Abstract
Idiopathic inflammatory myopathies, notably polymyositis and dermatomyositis are comparatively uncommon diseases and few randomised, double blind placebo controlled trials have been done. Final validation of measures to assess outcome and response to treatment is awaited. Corticosteroids are an effective initial treatment, although rarely tested in randomised controlled trials. Unfortunately, not all patients respond to them and many develop undesirable side effects. There is thus a need for second line agents notably immunosuppressives or intravenous immunoglobulin. There are no defined guidelines or best treatment protocols agreed internationally and so the medical approach must be individualised, based on the severity of clinical presentation, disease duration, presence of extramuscular features, and prior therapy and contraindications to particular agents. There is still a significant percentage of non‐responders (around 25%) and clinical relapses. Novel therapeutic approaches are now directed towards cytokine modulation and the use of monoclonal antibodies targeting B and T cells.
Keywords: polymyositis, dermatomyositis
Treating inflammatory muscle diseases is challenging and can become extremely difficult in refractory cases. It is essential that the correct diagnosis be made and this entails an assessment of clinical features, serological tests, electromyogram evidence, and biopsy or imaging changes. To gauge the totality of the effect of multisystemic disease measures/indices, which distinguish activity (implying ongoing inflammation), damage (signifying permanent damage), and the patients' own perception of their disease are required.1
Poor prognostic factors common to several studies include old age, non‐white race, bulbar involvement, delayed treatment, and cardiovascular and pulmonary involvement.2
The main objective of treatment is to improve muscle strength3 and to obtain remission, or at least clinical stabilisation. To assess muscle strength clinical and laboratory criteria should be routinely assessed. Major international efforts (discussed later) are proceeding to provide reliable measures of function and disability. The use of formal manual muscle strength testing, timed functional tests, and the use of endurance parameters performing some everyday activities are helpful assessment tools. In addition an isokinetic dynamometer should provide more accurate data.4,5
Laboratory tests, notably muscle enzymes, are of some use in monitoring inflammation, while renal, liver, and haematological tests are also required to check on any toxicity from prescribed drugs. The muscle enzymes creatine kinase (CK), aldolase, aspartate aminotransferase (AST), alanine aminotransferase (ALT), and lactate dehydrogenase (LDH) are used to monitor disease activity but may be unpredictable4,6 or only slightly raised despite clinical disability. Despite these limitations the serum CK level remains a widely used biochemical indicator of disease activity,3,5 and should be monitored at least monthly after starting treatment.
A decline in the CK level invariably precedes objective clinical improvement for several weeks4,5 and mild to moderate CK level increases may persist for some time despite functional recovery. A rise in this muscle enzyme may be the first indicator of disease flare, before worsening of muscle weakness.4,5 A normal CK level in a patient thought to have active disease may reflect the underlying severe impairment—that is, few functioning muscle fibres are left intact, or muscle atrophy.3,7
Muscle MRI can be very useful in diagnosing and assessing activity in patients with myositis because of its sensitivity on measuring the tissue's water content. Muscle oedema as detected by MRI correlates well with inflammatory changes.5,7 A comparison of the T1 and T2 weighted fat suppressed sequences is used to interpret whether weakness is attributable to ongoing inflammation (sometimes patchy), a mixed picture of both inflammation and damage, or muscle atrophy with fat replacement.5,8,9
Polymyositis (PM) and dermatomyositis (DM)
As idiopathic muscle diseases are rare, descriptions of the use of drugs are restricted to small series case reports. Few controlled trials, most of then with a small number of patients have been published.4,6
Corticosteroids and immunosuppressive agents currently accepted as treatment for DM and PM are not always effective and both may cause serious side effects.9,16 The systemic manifestations, pulmonary involvement in particular, may account for additional therapeutic challenges and increased mortality.
Around a third of patients will not respond or respond poorly to conventional therapy and remain significantly disabled.2,9,17 Some reports show that those with an associated autoimmune rheumatic disease are more likely to respond inadequately.4,16 This is not, however, a universal finding.18 DM is the most treatable subset, in the majority of cases responding to corticosteroids, immunosuppressives, or intravenous immunoglobulin (IVIG),3,19 but the increased risk of associated malignancy should not be overlooked in refractory cases or relapses.7,8 Several new agents are under investigation targeting cytokines, activation molecules, and adhesion receptors.19
Corticosteroids
Corticosteroids are the standard main treatment for inflammatory myositis. Although their efficacy has not been fully established in randomised, placebo controlled trials,2,4,5,6,7,9,20,21,22,23 their clinical efficacy is recognised in most cases,2,5,6,11,24,25,26 especially in newly diagnosed patients.20 Several regimens have been studied.6 High doses of corticosteroids (1 mg/kg/day) have been used for the past three decades with some success.27,28,29 However, the frequent side effects have led to the use of lower doses for shorter periods of time. A study, by Nzeusseu,30 showed the same functional outcome in a small group of patients receiving a low dose regimen (⩽0.5 mg/kg/day) compared with those receiving higher doses (>0.5 mg/kg/day),2,4 although doubts have been expressed about the assessment of statistical differences.2
Noting that the regimen should be individualised, in practice we use prednisolone (PDN) starting with about 0.75 mg/kg/day in single or divided doses (average 40–60 mg/day)4,5,6,8 for one to two months until achieving clinical benefit. In mild cases this dose may be lower (20–40 mg/day).4 Progressive reductions by 5 to 10 mg per month over a three months period should be aimed at,5 with a slower reduction rate when reaching doses below 15 mg/day and adapted to the patients response,5 until achieving a maintenance dose of 5–10 mg per day.4 It is important to promote bone protection during this time and some centres recommend an annual dexascan of patients with myositis to monitor bone loss.
In acutely ill and severe clinical manifestations intravenous pulses of one gram of methylprednisolone for three consecutive days may be given to achieve rapid disease control.2,4,5,6,7,31,32
In severe cases, notably those not achieving a good response despite adequate immunosuppression over a three month period (and in patients who relapse during corticosteroid tapering), other pharmacological options must be considered (see below).5,6,33
Prolonged corticosteroid administration should be avoided if possible.5,8 On occasion corticosteroid induced myopathy should be suspected,6 particularly when weakness persists in the proximal muscles of patients with normal muscle enzyme activities.4,5 This myopathy generally improves upon corticosteroid reduction associated with a physical exercise programme.5
Immunosuppressive agents
Most patients will respond favourably to PDN alone or in combination with an immunosuppressive agent and achieve a complete or worthwhile remission.8 There is however no agreement about the best regimen or combination of immunosuppressant agents.6 The choice depends on the severity of the disease, possible extramuscular manifestations, personal experience, and the relevant relative efficacy/safety profile ratio of the drug.3 Patients who are diabetic, elderly, immunodeficient, have an associated interstitial lung disease (ILD), and those with bulbar or respiratory muscle dysfunction pose particular difficulties.8 Superiority of a specific combination remains unproved.2,3
Combined therapy is more likely to lead to a remission and better outcome with a lower relapse rate and lower PDN doses can be used.5,8
The introduction of immunosuppressive agents is usually considered if a patient shows3,4,6,20: (1) a poor response or refractory to therapy with corticosteroids alone; (2) rapidly progressive disease; (3) internal/severe organ involvement; (4) relapse during corticosteroid reduction; (5) evidence of corticosteroid side effects (diabetes, hypertension osteoporosis).
Methotrexate (MTX): is widely used in inflammatory myopathies as the first immunosuppressive agent4 and its efficacy is reported in a large percentage of patients.29,34 It has a good response rate in childhood and adult inflammatory myositis and recalcitrant DM.2,17,35,36 It can be given up to 20–25 mg orally, subcutaneously, or intramuscularly in a weekly dose, generally in association with folic acid to minimise its side effects, which include nausea, stomatitis, alopecia, liver toxicity, bone marrow suppression, increased risk of infection and lymphoma, and pneumonitis. The concomitant use of trimethoprim should be avoided. The risk of pulmonary fibrosis is a limitation for its use in patients with associated interstitial lung disease.3,6 It is often preferred to azathioprine (AZA) because of its more rapid onset of action.3,4,6 Retrospective studies29,37 suggested that MTX is more effective than AZA in male patients with antisynthetase antibodies unresponsive to corticosteroids alone.4,5,6 Another study38 reported a positive beneficial trend in a combination of MTX plus AZA compared with intravenous MTX. There were patients who responded to this combined therapy that had previously failed each drug separately.4,5 MTX did not demonstrate efficacy in patients with inclusion body myositis (IBM).39
AZA: may also be the first immunosuppressive/corticosteroid sparing drug started4,7 and can be as effective and well tolerated as MTX,5 but seems to take longer to be effective (up to four to six months).3,6 Normal dose range varies from 1.5 to 3 mg/kg/day, orally in divided doses. Its major side effects are nausea, abdominal pain, bone marrow suppression, liver toxicity, increased risk of infection and malignancy, and concomitant use of allopurinol should be avoided.6 Studies with AZA have shown efficacy29,40,41,42 with lower requirement of PDN.2,4,6,29,41,42
Cyclosporine (CyA): is an immunosuppressive agent with selective effect on T cell activation and cytokine production.5 It has the same treatment potential effect as MTX,4,43 acts faster than AZA,33 and is a useful additional second line agent in PM and DM, including those with juvenile DM6,22,26,44,45,46,47,48,49,50 previous unresponsive to other immunosuppressives and in associated cases of ILD. Its combination with MTX seems to be beneficial in patients with refractory DM.44 Its combination with PDN and IVIG has recently been shown to be effective, and with a sustained response, compared with CyA alone.20 Average doses range from 2 to 3.5 mg/kg/day, higher doses can be used but with increased risk of renal impairment. Other side effects include hypertension, hypertrychosis, gingival hyperplasia tremor, and increased risk of infection.6,20
IVIG: is derived from large pools of serum from healthy people, providing a large range of antibodies and has an immunomodutatory effect. IVIG treatment leads to a decrease in class I major histocompatibility complex (MHC), intracellular adhesion molecule 1 and pathological cytokines, blocks IgG receptors on phagocytic cells, down‐regulates transforming growth factor β1 involved in chronic inflammation, fibrosis, and prevents the accumulation of extracellular matrix in patients with DM (but not in IBM).9,51 In patients with myositis it also prevents activated complement from further cutaneous and muscular damage, inhibits serum SC5b‐9 complex levels, prevents membrane attack complex (MAC) deposits from entering the endomysial capillaries, and restores the capillary network.9 IVIG is effective in DM but there are only uncontrolled studies in patients with PM.6,9,51,52 Although not used as a first line agent53 it is considered in refractory cases of DM/PM.4,5,7,51 It causes no significant improvement in patients with IBM, except for the dysphagia.51,52 In a randomised placebo controlled study by Dalakas54 patients with refractory DM showed improvements in muscle strength and rash,2,6,7,51,52 especially in an early disease phase. Muscle biopsies repeated in these IVIG treated patients showed increase in muscle fibre diameter, reduction in capillary diameter and complement deposits, especially C3 and MAC on capillaries, decreased muscle necrosis and endomysial lymphocytic infiltrates.6,7,51,52,54,55 In patients with PM, uncontrolled studies led to muscle power increase, improvement in muscle disability scores and oesophageal disorders with a decrease in CK levels.51 A combination of IVIG, CyA, and PDN also proved effective in patients with relapsing or refractory DM/PM.20
Most investigations have used IVIG at a dose of 2 g/kg given either in 1 g/kg/day for two days every four weeks2,51 or alternatively 0.4 mg/kg/day for five days initially and than for three days monthly for three to six months.8 However, more investigation is required to establish the optimal dose, schedule, and duration of treatment.9 IVIG is expensive with duration of action between three to four weeks, but better tolerated than PDN, less toxic than other immunosuppressives, and can be used in immunocompromised patients. Side effects are rare and benign, occurring usually during infusions or shortly afterwards, notably mild headaches, shivering, sweating, myalgias, anaphylactic reactions, hypotension, fever, and nausea. There may be a risk of causing an immune mediated deterioration in renal function and aseptic meningitis.6,51
Cyclophosphamide (CyC): although effective in other autoimmune diseases, in patients with inflammatory myositis CyC has had variable results.3,4,5,6,56,57,58,59,60 In view of these uncertain results it is mostly reserved for cases resistant to other immunosuppressives and IVIG.2 There may be a strong case for its use in patients with DM, particularly when associated with vasculitis, IDL, and involvement of respiratory or bulbar muscles. It is given intravenously (0.5–1 g/m2), which is as effective as the oral formulation but causes fewer side effects.5 This dose is often repeated monthly for three to six months. Its major recognised side effects include bone marrow toxicity, haemorrhagic cystitis, teratogenicicty, ovarian failure and azoospermia, increased risk of infections and secondary malignancies.6
Tacrolimus (FK506): initially used as a transplant rejection agent, it has similarities to CyA inhibiting activation of CD4+ T‐helper cells4,5,61 and TNFα production.4 Its clinical application in inflammatory myopathies has been shown in a few patients for refractory myositis with ILD and antisynthetase antibodies4,5,6,62,63 and by ointment formulation on recalcitrant cutaneous lesions.61,64 In a small group of patients with refractory PM, most anti‐Jo1 antibody positive, tacrolimus was given in a dose of 0.075 mg/kg/day, in two divided doses, to maintain a plasma concentration between 5 and 10 ng/ml.4,63 It improved manual muscle strength in all patients, including those anti‐Jo1 positives, regaining normal strength. Some patients showed improvement in lung function tests. Serum CK level and PDN requirements both decreased. There was also improvement in extramuscular manifestations such as fever, polyarthritis, and mechanic hands (lateral and palmar darkened lines in the fingers).4,63
Mycophenolate mofetil (MMF): inhibits the de novo guanosine nucleotide synthesis and therefore impairs the function of T and B lymphocytes.6,16,21 Mycophenolate is being tried as a second line agent for refractory disease with promising results.65,66 It was shown to improve muscle strength66 and rash,16,21,66 but controlled trials are inevitably lacking.6,8 A dose of 2 g per day, orally (about 30 mg/kg/day) is well tolerated and effective as a corticosteroid sparing agent, although it has a slow mode of action.5,8,21 Adverse effects include cytopenias, gastrointestinal intolerance, and increased risk of infection.
Chlorambucil: there are few data describing any benefits in patients refractory to other immunosuppressive agents.1,2,6 It was effective at 4 mg daily dose, as a corticosteroid sparing agent in five patients with DM refractory to PDN, MTX, and AZA2,67 and was also reported to be effective in association with MTX and PDN.34,68 The common side effects include hypersensibility reactions, infection, liver toxicity, gastrointestinal disturbance, and teratogenicity.6 The risks of secondary malignancy, liver and bone marrow toxicity are likely to be increased, as the drug is an alkylating agent.
Fludarabine: adenine analogue used commonly as an antineoplasic agent for haematological malignancies. A pilot study47 using a three day regimen of 20 mg/m2/month for six months failed to show significant improvement in patients with refractory PM/DM. Nevertheless when re‐examined with less stringent criteria some response was claimed.4,5,6
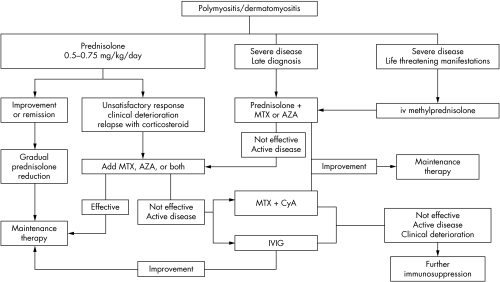
Figure 1 is a resume of the sequential treatment described above.
Figure 1 Treatment algorithm for inflammatory muscle diseases. MTX, methotrexate; AZA, azathioprine; iv, intravenous; IVIG, intravenous immunoglobulin; CyA, cyclosporine.
New treatments
As a significant number of patients with inflammatory muscle disease respond adequately to treatment with corticosteroids and immunosuppressive agents or IVIG, investigations continue for more effective drugs with fewer side effects.
Blockage of signal transduction in T lymphocytes (FK 506, rapamycin, CAMPATH), monoclonal antibodies against cytokines (for example, to TNFα and IL1, soluble receptors of TNFα and beta interferon) or costimulatory molecules (CD28/CTLA4 Ig) and interference with cell adhesion molecules (integrins and their receptors) and matrix metalloproteinases have been the main subjects of recent research.3,5
Increased expression of TNFα in muscle fibres of patients with myositis has been reported implicating it in the pathogenesis of myositis.4,23 There have been several anedoctal and case reports of the safe use of TNFα blockers (etanercept and infliximab) in refractory cases of inflammatory myopathies (DM, PM, juvenile and amyopathic DM) with claims of rapid clinical benefit in disease activity with a decrease in serum CK levels.4,5,6,23,69,70 Improvement of muscle strength and electromyography pattern, a decrease in serum CK, and reduction of necrotic muscular fibres and extent of inflammation in repeated muscle biopsy, was seen in a case report of two “naive” patients treated with infliximab (10 mg/kg—three infusions separated by two weeks), without any side effects.23 Clearly more studies are warranted in larger groups of patients.
Rituximab is a depleting chimeric monoclonal antibody against the B lymphocyte marker CD20 and has shown promising results in patients with rheumatoid arthritis and systemic lupus erythematosus and IgM mediated neuropathies.71,72,73,74 Levine has reported the use of rituximab in five patients with longstanding DM previously treated with at least three immunosuppressive agents with incomplete response and in one newly diagnosed patient.74 In this open label pilot study all patients were given 375 mg/m2 of rituximab in a four weekly dose (in the first week only 100 mg/m2 was given as a safety prerequisite). There was a sustained improvement in muscle strength and rash up to one year. Three patients with impaired pulmonary function improved their forced vital capacity. Side effects are usually mild and related to the infusion.73 Relapses do occur but only after the return of B cells.73,74 The optimal dose and re‐treatment schedules are still under study. Nevertheless, rituximab is a new therapeutic agent to be considered in refractory cases.4,74
Eculizumab (h5G1.1‐mAb) is a high affinity humanised monoclonal antibody to C5 that has the ability to inhibit the cleavage of the complement sequence C5 to C5a and C5b‐9, implicated in the pathogenesis of DM.4,75 It produced encouraging clinical effects on skin scores in a double blind, placebo controlled pilot study with 10 patients receiving 8 mg/week for five weeks and then every two weeks for two months.75
Anti‐T lymphocyte globulin (ATG) treatment in combination with MTX and PDN has been tried in patients with IBM in a controlled and randomised but unblinded, pilot study with 10 patients. The results showed a mild mean overall increase in muscle strength in the ATG group compared with MTX alone, with a slight decrease in serum CK levels and minimal biopsy changes.76
Other therapies
Plasmapheresis removes circulating immunocomplexes and antibodies and has been tried in patients with myositis but is of dubious benefit.3,20,77 It did not improve muscle strength or functional capacity in a double blind placebo controlled study,3,20,77,78 although in other reports79,80 showed some help when it was used in association with IVIG or immunosuppressive therapies, respectively in severe refractory cases of PM and DM. More recent investigations,20 comparing the use of plasma exchange with IVIG to IVIG alone in refractory cases of PM and DM (after treatment with corticosteroids and cyclosporine) showed that no additional benefit was achieved by adding plasmapheresis to IVIG.20 There is little justification for its use.
Autologous haematopoietic stem cell transplantation has been used as a rescue therapeutic option in the most severe cases of autoimmune disorders, but there are scant data about its use in myositis.81
There are some literature reports about the benefits of several other therapies such as whole body irradiation and thymectomy in severely affected patients.8 Total lymphoid irradiation has helped in a few patients2,82,83,84 but its long term side effects (increased risk of malignancy) restrict its use.3,85
Thymectomy and extracorporeal photochemotherapy for refractory PM and DM have been reported, but in a small number of cases and with dubious benefit.2
Exercise
The use of exercise has been controversial,4,86 particularly in patients with juvenile DM. However, concerns of increasing inflammation, contribution to calcinosis, and increasing CK levels have been allayed.4 Coordination of medical treatment with an appropriate physical program in PM/DM is effective.4,5,6,86,87 It helps to improve muscle strength and fatigue (better cardiovascular fitness with higher aerobic capacity and exercise tolerance), maintain adequate range of joint movement, prevent joint contractures (resulting from fibrotic healing of inflamed muscles), and prevent muscle atrophy. The eventual rise in the serum CK levels in a post‐exercise phase is transient, of no clinical relevance, and followed by a return to baseline levels.5
The training programmes should include isometric, isotonic, concentric, and eccentric (isokinetic) exercises,4,5,86 resisted and weight bearing exercises but should be adapted to the patient's condition and degree of muscle strength. A bedridden patient cannot perform an active resisted programme but may benefit from heat and massage before passive exercises, stretching muscles and tendons initially just to the point of mild discomfort.4 Patients should also be advised about preventing excessive weight gain.6
Key points
Corticosteroids are still the first line treatment approach
Use intravenous methylprednisolone pulses in severe clinical manifestations
Avoid long term use of corticosteroids
Consider immunosuppression if disease control is not achieved with corticosteroids alone and in rapidly progressive disease or internal organ involvement
Methotrexate is effective and is usually the first line immunosuppressive option
Cyclosporin A alone or in combination with methotrexate is a second line option treatment
Azathioprine can also be used as a first line approach
Intravenous immunoglobulin is considered for refractory cases and dysphagia
Cyclophosphamide is reserved for refractory cases, for patients with vasculitis, interstitial lung disease, and involvement of respiratory/bulbar muscles
Regimens using tacrolimus and mycophenolate mofetil have shown good results and await controlled trials
New approaches using cytokine modulation and monoclonal antibodies are promising treatment tools
Subsets of disease
Pulmonary disease
Weakness of respiratory muscles and ILD occurs in some patients with severe PM/DM. It is more common in patients with anti‐Jo1 antibodies,3,5 but in both cases respiratory involvement can be severe with alveolitis and adult respiratory distress syndrome. Dyspnoea if pronounced is a worrying sign.
ILD contributes to morbidity and mortality in inflammatory muscle diseases, thus its early recognition and rapid onset of adequate and aggressive immunosuppressants may help improving the patient's outcome.4,88 The response to treatment is in most cases unsatisfactory, worse in the patients with associated antisynthetase antibodies.8
Initial therapy includes corticosteroids in a daily dose of 0.5 to 0.75 mg/kg/day and if necessary intravenous methylprednisolone pulses (1 g for three consecutive days). Some patients will respond when CyC, AZA, or CyA is added4,8 and tacrolimus has also been reported to be effective. Recent data showed no consistent beneficial results with intravenous cyclophosphamide, showing it only to be inconstantly effective for reversing longstanding ILD, but nevertheless permitting stabilisation of functional tests in early limited ILD cases.56 There is some evidence that CyC may help in refractory cases of ILD.4
Autologous stem cell transplantation was tried and showed to be effective in two patients with PM with associated ILD and anti‐Jo1 antibodies.4,89,90
Cutaneous disease
The treatment of cutaneous manifestations of classic DM and amyopathic DM includes sunlight protection with broad sunscreens and anti‐inflammatories as initial management.69 There may be a different evolution between muscle and cutaneous manifestations, because cutaneous lesions can remain active and be unresponsive to antimalarials and immunosuppressive therapies despite improvement in the muscle.4,17,91
Hydroxychloroquine, an antimalarial drug, is usually the initial pharmacological agent chosen, given its safety profile, in daily doses ranging from 200 to 400 mg/day.4,17,69 Alternately chloroquine (250–500 mg per day) may be tried. Subsequently quinacrine 100 mg (per day or twice a day)17 and isotretionine 0.5–1 mg/kg/day can also be added.4 Topical corticosteroids can also be tried.64
MTX is usually effective as shown in two reviews25,35 with reduction in corticosteroid dose.2,36 Recently mycophenolate mofetil has provided promising results in recalcitrant skin lesions,1,16,21 controlling cutaneous activity, and decreasing the corticosteroid dose required.
IVIG may also ameliorate skin lesions,17,51 in some studies with doses even low as 0.1 mg/kg/day (five days) in previous unresponsive cases2 and in normal range doses in several patients with DM in a randomised controlled study.4,5,9,54
The use of topical tacrolimus has been reported and may be of value in patients with DM or ADM with previous unresponsive cutaneous lesions.4,61,64,92,93 The ointment 0.1% applied twice a day showed improvement of heliotrope erythema, Gottron papules, “mechanics hands”, and even in more “exuberant” poikilodermatous manifestations. A second generation agent, pimecrolimus, can also be used.4
There is a report of the use of dapsone in DM cutaneous lesions previously refractory to PDN, hydroxychloroquine, quinacrine, and other immnunosupressive agents such as MMF, MTX, and CyA, showing rapid improvement of skin lesions and their exacerbation with dapsone's withdrawal.64,91 Dapsone is a sulphur based antibiotic with anti‐inflammatory properties, particularly directed against leucocytes and complement activation.91 Its side effects are uncommon, usually minor, and dose related (gastrointestinal intolerance, haemolysis—minimised by concomitant administration of cimetidine). Severe rare side effects include aplastic anaemia, hypoalbuminaemia, exfoliative dermatitis, peripheral neuropathy, and allergic hypersensitivity syndrome.91
In juvenile dermatomyositis (JDM) skin care is of extreme importance, particularly in those presenting with calcinosis because of risk of ulcerations, fissures, and with higher risk of secondary infection and abscesses. Calcinosis is particularly common in JDM but its treatment remains unsatisfactory.17,94 Early and aggressive immunosuppression may help and the administration of intravenous methylprednisolone pulses may be useful.17,95 The other available drugs reported with some success include colchicine (0.6–1.2 mg daily),4 diltiazem (240–480 mg/day),4,96 pamidronate, and alendronate.97 Anedoctal beneficial effect was also reported with warfarin, probenecid, and aluminium hydroxide.4,17,94,98 There are also current attempts to control calcinosis with TNF blockage (infliximab).99
Other manifestations
Other internal organ involvement includes gastrointestinal disturbances, particularly dysphagia and vasculitis, the latest more frequent in JDM. The proximal pharyngeal weakness leading to dysphagia accounts for increased risk of aspiration. In these cases a feeding tube should be positioned as a preventive measure.1 The preferred drug for dysphagia in adults is IVIG,4,7,100 but in JDM the clinical approach usually consists in the increase of the DMARD dose.
Cardiovascular manifestations include conduction defects, myocarditis, and heart failure.3 In the presence of systemic vasculitis, immunosuppression is invariably required.33
Activity and damage assessment
As they are chronic conditions, idiopathic inflammatory muscle diseases, should ideally have reliable and validated measures to assess disease activity, implying ongoing inflammation but still reversible, and damage.10,11 Damage indicates irreversibility and reflects permanent changes in anatomy, physiology, pathology, or function resulting from prior active disease or complications of therapy and must be present for at least six months.10 These assessment tools would not only permit a clearer understanding of disease activity and severity, good enough, to support therapeutic decisions regarding immunosuppressive drugs, but also to standardise clinical trials and compare clinical outcomes.
The international study group IMACS (International Myositis and Clinical Studies Group) is undertaking efforts to reach consensus in this area. Achieving this aim is challenging because these diseases have heterogeneous manifestations with extramuscular features and children may also be affected, requiring special assessment measures.
Muscle strength on its own is not enough to assess activity because it does not discriminate between active myositis and disease damage (muscle atrophy, contractures) and does not correlate with extramuscular clinical manifestations.10 Furthermore, the level of serum CK does not always reflect disease activity and serum enzyme measurements are not fully validated.10,11 Some data suggest that serum lactate dehydrogenase (LDH) correlates best with global disease activity in patients with JDM.10 The LDH level in combination with one of other serum muscle enzyme (CK, aldolase, AST, ALT) predicts global disease activity as well as four serum muscle enzymes measured in combination in JDM.10 However, the CK level may be better for assessing adult onset muscle diseases, particularly PM.10,12,13,14
Nevertheless, muscle strength is an important tool in diagnosis and follow up of inflammatory muscle diseases,15 and therefore important to test. The methods used differ among clinical trials and have included manual muscle testing (MMT), measurement by a handheld pull gauge, sphygmomanometry, myometry, or electromyometry. The MMT in JDM has been validated10 and the childhood myositis assessment scale (CMAS) is a tool of muscle function, strength, and endurance with good validity with global disease activity, muscle strength, patient assessed physical function, and CK.10
Maximal isometric muscle strength is performed in a predefined position to avoid interference from gravitational forces and in a predefined angulation (90 degrees) between the part of the body assessed and the position of the equipment.15 The results of the assessment may be compared with the muscle group of the opposite side, with the centile curves of general population and with the results from previous assessments, helping to determine any clinical change.15
The evaluation of extraskeletal muscle disease, particularly articular, cardiac, and pulmonary manifestations is also important but lacks validated tools.10
Consensus about the assessment of disease activity confirms that several domains must be considerated, namely: (1) global disease activity, for which some use, patient/parent visual analogue scales (VAS), (2) muscle strength using MMT, (3) physical function assessed by HAQ/CHAQ (health assessment questionnaire/childhood health assessment questionnaire), (4) laboratory evaluation measuring at least two serum enzymes from CK, aldolase, LDH, AST, or ALT, and (5) extraskeletal muscle involvement.10
Extended set measures can be added to each of these five domains to achieve greater accuracy. These include tests like sphygmomanometry, dynamometry, pull gauge, myometry, maximum voluntary isometric contraction, timed tests, MRI (T2 weighted images), muscle biopsies, cutaneous assessment tool (rashes in patients with DM), periungueal nailfold capillary (capillary density correlates to skin and physician global activity), high resolution computed tomography, echocardiography, pulmonary function tests, and swallowing studies.10
The patient's own perception of their quality of life is also important, and may be assessed by the 36‐item short form (SF‐36).10
Disease damage remains difficult to assess and agreement and validation of a suitable index is awaited. The most probable tools to be used include physician global damage assessment, HAQ/CHAQ, VAS scales for the several organs involved, and a modification of SLICC/ACR (Systemic Lupus International Collaborative Clinics/American College of Rheumatology).10 The CHAQ has showed good validity with muscle strength and disease severity.10
An international consensus on disease activity and damage, partially validated, has just been published by the IMACS group.11 In assessing disease activity two indices were tested: (1) MITAX—myositis intention to treat index, which consists of a modification of the BILAG (British Isles Lupus Assessment Group) and is based on the principle of the physician's intention to treat and (2) MYOACT—myositis disease activity assessment VAS, by series of 10 cm VAS completed by the physician assessing the patient in the several systems that may be affected in myositis.11 Both showed good results but with limitation and further validation is awaited.11
For the assessment of damage a myositis damage index has been suggested. This index evaluates the extent and severity of damage in the different organs that might be affected using a modification of SLICC/ACR damage index. In addition the MYODAM index has been developed. In this index a myositis damage score, represented by series of 10 cm VAS is used to quantify the severity of damage in the various organs affected. However, formal validation and reliability studies are awaited.11
Despite the absence of completely validated measures there are already several tools to assess patients with inflammatory muscle diseases that are being used in clinical trials.
Conclusions
Continuous efforts are been undertaken to achieve the best possible treatment for patients with inflammatory myopathies, but more specific immunotherapy still awaits a precise understanding of target antigen molecules and the immunopathological process responsible for these disorders.5 However, the availability of new agents coupled with the imminent development of validated reliable assessment tools to discern activity and damage offers the realistic prospect of more effective treatment.
Abbreviations
CK - creatine kinase
AST - aspartate aminotransferase
ALT - alanine aminotransferase
LDH - lactate dehydrogenase
DM - dermatomyositis
PM - polymyositis
IVIG - intravenous immunoglobulin
PDN - prednisolone
MTX - methotrexate
AZA - azathioprine
CyA - cyclosporine A
IBM - inclusion body myositis
CyC - cyclophosphamide
IDL - interstitial lung disease
JDM - juvenile dermatomositis
Footnotes
Funding: none.
Conflicts of interest: none declared.
References
- 1.Isenberg D A, Ramsey‐Goldman R. Assessing patients with lupus: towards a drug responder index. Rheumatology 1999381045–1049. [DOI] [PubMed] [Google Scholar]
- 2.Choy E H S, Isenberg D A. Treatment of dermatomyositis and polymyositis. Rheumatology 2002417–13. [DOI] [PubMed] [Google Scholar]
- 3.Dalakas M. Polymyositis and dermatomyositis. Lancet 2003362971–982. [DOI] [PubMed] [Google Scholar]
- 4.Oddis C. Idiopathic inflammatory myopathies: a treatment update. Curr Rheumatol Rep 20035431–436. [DOI] [PubMed] [Google Scholar]
- 5.Mastaglia F, Garlepp M, Phillips B.et al Inflammatory myopathies: clinical, diagnostic and therapeutic aspects. Muscle Nerve 200327407–425. [DOI] [PubMed] [Google Scholar]
- 6.Amato A A, Grigs R C. Treatment of idiopathic inflammatory myopathies. Curr Opin Neurol 200316569–575. [DOI] [PubMed] [Google Scholar]
- 7.Kiely P, Heron C, Bruckner F. Presentation and management of idiopathic inflammatory muscle disease: four case reports and commentary from a series of 78 patients. Rheumatology 200342575–582. [DOI] [PubMed] [Google Scholar]
- 8.Mastaglia F, Zilko P. Inflammatory myopathies: how to treat the difficult cases. Journal of Clinical Neuroscience 20031099–101. [DOI] [PubMed] [Google Scholar]
- 9.Cherin P, Pelletier S, Teixeira A.et al Results and long‐term follow‐up of intravenous immunoglobulin infusions in chronic, refractory polymyositis. Arthritis Rheum 200246467–474. [DOI] [PubMed] [Google Scholar]
- 10.Miller F W, Rider L G, Chung Y‐L, for the International Myositis Outcome Assessment Collaborative Study Group et al Proposed preliminary core set measures for disease outcome assessment in adult and juvenile idiopathic inflammatory myopathies. Rheumatology 2001401262–1273. [DOI] [PubMed] [Google Scholar]
- 11.Isenberg D A, Allen E, Farewell V, for the International Myositis and Clinical Studies Group (IMACS) et al International consensus outcome measures for patients with idiopathic inflammatory myopathies. Development and initial validation of myositis activity and damage indices in patients with adult onset disease. Rheumatology 20044349–54. [DOI] [PubMed] [Google Scholar]
- 12.Rider L, Prasad K, Feldman B et al, for the JDM Disease Activity Collaborative Study Group Relationships among laboratory tests and global disease activity assessments in juvenile dermatomyositis. Arthritis Rheum 199639(suppl)S191. [DOI] [PubMed] [Google Scholar]
- 13.Rider L G, Miller F W. Laboratory evaluation of the inflammatory myopathies. Clin Diag Lab Immuol 199521–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guzman J, Petty R E, Malleson P N. Monitoring disease activity in juvenile dermatomyositis: the role of von Willebrand factor and muscle enzymes. J Rheumatol 199421739–743. [PubMed] [Google Scholar]
- 15.Stoll T.Isometric muscle strength measurement. Stuttgart: Thieme Flexibook, 2002136–137.
- 16.Schneider C, Gold R, Schäfers M.et al Mycophenolate mofetil in the therapy of polymyositis associated with a polyautoimmune syndrome. Muscle Nerve 200225286–288. [DOI] [PubMed] [Google Scholar]
- 17.Callen J P. Dermatomyositis. Lancet 200035553–57. [DOI] [PubMed] [Google Scholar]
- 18.Garton M J, Isenberg D A. Clinical features of lupus myositis versus idiopathic myositis: a review of 30 cases. Br J Rheumatol 1997361067–1074. [DOI] [PubMed] [Google Scholar]
- 19.Dalakas M C. Therapeutic approaches in patients with inflammatory myopathies. Semin Neurol 200323199–206. [DOI] [PubMed] [Google Scholar]
- 20.Danieli M, Malcangi G, Palmieri C.et al Cyclosporine A and intravenous immunoglobulin treatment in polymyositis/dermatomyositis. Ann Rheum Dis 20026137–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gelber A, Nousari H, Wigley F. Mycophenolate mofetil in treatment of severe skin manifestations of dermatomyositis: a series of 4 cases. J Rheumatol 2000271542–1545. [PubMed] [Google Scholar]
- 22.Dalakas M C. Current treatment of the inflammatory myopathies. Curr Opin Rheumatol 19946595–601. [DOI] [PubMed] [Google Scholar]
- 23.Hengstman G, van der Hoogen F, Barrera P.et al Successful treatment of dermatomyositis and polymyositis with anti‐tumor‐necrosis‐factor‐alpha: preliminary observations. Eur Neurol 20035010–15. [DOI] [PubMed] [Google Scholar]
- 24.DeVere R, Bradley W G. Polymyositis: its presentation, morbidity and mortality. Brain 197598637–666. [DOI] [PubMed] [Google Scholar]
- 25.Ansell B M. Management od polymyositis and dermatomyositis. Clin Rheum Dis 198410205–213. [PubMed] [Google Scholar]
- 26.Mastaglia F L, Phillips B A, Zilko P. Treatment of inflammatory myopathies. Muscle Nerve 199720651–654. [DOI] [PubMed] [Google Scholar]
- 27.Carpenter J R, Bunch T W, Engel A G.et al Survival in polymyositis: corticosteroids and risk factors. J Rheumatol 19774207–214. [PubMed] [Google Scholar]
- 28.Amato A A, Barohn R J. Idiopathic inflammatory myopathies. Neurol Clin 199715615–648. [DOI] [PubMed] [Google Scholar]
- 29.Joffe M M, Love L A, Leff Rl.et al Drug therapy of the idiopathic inflammatory myopathies: predictors of response to prednisolone, azathioprine and methotrexate and a comparison of their efficacy. Am J Med 199394379–387. [DOI] [PubMed] [Google Scholar]
- 30.Nzeusseu A, Brion F, Lefebvre C.et al Functional outcome of myositis patients can a low‐dose glucocorticoid regimen achieve good functional results? Clin Exp Rheumatol 199917441–446. [PubMed] [Google Scholar]
- 31.Villalba L, Adams E M. Update on therapy for refractory dermatomyositis and polymyositis. Curr Opin Rheumatol 19968544–551. [DOI] [PubMed] [Google Scholar]
- 32.Hirano F, Tanaka H, Nomura y.et al Sucessful treatment of refractory polymyositis with pulse intravenous cyclophosphamide and low‐dose weekly oral methotrexate therapy. Intern Med 199332749–752. [DOI] [PubMed] [Google Scholar]
- 33.Gold R, Dalakas M, Toyka K. Immunotherapy in autoimmune neuromuscular disorders. Lancet Neurology 2003222–32. [DOI] [PubMed] [Google Scholar]
- 34.Cagnoli M, marchesoni A, Tosi S. Combined steroid, methotrexate, and chlorambucil therapy for steroid resistant dermatomyositis. Clin Exp Rheumatol 19919658–659. [PubMed] [Google Scholar]
- 35.Kasteler J S, Callen J P. Low‐dose methotrexate administered weekly is an effective corticosteroid‐sparing agent for the treatment of the cutaneous manifestations of dermatomyositis. J Am Acad Dermatol 19973667–71. [DOI] [PubMed] [Google Scholar]
- 36.Zieglschmid‐Adams M E, Pandya A G, Cohen S B.et al Treatment of dermatomyositis with methotrexate. J Am Acad Dermatol 199532754–757. [DOI] [PubMed] [Google Scholar]
- 37.Metzger A L, Bohan A, Goldberg L S.et al Polymyositis and dermatomyositis: combined methotrexate and corticosteroid therapy. Ann Intern Med 197481182–189. [DOI] [PubMed] [Google Scholar]
- 38.Villalba L, Hicks J E, Adams E M.et al Treatment of refractory myositis: a randomised crossover study of two new cytotoxic regimens. Arthritis Rheum 199841392–399. [DOI] [PubMed] [Google Scholar]
- 39.Badrising U, Maat‐Schieman M, Ferrari M.et al Comparison of weakness progression in inclusion body myositis during treatment with methotrexate or placebo. Ann Neurol 200251369–372. [DOI] [PubMed] [Google Scholar]
- 40.Bunch T W, Worthington J W, Combs J J.et al Azathiprine with prednisolone for polymyositis. A controlled, clinical trial. Ann Intern Med 198092365–369. [DOI] [PubMed] [Google Scholar]
- 41.Bunch T W. Prednisolone and azathioprine for polymyositis: long‐term follow‐up. Arthritis Rheum 19812445–48. [DOI] [PubMed] [Google Scholar]
- 42.Tymms K E, Webb J. Dermato/polymyositis and other connective tissue diseases: a review of 105 cases. J Rheumatol 1985121140–1148. [PubMed] [Google Scholar]
- 43.Vencovsky J, Jarosova K, Machacek S.et al Cyclosporine A versus methotrexate in the treatment of polymyositis and dermatomyositis. Scand J Rheumatol 20002995–102. [DOI] [PubMed] [Google Scholar]
- 44.Reiff A, Rawlings D J, Shaham B.et al Preliminary evidence for cyclosporin A as an alternative in the treatment of recalcitant juvenile rheumatoid arthrirtis and juvenile dermatomyositis. J Rheumatol 1997242436–2443. [PubMed] [Google Scholar]
- 45.Maeda K, Kimura R, Komuta K.et al Cyclosporin treatment for polymyositis/dermatomyositis: is it possible to rescue the deteriorating cases with interstitial pneumonitis? Scand J Rheumatol 19972624–29. [DOI] [PubMed] [Google Scholar]
- 46.Saaded C, Bridges W, Burwick F. Dermatomyositis: remission induced with combined oral cyclosporin and high‐dose intravenous immune globulin. South Med J 199588866–870. [DOI] [PubMed] [Google Scholar]
- 47.Adams E M, Pucino F, Yarboro C.et al A pilot study: use of fludarabine for refractory dermatomyositis and polymyositis, and examination of endpoint measures. J Rheumatol 199926352–360. [PubMed] [Google Scholar]
- 48.Lueck C J, Trend P, Swash M. Cyclosporin in the management of polymyositis and dermatomyositis. J Neurol Neurosurg Psychiatry 1991541007–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Casato M, Bonomo L, Caccavo D.et al Clinical effects of cyclosporin in dermatomyositis. Clin Exp Dermatol 199015121–123. [DOI] [PubMed] [Google Scholar]
- 50.Kavanagh G M, Ross J S, Black M M. Dermatomyositis treated with cyclosporin. J R Soc Med 199184306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Braun‐Moscovici Y, Furst D. Immunoglobulin for rheumatic diseases in the twenty‐first century: take it or leave it? Curr Opin Rheumatol 200315237–245. [DOI] [PubMed] [Google Scholar]
- 52.Dalakas M C. High‐dose intravenous immunoglobulin in inflammatory myopathies: experience based on controlled clinical trials. Neurol Sci 200324(suppl 4)S256–S259. [DOI] [PubMed] [Google Scholar]
- 53.Cherin P, Piette J C, Wechsler B.et al Intravenous immunoglobulin as first line therapy in polymyositis and dermatomyositis: an open study in 11 adult patients. J Rheumatol 1994211092–1097. [PubMed] [Google Scholar]
- 54.Dalakas M C, Illa I, Dambrosia J M.et al A controlled trial of high dose intravenous immune globulin, infusions as treatment for dermatomyositis. N Engl J Med 19933291993–2000. [DOI] [PubMed] [Google Scholar]
- 55.Basta M, Dalakas M C. High‐dose intravenous immunoglobulin exerts its beneficial effect in patients with dermatomyositis by blocking endomysial deposition of activated complement fragments. J Clin Invest 1994941729–1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Meyer O, Hayem G, Palazzo E.et al Treatment of interstitial lung disease due to polimyositis with a 6 month course of intravenous pulse cyclophosphamide. Arthritis Rheum 200348(suppl)S308 [Google Scholar]
- 57.Cronin M E, Miller F W, Hicks J E.et al The failure of intravenous cyclophosphamide therapy in refractory idiopathic inflammatory myopathy. J Rheumatol 1989161225–1228. [PubMed] [Google Scholar]
- 58.De Vita S, Fossaluzza V. Treatment of idiopathic inflammatory myopathies with cyclophosphamide pulses: clinical experience and a review of literature. Acta Neurol Belg 199292215–227. [PubMed] [Google Scholar]
- 59.Kono D H, Klashman D J, Gilbert R C. Sucessful IV pulse cyclophosphamide in refractory PM in 3 patients with SLE. J Rheumatol 199017982–983. [PubMed] [Google Scholar]
- 60.Al‐Janadi M, Smith C D, Karsh J. Cyclophosphamide treatment of interstitial pulmonary fibrosis in polymyositis/dermatomyositis. J Rheumatol 1989161592–1596. [PubMed] [Google Scholar]
- 61.Nézondet‐Chetailee A ‐ L, Nigrovic P A, Woodward A L.et al Efficacy of tacrolimus ointment for the treatment of cutaneous manifestations of juvenile dermatomyositis. Arthritis Rheum 200246(suppl)S306 [Google Scholar]
- 62.Wilkes M, Sereika S, Fertig N.et al Treatment of antisynthetase associated interstitial lung disease with tacrolimus in patients with idiopathic inflammatory myopathy. Arthritis Rheum 200348(suppl)S434. [DOI] [PubMed] [Google Scholar]
- 63.Oddis C V, Sciurba F, Elmagd K.et al Tacrolimus in refractory polymyositis with interstitial lung disease. Lancet 19993531762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ueda M, Makinodan R, Matsmura M.et al Successful treatment of amyopathic dermatomyositis with topical tacrolimus. Br J Dermatol 2003148595–596. [DOI] [PubMed] [Google Scholar]
- 65.Chaudhry V, Cornblath D R, Griffin J W.et al Mycophenolate mofetil: a safe and promising immunosuppressant in neuromuscular diseases. Neurology 20015694–96. [DOI] [PubMed] [Google Scholar]
- 66.Majithia V, Harisdangkul V. Mycophenolate mofetil (CellCept): an alternative therapy for autoimmune inflammatory myopathy. Rheumatology 200544386–389. [DOI] [PubMed] [Google Scholar]
- 67.Sinoway P A, Callen J P. Chlorambucil: an effective corticosteroid‐sparing agent for patients with recalcitrant dermatomyositis. Arthritis Rheum 199336319–324. [DOI] [PubMed] [Google Scholar]
- 68.Wallace D J, Metzger A L, White K K. Combination immunosuppressive treatment of steroid‐resistant dermatomyisitis/polymyositis. Arthritis Rheum 198528590–592. [DOI] [PubMed] [Google Scholar]
- 69.Sontheimer R D. Dermatomyositis: an overview of recent progress with emphasis on dermatologic aspects. Dermat Clin 200220387–408. [DOI] [PubMed] [Google Scholar]
- 70.Miller M, Smith R, Abbott K.et al Use of etanercept in chronic juvenile dermatomyositis. Arthritis Rheum 200246(suppl)S306 [Google Scholar]
- 71.Edwards J C W, Cambridge G. Sustained improvement in rheumatoid arthritis following a protocol designed to deplete B lymphocytes. Rheumatology 200140205–211. [DOI] [PubMed] [Google Scholar]
- 72.Levine T D, Pestronk A. IgM antibody‐related polyneuropathies: B‐cell depletion chemotherapy using rituximab. Neurology 1999521701–1704. [DOI] [PubMed] [Google Scholar]
- 73.Leandro M J, Edwards J C, Cambridge G.et al An open study of b lymphocyte depletion in systemic lupus erythematosus. Arthritis Rheum 2002462673–2677. [DOI] [PubMed] [Google Scholar]
- 74.Levine T. Rituximab in the treatment of dermatomyositis—an open label pilot study. Arthritis Rheum 200552601–607. [DOI] [PubMed] [Google Scholar]
- 75.Takada K, Bookbinder S, Furie R.et al A pilot study of eculizumab in patients with dermatomyositis. Arthritis Rheum 200246(suppl)S489 [Google Scholar]
- 76.Lindberg C, Trysberg E, Tarkowski A.et al Anti‐T‐lymphocyte globulin treatment in inclusion body myositis—a randomised pilot study. Neurology 200361260–262. [DOI] [PubMed] [Google Scholar]
- 77.Fukunaga E, Kunishige M, Mitsui T.et al Severe dermatomyositis with rabdomyolisis and paralytic ileus: a case successful treated with plasmapheresis and intravenous immunoglobulin. Eur J Neurol 20029697–698. [DOI] [PubMed] [Google Scholar]
- 78.Miller F W, Leitman S F, Cronin M E.et al Controlled trial of plasma exchange and leukapheresis in polymyositis and dermatomyositis. N Engl J Med 19923261380–1384. [DOI] [PubMed] [Google Scholar]
- 79.Herson S, Cherin P, Coutellier A. The association of plasma exchange synchronized with intravenous gamma globulin therapy in severe intractable polymyositis. J Rheumatol 199219828–829. [PubMed] [Google Scholar]
- 80.Cherin P, Auperin I, Bussel A.et al Plasma exchange in polymyositis and dermatomyositis: a multicenter study of 57 cases. Clin Exp Rheumatol 199513270–271. [PubMed] [Google Scholar]
- 81.Farge D, Marjanovic Z, Henegar C.et al Autologous hematopoietic stem cells (hsc) transplantation in auto‐immune disease: extended results from French multicenter phaseI‐II study. Arthritis Rheum 200348(suppl)S563 [Google Scholar]
- 82.Engel W K, Lighter A S, Galdi A P. Polymyositis, remarkable response to total body irradiation. Lancet 1981i658. [DOI] [PubMed]
- 83.Kelly J J, Madoc‐Jones H, Adelman L S.et al Response to total body irradiation in dermatomyositis. Muscle Nerve 198811120–123. [DOI] [PubMed] [Google Scholar]
- 84.Morgan S H, Bernstein R M, Coppen J.et al Total body irradiation and the cause of polymyositis. Arthritis Rheum 198528831–835. [DOI] [PubMed] [Google Scholar]
- 85.Dalakas M C, Engel W K. Total body irradiation in the treatment of intractable polymyositis and dermatomyositis. In: Dalakas MC, ed. Polymyositis and dermatomyositis. Stoneham: Butterworth, 1988281–291.
- 86.Maillard S, Jones R, Owens C.et al Benefits of exercise in the management of juvenile dermatomyositis—a new approach to physiotherapy. Arthritis Rheum 200348(suppl)S433 [Google Scholar]
- 87.Dastmalchi M, Esbjornsson‐Liljedahl M, Alexanderson H.et al Improved muscle function and altered muscle quality in myositis patients following physical exercise program. Arthritis Rheum 200348(suppl)S312 [Google Scholar]
- 88.Azuma K, Yamasaki Y, Ogawa H.et al Immunosupprpressive agents for the treatment of interstitial pneumonitis associated with amyopathic dermatomyositis and polymyositis: prognostic factors of the clinical outcome. Arthritis Rheum 200348(suppl)S310 [Google Scholar]
- 89.Baron F, Ribbens C, Kaye O.et al Effective treatment of Jo‐1 associated polymyositis with T‐cell‐depleted autologous peripheral blood stem cell transplantation. Br J Haematol 2000110339–342. [DOI] [PubMed] [Google Scholar]
- 90.Bingham S, Griffiths B, McGonagle D.et al Autologous stem cell transplantation for rapidly progressive Jo‐1‐positive polymyositis and long‐term follow‐up. Br J Haematol 2001113840–841. [DOI] [PubMed] [Google Scholar]
- 91.Cohen J. Cutaneous involvement of dermatomyositis can respond to dapsone therapy. Int J Dermatol 200241182–184. [DOI] [PubMed] [Google Scholar]
- 92.Dawkins M A, Jorizzo J L, Walker F O.et al Dermatomyositis: a dermatology‐based case series. J Am Acad Dermatol 199838397–404. [DOI] [PubMed] [Google Scholar]
- 93.Yoshimasu T, Ohtani T, Sakamoto T.et al Topical FK506 (tacrolimus) therapy for facial erythematous lesions of cutaneous lupus erythematosus and dermatomyositis. Eur J Dermatol 20021250–52. [PubMed] [Google Scholar]
- 94.Ichiki Y, Akiyama T, Shimozawa N.et al An extremely severe case of cutaneus calcinosis with juvenile dermatomyositis, and sucessful treatment with diltiazem. Br J Dermatol 2001144894–897. [DOI] [PubMed] [Google Scholar]
- 95.Callen A M, Pachman L M, Hayford J.et al Intermitent high dose intravenous methylprednisolone (IV pulse) prevents calcinosis and shortens disease course in juvenile dermatomyositis (JDMS). (Abstract). Arthritis Rheum 199437R10 [Google Scholar]
- 96.Vinen C S, Patel S, Bruckner F E. Regression of calcinosis associated with adult dermatomyositis following diltiazem therapy. Rheumatology 200039333–334. [DOI] [PubMed] [Google Scholar]
- 97.Mukamel M, Horev G, Mimouni M. New insight into calcinosis of juvenile dermatomyositis: A study of composition and treatment. J Pediatr 2001138763–766. [DOI] [PubMed] [Google Scholar]
- 98.Harel L, Harel G, Konrenreich L.et al Treatment of calcinosis in juvenile dermatomyositis with probenecid: the role of phosphorus metabolism in the development of calcifications. J Rheumatol 2001281129–1132. [PubMed] [Google Scholar]
- 99.Maillard S, Wilkinson N, Riley P.et al The treatment of persistent severe idiopathic inflammatory myositis (IIM) with anti‐TNFα therapy. Arthritis Rheum 200246(suppl)S307 [Google Scholar]
- 100.Marie I, Hachulla E, Levesque H.et al Intravenous immunoglobulin as treatment of life threatening esophageal involvement in polymyositis and dermatomyositis. J Rheumatol 1999262706–2709. [PubMed] [Google Scholar]