Abstract
Limited data are available on the frequency of inflammatory bowel diseases in East European countries. A recent study from Hungary reported an increasing incidence rate for ulcerative colitis (from 1.6 to 11.0) and for Crohn's disease (from 0.4 to 4.7) from 1977 to 2001. A similar trend was seen in Croatia. In contrast, other countries (for example, Czech Republic, Poland, Romania, Slovakia, and Baltic countries) reported low incidence and prevalence rates. This review will discuss the available data on the epidemiology of inflammatory bowel diseases in Eastern Europe, as well as consider the possible factors responsible for the differences seen between countries and epidemiological trends.
Keywords: inflammatory bowel disease, ulcerative colitis, Crohn's disease, incidence, prevalence, epidemiology
The pathogenesis of ulcerative colitis (UC) and Crohn's disease (CD) has only been partly understood. Inflammatory bowel disease (IBD) is a multifactorial polygenic disease with probable genetic heterogeneity.1,2,3,4 Based on this hypothesis, the disease may develop in a genetically predisposed host as a consequence of disregulated immune response to environmental, in particular, enteric antigens, resulting in continuous immune mediated inflammation.
IBD represents an important public health problem, as it tends to afflict young people and have a protracted and relapsing clinical course, affecting education, working abilities, social life, and quality of life.
Several studies have been conducted on the epidemiology of IBD.5,6,7,8,9 The geographical incidence of IBD varies considerably; the highest incidence rates were reported in Northern and Western Europe as well as North America, whereas lower rates were recorded in Africa, South America, and Asia, including China.10 It is more common in developed, more industrialised countries, pointing at urbanisation as a risk factor. The incidence rate of UC varies greatly between 0.5 and 24.5/100 000 inhabitants, while that of CD varies between 0.1 and 11/100 000 inhabitants worldwide, with prevalence rates rising up to 396/100 000 inhabitants.7
Previous studies in Europe suggested that the incidence was decreasing from north to south,11,12,13,14 but in the early 1990s the European IBD Study Group found comparable rates between Southern and Northern Europe.15 This tendency may be explained by the relative stable incidence in previously high incidence areas, whereas in formerly low incidence areas the incidence continuously rose. A further difference is that the previously reported predominance of UC is diminishing, as CD is becoming more prevalent. The average reported incidence of UC in Western and Southern Europe is 10.4, whereas that of CD is 5.6. In contrast, a French group recently reported a 23% increase in the incidence of CD (from 5.2 to 6.4) along with a 17% decrease in the incidence of UC (from 4.2 to 3.5) between 1988 and 1999 in Northern France.16 Until recently, only few data were available on the epidemiology of IBD in East European countries. As Ekbom wrote: “The disappearance of the north‐south gradient in Europe might be an illustration of what will happen when society gains affluence. It is therefore of extreme interest to follow the temporal trends for IBD in Eastern Europe.”17
Hungary
This change is most evident in Hungary. Lakatos L et al18,19 published a population based epidemiology survey from Veszprem province (about 400 000 inhabitants). The study was retrospective from 1977 to 1985 with prospective data collection in the Veszprem Hospital only. From 1985 to 2001 (and onwards) data were collected prospectively. Simultaneously, an IBD registry was established in Veszprem and data were collected from the seven general hospitals in the province (internal medicine departments, surgery departments, paediatric departments, outpatient units) and family doctors. Most patients (76% of UC patients and 94% of CD patients) were monitored in the Csolnoky F Province Hospital in Veszprem. This hospital also serves as secondary referral centre for IBD patients in the province. A systematic search was performed; every centre was contacted at least two to three times a year. Diagnoses (based on hospitalisation records, outpatient visits, endoscopic, radiological, and histological evidence) generated in each hospital and outpatient unit were thoroughly reviewed, using the Lennard‐Jones criteria.20 During the observation period, 560 new patients with UC (M/F: 288/272, ratio: 1.058) and 212 new patients with CD (M/F: 108/104, ratio: 1.038) were diagnosed. The mean incidence rate for UC was 5.89 (95%CI: 2.15 to 9.63) cases per 100 000 inhabitants per year (men: 6.19, 95%CI: 2.30 to 10.08, women: 5.64, 95%CI: 2.39 to 8.89). In CD, the average 25 year incidence was 2.23/105 (95%CI: 0.5 to 3.96). Indeterminate colitis was diagnosed in 40 cases (M/F: 22/18); the mean incidence rate was 0.42 cases per 100 000 persons per year.
A sharp increase in incidence of UC was seen from 1.66/105 during 1977–81 to 11.01/105 during 1997–2001. In CD, a similar trend was seen (from 0.41/105 to 4.68/105). The ratio of UC/CD incidence rates decreased from 4.05 to 2.35 during the observed periods. The prevalence was high; for UC the prevalence was 142.6/105, while for CD 52.9/105 inhabitants at the end of 2001.
The mean (SD) age at diagnosis was 31.7 (12.8) years for CD, 38.9 (15.5) years for UC and 36.8 (13.9) years for IC. Only one peak incidence was seen. For UC the highest incidence rate was in 31–40 year olds, but the 21–30 year olds incidence rate was in the same range (10.96 and. 9.26). For CD the peak incidence was in the 21–30 year olds.
In UC, the proportion of extensive colitis was higher, while the percentage of proctitis cases was lower than those seen in the recent EC‐IBD study.15 The location of UC cases only slightly changed in the observed period. The percentage of pancolitis cases decreased, as procitis became more prevalent; however, the rates were still lower than those seen in the West European epidemiological studies.15
In CD, ileal disease was found in one third, and ileocolonic in 41.0% of the patients. Colonic involvement was seen in almost two thirds of patients, and there seemed to be a shift from ileal to colonic location during the observed period. The EC‐IBD study reported a higher frequency of ileocolonic disease while the percentage of ileal disease was lower.15
The retrospective study of Nagy et al21 reported similar trends: the average incidence has risen in UC from 3.1 and in CD from 0.43 in the years 1962–82 to 3.6 and 1.0 in 1982–92 in Borsod‐Abauj‐Zemplen county (about 770–800 000 inhabitants), respectively. Prevalence was not calculated by the authors, but based on the published data one could estimate a prevalence of about 100/105 of UC and about 20/105 for CD in 1992. In this study however, the epidemiology data were mainly based on inpatient hospital records. As Veszprem province is in west Hungary, while Borsod‐Abauj‐Zemplen county is in the east part of the country, a west‐east gradient can be suspected within the country, concomitant with a tendency for increasing IBD frequency, seen in both areas.
Croatia
In the early 1980s, Vucelic et al22,23 conducted a prospective study on the incidence of IBD in Zagreb between 1980 and 1989. The study was population based, including inpatient and outpatient data, as well as general practitioners' reports. Participating centres were contacted every two months. An area including 1 175 000 inhabitants was investigated. The authors reported an unchanged incidence rate of 1.5/105 inhabitants in UC and 0.7/105 in CD. The prevalence of UC was 21.4/105 and that of CD was 8.3/105 at the end of 1989.
In CD, only one peak incidence was seen in 15–30 year olds. In UC, the highest incidence rate was in 25–34 year olds, with a second peak detected in the 55–64 year olds.
A long term population based study was published on the epidemiology of CD covering the area of Istra and Rijeka (575 000 inhabitants).24 A total of 197 patients were diagnosed from 1973–1994. This gave an annual incidence of 0.34/105 in 1973 and 3.47/105 in 1994. The most frequent age groups affected were 15–25 and 50–60 years olds; 54% of the patients were younger than 30 years. The small bowel was involved in 49.7% of the patients, the large bowel in 23.3%, and both the small and large bowels in 25.8%.
A more recent retrospective study was conducted by Mijandrusic Sincic et al25 at the Adriatic Sea coastal area including 305 000 inhabitants from 1995 to 2001. Much higher incidence and prevalence rates were reported compared with the earlier study from Zagreb.22,23 The data were based however, on the records of a single hospital; both inpatient and outpatient data of the gastroenterology, surgery, paediatric, and infectious diseases departments were registered but no systematic search for other patients was conducted, thus incidence may have been underestimated. The average incidence of UC was 3.88/105 (with 5.97/105 in 2001), while that of CD was 3.92/105 (5.71/105 in 2001). The prevalence of the two diseases was also comparable: 53.9/105 for UC and 46.4/105 for CD. In a prospective follow up of the same area, using the same method, an age specific incidence for CD of 7.0 (100 patients) and 4.3 for UC (70 patients) for the period 2000–2004 was uncovered,26 supporting an increase in the incidence of both UC and CD in Croatia, a similar trend to that seen in Hungary.
Czech republic
The first report was published in 1967 by Nedbal and Mařatka.27 The incidence of UC in Czechoslovakia was estimated to be 1.4, while the prevalence of UC at 10.4. This was a hospital based single centre survey in Prague spanning five years. Data were summarised retrospectively. Both inpatient and outpatient data were included.
At the beginning of 1990s Bitter estimated the incidence of UC to be 3.1 and prevalence 39.2,28 based on the results of a prospective population based survey conducted in the northern part of Czech Republic covering an area of about one million inhabitants for 15 years (1975–90). The study was based only on data reported by gastroenterology centres.
Four years ago, Kolek et al29 published the results of a prospective population based survey conducted in the 1990s in Moravia, in children and adolescents (eastern part of Czech Republic, 232 000 children and adolescents from 1990 to 1994 and 180 000 from 1995 to 1999). Data were collected from three regional hospitals in the northern part of Moravia, including inpatient and outpatients. He found a dramatic rise in the incidence of UC (from 0.5 to 1.5, based on the detection of 5 and 15 patients) at the second part of the decade. The same trend was seen in adolescents with CD (from 0.3 to 1.5, equalling 1 and 14 patients).
The incidence of CD, in children under the age of 15 years, has risen from 0.11 to 0.91, while in adolescents (16–18 year old) increased from 0 to 3.83 when comparing periods 1990–1994 and 1995–1999. In UC, the incidence has risen in children under the age of 15 years from 0.55 to 1.43 and from 0 to 2.19 in 16–18 year old adolescents for the same period.
Poland
A single hospital based observational case series was published in 2005 by Wierska‐Drapalo et al.30 They reported diagnosing a total of 248 IBD patients between 1990 and 2003 covering an area of about 1 000 000 inhabitants in north east Poland. However, no incidence or prevalence rates were reported, none the less, based on the published data the average incidence of UC may be estimated at around 1.8/105 while that of CD at 0.1/105 (15 patients altogether). The peak incidence of UC was in the 20–40 year olds with a less apparent second peak in the 60–70 year olds. Most of the patients were diagnosed to have left sided colitis. The authors reported however that some other centres were also treating IBD patients; hence the number of the patients might have been underestimated.
Only case series were published previously. Data from four hospitals in north west Poland collected between 1965 and 1972 referred to 30 patients with UC and three with CD.31 Another study analysed 215 IBD patients in 1955–1970.32 Bartnik et al33 reported the experiences of the treatment of 406 UC patients between 1955 and 1970. However, these reports may not be considered as epidemiological investigations.
Romania
A single study, published in 2004, is available on the epidemiology of IBD in Romania. A nationwide epidemiological survey34 was conducted in Romania over a period of one year between June 2002 and June 2003. It was a multicentre study, in which 18 secondary and tertiary centres were included. The patient data were collected through a questionnaire from gastroenterology departments. It is not stated however, which area(s) these hospitals were referred from; the authors extrapolated the results as national data. During the study period, 163 incident UC cases and 85 incident CD cases were reported equalling an incidence of 0.97/105 and 0.50/105, respectively. Limitations of methodology are apparent in the detection of prevalent cases. The reported prevalence was 2.25/105 in UC and 1.51/105 in CD. Most patients had mild to moderate disease. The authors saw one peak onset in CD in the 21–40 year olds, while the distribution according to the age at onset was more balanced in UC.
Slovakia
Only a single report35 was published from Slovakia on the epidemiology, socioeconomic, and psychological factors associated with IBD. The prevalence of UC was reported to be 6.75/105 in a survey based on data of gastroenterology centres. A total of 357 patients were registered. Regional differences, expressed in a west‐east gradient, were seen (west Slovakia: 9.5/105, central: 5.3/105, east: 4.38/105). The authors also tried to analyse the socioeconomic and psychological status of CD patients by filling out a questionnaire; however, the response rate was too low (27.4%) to reach a definite conclusion.
Baltic countries
It is interesting to discuss the epidemiological data available from Baltic countries. Although they are located in Northern Europe close to Finland or Sweden, the incidence of IBD is showing pronounced differences compared with the high incidence in Scandinavian countries.12,15
Low incidence was reported in a population based prospective study from Estonia.36 The data were collected from 1993 to 1998 in Tartu country (151 301 inhabitants) from internal medicine, paediatrics, and surgery departments. A total of 16 UC and 13 CD patients were diagnosed, equalling an average incidence of 1.7/105 in UC and 1.4/105 in CD; however, the comparative small area and small absolute number of patients might have biased the data.
A previous retrospective study by Kull et al37 between 1973 and 1992 showed similar results for UC: the mean annual incidence of UC was 1.5/105. The reported incidence of CD was lower (0.27/105), which also supports a change in Estonia, at least in the frequency of CD. This is even more interesting as both studies were carried out in Tartu country.
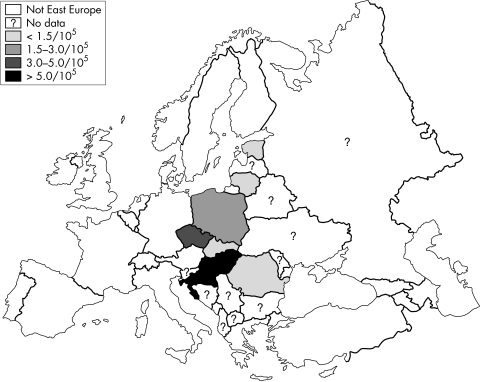
Only a case series was published from Lithuania.38 During a six year period, from 1995 to 2001, 273 IBD patients (218 UC and 55 CD) were hospitalised in Kaunas (about 400 000 inhabitants). Seventy five per cent of UC and 97% of CD patients were followed up, at least once per year. Accepting the limitations of extrapolating the results one may estimate the prevalence of UC at 30–40/105 and that of CD around 10/105. Figure 1 shows the incidence of IBD in Eastern Europe and table 1 the epidemiology of IBD in Eastern Europe and the Baltic countries.
Figure 1 Incidence of IBD in Eastern Europe.
Table 1 Epidemiology of IBD in Eastern European and Baltic countries.
| Observation period | Study design | UC | CD | |||
|---|---|---|---|---|---|---|
| incidence | prevalence | incidence | prevalence | |||
| Hungary | ||||||
| Lakatos et al18 | 1977–2001 | Population based, prospective | 5.9 (1.6 to 11.0) | 142.6 (2001) | 2.2 (0.4 to 4.7) | 52.9 (2001) |
| Nagy et al21 | 1962–1992 | Hospital based, retrospective | 3.1 to 3.6 | 0.4 to 1.0 | ||
| Croatia | ||||||
| Jovanovic24 | 1973–1994 | Population based, retrospective | 0.34 to 3.47 | |||
| Vucelic et al22,23 | 1980–1989 | Population based, prospective | 1.5 | 21.4 (1989) | 0.7 | 8.3 (1989) |
| Mijandrusic Sinic et al25 | 1995–2001 | Population based, retrospective | 3.9 to 5.9 | 53.9 (2001) | 3.9 to 5.7 | 46.4 (2001) |
| Czech Republic | ||||||
| Nedbal et al26 | 1960–1965 | Single centre, retrospective | 1.4 | 10.4 | ||
| Bitter et al27 | 1975–1990 | Population based, prospective | 3.1 | 39.2 | ||
| Kolek et al28 | 1990–1999 | Population based, prospective (children) | 0.5 to 1.5 | 0.3 to 1.5 | ||
| Poland | ||||||
| Wierska‐Drapalo et al29 | 1990–2003 | Single centre, retrospective | 1.8 | 0.1 | ||
| Romania | ||||||
| Gheorghe et al33 | 2002 | Hospital based, prospective | 0.97 | 2.4 | 0.5 | 1.5 |
| Slovakia | ||||||
| Prikazka et al34 | 1994 | Hospital based, prospective | 6.75 | |||
| Baltic countries | ||||||
| Salupere et al35 | 1993–1998 | Population based, prospective | 1.7 | 1.4 | ||
| Kull et al36 | 1973–1992 | Hospital based, retrospective | 1.5 | 0.3 | ||
How can we explain the differences between East European countries?
The incidence of IBD varies greatly worldwide. Genetic and environmental factors are assumed to play a significant part in the aetiology of the disease.1,2,3 The role of genetic factors is supported by ethnic and familial differences, as well as twin studies,4,39,40,41 while the differences in incidence rates among various geographical areas suggest a role for certain environmental factors. There has been an important change in the incidence of IBD in the past few decades. In high incidence countries in Western Europe, the incidence rate remained comparatively stable or even decreased,16 while in previously low incidence areas—as supported by Hungarian and Croatian data as well18,21,22,23,24,25—the disease has become more prevalent.
We have to note that until now the role of private practice was limited in Eastern European countries and most patients were managed in the public healthcare system, permitting population based epidemiological investigations. Moreover, because of Hungarian health authority regulations, a follow up visit is obligatory for IBD patients at a specialised gastroenterology centre every six months. Otherwise, the conditions of the health insurance policy change and they forfeit their ongoing subsidised treatment. Consequently, the relationship between IBD patients and specialists is a close one. Most patients are regularly seen at primary and secondary referral gastroenterology centres and, although a centralised database is not available, it is comparatively easy and reliable to construct a database based on inpatient and outpatient reports in any given area. The diligent collection of the data and case ascertainment is, of course, crucial.
The increase in incidence rates of both UC and CD seen in Hungary and Croatia raises further questions. What could be the cause of this change? In the 1970s and early 1980s the lower incidence rates could be partially explained by the use of fewer up to date diagnostic procedures (for example, the comparative low availability of selective enterography or colonoscopy). It is also possible that better awareness, either by physicians or by patients, may result in the diagnosis of mild cases that previously might have gone unnoticed. There has also been an important change in patients' behaviour in Hungary, as patients tend to seek medical advice more often and with milder symptoms than they did two decades ago.
It is important that in the past several years, because of various and multiple causes, the healthcare system is less funded in most of these countries, yet with continuously increasing costs. Only currently are we experiencing a reform from a public healthcare system to a more private one. In addition, since joining the European Union (EU) some of these countries also have to face a shortage of young doctors, because of better work conditions in the other EU member states. Thus, access to health care has not improved much. In addition, the role of improved diagnostic tools in the epidemiological changes apparent in East Europe may only be limited. The increase in the percentage of severe cases also strongly opposes a major role for better diagnostic means or better healthcare access.
Hungarian (the second Hungarian study was hospital based) and Croatian studies were population based, prospective or partially prospective, with extensive search for IBD cases. Through a review of the cases they also incorporated quality assurance. We believe that the increase in the incidence of IBD in the late 1980s and 1990s is real and not solely attributable to improved diagnosis or more extensive search. This notion is also supported by the increase in more severe cases, which can only be interpreted as real. Hungarian incidence rates of UC (11.01) and CD (4.68) in the 1997–2001 period and the reported 5.71 incidence rate for CD and 5.97 for UC in 2001 in Croatia were in the range as previously seen in high incidence Nordic countries.13,15,42,43 Furthermore, they were much higher than reported in Hungary two decades ago.21
In contrast, other countries (for example, Czech Republic, Poland, Romania, Slovakia) still reported low incidence rates. These studies however, had several limitations. The Czech data were mainly hospital based, both prospective and retrospective surveys.26,27,28 In contrast, although the data from Poland29 were prospective, they were based on the inpatient and outpatient records of a single hospital, extrapolating the results to the investigated area. Patients might have been unnoticed; thus the incidence rate is clearly underestimated.
The Romanian study33 was a one year long, multicentre survey, but there is no assurance that the hospitals reported data representative of the entire country, particularly because these were secondary and tertiary referral centres. A further limitation is that cases were reported by a voluntarily questionnaire, with the knowledge that the self report frequency of such questionnaires is limited. It may be assumed that the low prevalence reported in the same study is partly a consequence of a low reporting frequency. However, it may also be hypothesised that at present time, the prevalence of the disease in Romania is changing. It would be interesting to review follow up data of this work. The single study from Slovakia34 is also biased by methodological shortcomings. Again, similar to the study from Romania, gastroenterology centres were contacted and data were obtained from the centres by questionnaires, no systematic search was performed. It is worth mentioning that patients were contacted by mail after the database was established and the response rate of the patients was as low as 27.5%, which highlights the limitations of this methodology.
The situation is somewhat different in the Czech Republic and in the Baltic states. One of the Estonian papers was prospective;35 however, results might have been biased by the comparatively short study duration and the population size was also too small to reach a firm conclusion. Although the reported incidence in the Czech Republic26,27,28 and Baltic countries was similarly low, a tendency for an increasing frequency was also noted. In the Estonian studies, this difference may at least be partly explained by the difference in study design (prospective and retrospective).
The rapid increase in incidence rates in Hungary and Croatia supports a role for possible environmental factors.44 Diet, as a luminal antigen was thought to be an important factor in the pathogenesis of IBD.1,45 In the past two decades there has been a change in the lifestyle in Hungary and likewise in Croatia, as the standard way of living, including the diet, became more “Westernised”. This possibility is further supported by the differences in incidence and prevalence found within one country. In Hungary18,21 and Slovakia34 the prevalence was clearly different between the more “Western” type living in the western regions of the respective countries, in contrast with the less rapidly changing eastern parts. This raises the possibility that the prevalence of IBD in Romania and north east Poland is also capable of changing in the next one or two decades. The discrepancy between prevalence and incidence data in Romania33 and the change in the incidence of CD in Estonia,35,36 although minor, further support this hypothesis. Notwithstanding, this does not explain the high incidence seen at the Adriatic Sea coastal area,25 where the diet traditionally contains large amounts of fruit and fish.
Other possible environmental factors, such as perinatal events, infections in childhood or measles have not been investigated in any of the studies.43,46,47 Measles vaccination is however, universal in Hungary making the disease rare, as well as in Czech Republic, Croatia, and Poland. The birth rate in Hungary is also one of the lowest in Europe. Early childhood hygiene is also well developed, supporting a possible role for the “oversheltered child” theory.48 The hygiene hypothesis suggests that skewing of the Th1/Th2 balance in early life is an important cause for the recent increase in allergic and autoimmune diseases. However, as early high level of childhood hygiene has existed in most of these countries since the early 1970s, it does not explain the epidemiological trend seen in the late 1990s.
One of the most important environmental factors considered in the aetiology of IBD was smoking.1,49 In concordance with previous data in the study by Lakatos et al,18 smoking was identified as a protective factor in patients with UC (OR: 0.25). In contrast, smoking increased the risk for CD by almost twofold. It is even more interesting, taking into account the fact that smoking's prevalence has increased in Hungary, especially in young adults. The other Eastern European studies did not assess the role for possible risk factors.
In conclusion, an increasing amount of data are available on the epidemiology of IBD in East European countries. The quality of the surveys is however variable. Hungary and Croatia reported high incidence and prevalence rates in the past decade, comparable to that in West European countries. In contrast, IBD is still infrequent in other countries (for example, Czech Republic, Poland, Romania, Slovakia, and Baltic countries). The cause of the continuous and rapid increase in the incidence of IBD is unknown, but the evidence supports a possible role for environmental (for example, diet, lifestyle) factors.
Acknowledgements
The authors Dr Milan Lukas (Prague, Czech Republic) and Dr Limas Kupcinskas (Kaunas, Lithuania) for their help in the collection of national epidemiological data.
Abbreviations
IBD - inflammatory bowel disease
CD - Crohn's disease
UC - ulcerative colitis
Footnotes
Funding: none.
Conflicts of interest: none.
References
- 1.Podolsky D K. Inflammatory bowel disease. N Engl J Med 2002347417–428. [DOI] [PubMed] [Google Scholar]
- 2.Shanahan F. Crohn's disease. Lancet 200235962–69. [DOI] [PubMed] [Google Scholar]
- 3.Lakatos L, Lakatos P L. Etiopathgenesis of inflammatory bowel diseases (IBD). Orv Hetil 20031441853–1860. [PubMed] [Google Scholar]
- 4.Lakatos P L, Szalay F, Tulassay Z.et al Clinical presentation of Crohn's disease: Association between familial disease, smoking, disease phenotype, extraintestinal manifestations and need for surgery. Hepatogastroenterology 200552817–822. [PubMed] [Google Scholar]
- 5.Mayberry J F, Rhodes J, Newcombe R G. Crohn's disease in Wales, 1967–1976; an epidemiological survey based on hospital admissions. Postgrad Med J 198056336–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Delco F, Sonnenberg A. Commonalities in the time trends of Crohn's disease and ulcerative colitis. Am J Gastroenterol 1999942171–2176. [DOI] [PubMed] [Google Scholar]
- 7.Stone M A, Mayberry J F, Baker R. Prevalence and management of inflammatory bowel disease: a cross‐sectional study from central England. Eur J Gastroenterol Hepatol 2003151275–1280. [DOI] [PubMed] [Google Scholar]
- 8.Niv Y, Abuksis G, Fraser G M. Epidemiology of Crohn's disease in Israel: a survey of Israeli Kibbutz Settlements. Am J Gastroenterol 1999942961–2965. [DOI] [PubMed] [Google Scholar]
- 9.Probert C S J, Jayanthi V, Pnder D.et al Epidemiological study of ulcerative proctocolitis in Indian migrants and the indigenous population. Gut 199233687–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xia B, Shivananda S, Zhang G S.et al Inflammatory bowel disease in Hubei province of China. China Natl J New Gastroenterol 19973119–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tsianos E V, Masalas C N, Merkouropoulos M.et al Incidence of inflammatory bowel disease in North West Greece: rarity of Crohn's disease in an area where ulcerative colitis is common. Gut 199435369–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lennard‐Jones J E, Shivananda S. Clinical uniformity of inflammatory bowel disease at presentation and during first year of disease in the North and South Europe. Eur J Gastroenterol Hepatol 19979353–359. [DOI] [PubMed] [Google Scholar]
- 13.Stewenius J, Adnerhill I, Ekelund G.et al Ulcerative colitis and indeterminate colitis in the city of Malmö, Sweden. A 25‐year incidence study. Scand J Gastroenterol 19953038–43. [DOI] [PubMed] [Google Scholar]
- 14.Lapidus A, Bernell O, Hellers G.et al Incidence of Crohn's disease in Stockholm county 1955–89. Gut 199741480–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shivananda S, Lennard‐Jones J, Logan R.et al Incidence of inflammatory bowel disease across Europe: is there a difference between north and south? Results of the European collaborative study on inflammatory bowel disease (EC‐IBD). Gut 199639690–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Molinie F, Gower‐Rousseau C, Yzet T.et al Opposite evolution in incidence of Crohn's disease and ulcerative colitis in Northern France (1988–1999). Gut 200453843–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ekbom A. The changing faces of Crohn's disease and ulcerative colitis. In: Targan SR, Shanahan F, Karp LC, eds. Inflammatory bowel disease, from bench to bedside. Dordrecht: Kluwer Academic, 20035–21.
- 18.Lakatos L, Mester G, Erdelyi Z.et al Striking elevation in the incidence and prevalence of inflammatory bowel disease in a province of Western Hungary between 1977–2001. World J Gastroenterol 200410404–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lakatos L, Pandur T, David G.et al Association of extraintestinal manifestations of inflammatory bowel disease (IBD) in a province of Western Hungary with disease phenotype: results of a 25‐year follow‐up study. World J Gastroenterol 200392300–2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lennard‐Jones J E. Classification of inflammatory bowel disease. Scand J Gastroenterol 198924(suppl 170)2–6. [DOI] [PubMed] [Google Scholar]
- 21.Nagy G, Minik K, Ujszaszy L.et al Epidemiology of inflammatory bowel diseases in Borsod‐Abauj‐Zemplen county 1963–1992. LAM 19944424–430. [Google Scholar]
- 22.Vucelic B, Korac B, Sentic M.et al Ulcerative colitis in Zagreb, Yugoslavia: incidence and prevalence 1980–1989. Int J Epidemiol 1991201043–1047. [DOI] [PubMed] [Google Scholar]
- 23.Vucelic B, Korac B, Sentic M.et al Epidemiology of Crohn's disease in Zagreb, Yugoslavia: a ten year prospective study. Int J Epidemiol 199120216–220. [DOI] [PubMed] [Google Scholar]
- 24.Jovanovic Z. Epidemiology of Crohn's disease in the Rijeka‐Istra region. Lijec Vjesn 19991218–13. [PubMed] [Google Scholar]
- 25.Mijandrusic‐Sincic B, Vucelic B, Stimac D.et al The epidemiology of the inflammatory bowel diseases in Northern Costal county, Croatia. Falk symposium no 140, Dubrovnik (Croatia), 7–8 May 2004;No 48
- 26.Mijandrusic‐Sincic B, Vucelic B, Persic M.et al Incidence of inflammatory bowel disease in Primorsko‐Goranska county, Croatia, 2000–2004. Scand J Gastroenterol. (in press) [DOI] [PubMed]
- 27.Nedbal J, Mařatka Z. Colitis ulcerosa v Československu. Vnitřní Lékařtví 1967111054–1063. [PubMed] [Google Scholar]
- 28.Bitter J, Dyrhonová V, Komárková O.et al Nespecifické střevní záněty v České republice. Československá Gastroenterologie a Výľiva 199246313–321. [Google Scholar]
- 29.Kolek A, Janout V, Mathonová J. Increasing incidence of inflammatory bowel disease in childern and adolescents in six districts of Northen and Central Moravia (Czech Republic). Ceská a Slovenská Gastroenterologie a Hepatologie 200122173–177. [Google Scholar]
- 30.Wierska‐Drapalo A, Jaroszewicz J, Flisiak R.et al Epidemiological characteristics of inflammatory bowel disease in North‐Eastern Poland. World J Gastroenterol 2005112630–2633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marlicz K, Kopylow A, Cwajda H.et al Organic diseases of the large intestine in the patients of three clinics of internal diseases and the department of internal diseases of the City Hospital in Szczecin. Przegl Lek 197532452–456. [PubMed] [Google Scholar]
- 32.Ruzyllo E, Bartnik W, Bakowska Z. Ulcerative colitis‐epidemiological data and manifestations. Pol Arch Med Wewn 197249443–448. [PubMed] [Google Scholar]
- 33.Bartnik W, Regula J, Tomecki R.et al Results of the treatment of ulcerative colitis during the past 28 years. Pol Arch Med Wewn 198574340–345. [PubMed] [Google Scholar]
- 34.Gheorghe C, Pascu O, Gheorghe L.et al Epidemiology of inflammatory bowel disease in adults who refer to gastroenterology care in Romania: a multicenter study. Eur J Gastroenterol Hepatol 2004161153–1159. [DOI] [PubMed] [Google Scholar]
- 35.Prikazka M, Letkovicova M, Matejickova V. Crohns disease in Slovakia: prevalence, socioeconomic and psychological analysis. Eur J Epidemiol 19981449–53. [DOI] [PubMed] [Google Scholar]
- 36.Salupere R. Inflammatory bowel disease in Estonia: a prospective epidemiologic study 1993–1998. World J Gastroenterol 20017387–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kull K, Salupere R, Uibo R.et al Antineutrophil cytoplasmatic antibodies in Estonian pateints with inflammatory bowel disease: prevalence and diagnostic role. Hepatogastroenterology 1998452132–2137. [PubMed] [Google Scholar]
- 38.Zvirbliene A, Kiudelis G, Kupcinskas L. Retrospective analysis of case histories of patients with ulcerative colitis and Crohn's disease. Medicina(Kaunas) 200339745–750. [PubMed] [Google Scholar]
- 39.Loftus E V, Silverstein M D, Sandborn W J.et al Ulcerative colitis in Olmsted County, Minnesota, 1940–93: incidence, prevalence, and survival. Gut 200046336–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ahmad T, Tamboli C P, Jewell D.et al Clinical relevance of advances in genetics and pharmacogenetics of IBD. Gastroenterology 20041261533–1549. [DOI] [PubMed] [Google Scholar]
- 41.Zheng C Q, Hu G Z, Zeng Z S.et al Progress in searching for susceptibility gene for inflammatory bowel disease by positional cloning. World J Gastroenterol 200391646–1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Björnsson S, Johannsson J H. Inflammatory bowel disease in Iceland, 1990–1994: a prospective, nationwide, epidemiological study. Eur J Gastroenterol Hepatol 20001231–38. [DOI] [PubMed] [Google Scholar]
- 43.Lindberg E, Jarnerot G. The incidence of Crohn's disease is not decreasing in Sweden. Scand J Gastroenterol 199126495–500. [DOI] [PubMed] [Google Scholar]
- 44.Kugathasan S, Judd R H, Hoffmann R G.et al Epidemiologic and clinical characteristics of children with newly diagnosed inflammatory bowel disease in Wisconsin: a statewide population‐based study. J Pediatr 2003143525–531. [DOI] [PubMed] [Google Scholar]
- 45.Cashman K D, Shanahan F. Is nutrition an aetiological factor for inflammatory bowel disease? Eur J Gastroenterol Hepatol 200315607–613. [DOI] [PubMed] [Google Scholar]
- 46.Delco F, Sonnenberg A. Exposure to risk factors for ulcerative colitis occurs during an early period of life. Am J Gastroenterol 199994679–684. [DOI] [PubMed] [Google Scholar]
- 47.Elliman D A, Bedford H E. Measles, mumps and rubella vaccine, autism and inflammatory bowel disease: advising concerned parents. Paediatr Drugs 20024631–635. [DOI] [PubMed] [Google Scholar]
- 48.Gilat T, Hacohen D, Lilos P.et al Childhood factors in ucerative colitis and Crohn's disease. An international cooperative study. Scand J Gastroenterol 1987221009–1024. [DOI] [PubMed] [Google Scholar]
- 49.Picco M F, Bayless T M. Tobacco consumption and disease duration are associated with fistulizing and stricturing behaviors in the first 8 years of Crohn's disease. Am J Gastroenterol 200398363–368. [DOI] [PubMed] [Google Scholar]