J M, Maftouh M, Andrieu A.et al Structure and stereochemistry of the active metabolite of clopidogrel. Drug Metab Disp 2002301288–1295. [DOI] [PubMed] [Google Scholar]
- 2.CAPRIE Steering Committee A randomized, blinded, trial of clopidogrel versus aspirin in patients at risk of ischaemic events. Lancet 19963481329–1339. [DOI] [PubMed] [Google Scholar]
- 3.CURE Trial Investigators Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST‐segment elevation. N Engl J Med 2001345494–502. [DOI] [PubMed] [Google Scholar]
- 4.Steinhubl S R, Berger P B, Mann III J T.et al Early and sustained dual oral antiplatelet therapy following percutaneous coronary intervention. JAMA 20022882411–2420. [DOI] [PubMed] [Google Scholar]
- 5.Chen Z M, Jiang L X, Chen Y P.et al Addition of clopidogrel to aspirin in 45,852 patients with acute myocardial infarction: randomised placebo‐controlled trial. Lancet 20053661607–1621. [DOI] [PubMed] [Google Scholar]
- 6.Hochholzer W, Trenk D, Frundi D.et al Time dependence of platelet inhibition after a 600‐mg loading dose of clopidogrel in a large, unselected cohort of candidates for percutaneous coronary intervention. Circulation 20051112560–2564. [DOI] [PubMed] [Google Scholar]
- 7.Serebruany V L, Steinhubl S R, Berger P B.et al Variability in platelet responsiveness to clopidogrel among 544 individuals. J Am Coll Cardiol 200545246–251. [DOI] [PubMed] [Google Scholar]
- 8.Matetzky S, Shenkman B, Guetta V.et al Clopidogrel resistance is associated with increased risk of recurrent atherothrombotic events in patients with acute myocardial infarction. Circulation 20041093171–3175. [DOI] [PubMed] [Google Scholar]
- 9.Wenaweser P, Dorffler‐Melly J, Imboden K.et al Stent thrombosis is associated with an impaired response to antiplatelet therapy. J Am Coll Cardiol 2005451748–1752. [DOI] [PubMed] [Google Scholar]
- 10.Niitsu Y, Jakubowski J A, Sugidachi A.et al Pharmacology of CS‐747 (prasugrel, LY640315), a novel, potent antiplatelet agent with in vivo P2Y12 receptor antagonist activity. Semin Thromb Hemost 200531184–194. [DOI] [PubMed] [Google Scholar]
- 11.Wiviott S D, Antman E M, Winters K J.et al Randomized comparison of prasugrel (CS‐747, LY640315), a novel thienopyridine P2Y12 antagonist, with clopidogrel in percutaneous coronary intervention: results of the joint utilization of medications to block platelets optimally (JUMBO)‐TIMI 26 trial. Circulation 20051113366–3373. [DOI] [PubMed] [Google Scholar]
- 12.Ruggeri Z M. New insights into the mechanisms of platelet adhesion and aggregation. Semin Hemat 199431229–239. [PubMed] [Google Scholar]
- 13.Smith J W, Steinhubl S R, Lincoff A M.et al Rapid platelet‐function assay. An automated and quantitative cartridge‐based method. Circulation 199999620–625. [DOI] [PubMed] [Google Scholar]
- 14.Ault K A. Flow cytometric measurement of platelet function and reticulated platelets. Ann New York Acad Sci 1993677293–308. [DOI] [PubMed] [Google Scholar]
- 15.Serebruany V L, Gurbel P A. The relations of major platelet receptor expression during myocardial infarction. Monitoring efficacy of GPIIb/IIIa inhibitors by measuring P ‐ selectin? Thromb Haemost 199981314–316. [PubMed] [Google Scholar]
- 16.Serebruany V L, Midei M G, Malinin A I.et al Absence of interaction between atorvastatin or other statins and clopidogrel: results from the interaction study. Arch Intern Med 20041642051–2057. [DOI] [PubMed] [Google Scholar]
- 17.Gurbel P A, Cummings C C, Bell C R.et al Onset and extent of platelet inhibition by clopidogrel loading in patients undergoing elective coronary stenting: the plavix reduction of new thrombus occurrence (PRONTO) trial. Am Heart J 2003145239–247. [DOI] [PubMed] [Google Scholar]
- 18.Elsasser A, Nef H, Mollmann H.et al Clopidogrel in acute coronary syndrome: when, how much, how long? Z Kardiol 200594377–382. [DOI] [PubMed] [Google Scholar]
- 19.Kunapuli S P, Ding Z, Dorsam R T.et al ADP receptors‐‐targets for developing antithrombotic agents. Curr Pharm Des 200392303–2316. [DOI] [PubMed] [Google Scholar]
- 20.Sugidachi A, Asai F, Ogawa T.et al The in vivo pharmacological profile of CS‐747, a novel antiplatelet agent with platelet ADP receptor antagonist properties. Br J Pharmacol 20001291439–1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mills D C. ADP receptors on platelets. Thromb Haemost 199676835–856. [PubMed] [Google Scholar]
- 22.Sugidachi A, Asai F, Yoneda K.et al Antiplatelet action of R‐99224, an active metabolite of a novel thienopyridine‐type G(i)‐linked P2T antagonist, CS‐747. Br J Pharmacol 200113247–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brandt J T, Payne C D, Weerakkody G.et al Superior responder rate for inhibition of platelet aggregation with a 60 mg loading dose of prasugrel (CS‐747, LY640315) compared with a 300 mg loading dose of clopidogrel. J Am Coll Cardiol 200545(suppl A)87A [Google Scholar]
- 24.Wallentin L, Jernberg J, Leese P T.et al Inhibition of platelet aggregation with prasugrel (CS‐747, LY640315), a novel thienopyridine P2Y12 receptor antagonist, compared with clopidogrel in aspirin‐treated patients with atherosclerotic disease. J Am Coll Cardiol 200545(suppl A)416 [Google Scholar]
- 25.Serebruany V L, Malinin A I, Eisert R M.et al Risk of bleeding complications with antiplatelet agents: meta‐analysis of 338,191 patients enrolled in 50 randomized controlled trials. Am J Hematol 20047540–47. [DOI] [PubMed] [Google Scholar]
- 26.Chew D P, Bhatt D L, Sapp S.et al Increased mortality with oral platelet glycoprotein IIb/IIIa antagonists: a meta‐analysis of phase III multicenter randomized trials. Circulation 2001103201–206. [DOI] [PubMed] [Google Scholar]
- 27.Vinholt P, Poulsen T S, Korsholm L.et al The antiplatelet effect of clopidogrel is not attenuated by statin treatment in stable patients with ischemic heart disease. Thromb Haemost 200594438–443. [DOI] [PubMed] [Google Scholar]
- 28.Serebruany V L, Malinin A I, Ziai W.et al Effects of clopidogrel and aspirin in combination versus aspirin alone on platelet activation and major receptor expression in patients after recent ischemic stroke. For the plavix use for treatment of stroke (PLUTO‐stroke) trial. Stroke 2005362289–2292. [DOI] [PubMed] [Google Scholar]
- 29.Angiolillo D J, Fernandez‐Ortiz A, Bernardo E.et al Lack of association between the P2Y(12) receptor gene polymorphism and platelet response to clopidogrel in patients with coronary artery disease. Thromb Res 2005116491–497. [DOI] [PubMed] [Google Scholar]
- 30.Cattaneo M, Gachet C. ADP receptors and clinical bleeding disorders. Arterioscler Thromb Vasc Biol 1999192281–2285. [DOI] [PubMed] [Google Scholar]
- 31.Serebruany V L, Malinin A I, O'connor C M.et al Roxifiban oral compound kinetics evaluation trial‐I platelet substudy. Effects of roxifiban on platelet aggregation and major receptor expression in patients with coronary artery disease for the roxifiban oral compound kinetics evaluation trial‐I (ROCKET‐I platelet substudy). Am Heart J 200314691–98. [DOI] [PubMed] [Google Scholar]
- 32.Cupit L D, Schmidt V A, Bahou W F.et al Proteolitically activated receptor‐3. A member of an emerging gene family of protease receptors expressed on vascular endothelial cells and platelets. Trends Cardiovasc Med 1999942–48. [DOI] [PubMed] [Google Scholar]
- 33.Kahn M L, Nakanishi‐Matsui M, Shapiro M J.et al Protease activated receptors 1 and 4 mediate activation of human platelets by thrombin. Clin Invest 1999103879–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hechler B, Eckly A, Ohlmann P.et al The P2Y1 receptor, necessary but not sufficient to support full ADP‐induced platelet aggregation, is not the target of the drug clopidogrel. Br J Haematol 1998103858–866. [DOI] [PubMed] [Google Scholar]
- 35.Trumel C, Payrastre B, Plantavid M.et al A key role of adenosine diphosphate in the irreversible platelet aggregation induced by the PAR1‐activating peptide through the late activation of phosphoinositide 3‐kinase. Blood 1999944156–4165. [PubMed] [Google Scholar]
- 36.Martinson E A, Scheible S, Marx‐Grunwitz A.et al Secreted ADP plays a central role in thrombin‐induced phospholipase D activation in human platelets. Thromb Haemost 199880976–981. [PubMed] [Google Scholar]
- 37.Serebruany V L, Midei M G, Meilman H.et al Rebound platelet activation after termination of prasugrel and aspirin therapy due to confirmed non‐compliance in the patients enrolled in the JUMBO trial. Int J Clin Pract (in press) [DOI] [PubMed]
- 38.Gencheva E, Sloan M, Leurgans S.et al Attrition and non‐compliance in secondary stroke prevention trials. Neuroepidemiology 20042361–66. [DOI] [PubMed] [Google Scholar]
- 39.Serebruany V L, Hanley D F, Jr, Atar D.et al Noncompliance in antiplatelet trials: the AGATE trial perspective. Stroke 200435e143. [DOI] [PubMed] [Google Scholar]
- 40.Serebruany V L, Gurbel P A, Shustov A R.et al Heterogeneity of platelet aggregation and major surface receptor expression in patients with acute myocardial infarction. Am Heart J 1998136398–405. [DOI] [PubMed] [Google Scholar]