Abstract
Non‐alcoholic fatty liver disease (NAFLD) is common and may progress to cirrhosis and its complications. The pathogenesis of steatosis and cellular injury is thought to be related mostly to insulin resistance and oxidative stress. Therefore, management entails identification and treatment of metabolic risk factors, improving insulin sensitivity, and increasing antioxidant defences in the liver. Weight loss and exercise improve insulin sensitivity. Bariatric surgery may improve liver histology in patients with morbid obesity. Insulin sensitising drugs showed promise in pilot trials as have a number of hepatoprotective agents. Further randomised, well controlled trials are required to determine the efficacy of these drugs.
Keywords: non‐alcoholic fatty liver disease, insulin resistance, treatment, weight loss
Non‐alcoholic fatty liver disease (NAFLD) occurs across all age groups and ethnicities and is recognised to occur in 14%–30% of the general population.1,2 Primary NAFLD is related to insulin resistance and thus frequently occurs as part of the metabolic changes that accompany obesity, diabetes, and hyperlipidaemia. However, it is important to exclude secondary causes of hepatic steatosis (table 1) by clinical assessment. Treatment of these conditions differs and revolves around correcting the underlying cause.3
Table 1 Causes of non‐alcoholic fatty liver disease.
| Primary | Obesity, glucose intolerance, hypertriglyceridaemia, low HDL cholesterol, hypertension |
| Nutritional | Protein‐calorie malnutrition, rapid weight loss, gastrointestinal bypass surgery, total parental nutrition |
| Drugs | Glucocorticoids, oestrogens, tamoxifen, amiodarone, methotrexate, diltiazem, zidovudine, valproate, aspirin, tetracycline, cocaine |
| Metabolic | Lipodystrophy, hypopituitarism, dysbetalipoproteinaemia, Weber‐Christian disease |
| Toxins | Amanita phalloides mushroom, phosphorus poisoning, petrochemicals, bacillus cereus toxin |
| Infections | Human immunodeficiency virus, hepatitis C, small bowel diverticulosis with bacterial overgrowth |
Pathogenesis
The pathogenesis of NAFLD is not fully understood, however the finding that not all patients with steatosis develop hepatic inflammation and hepatocellular damage has led to the hypothesis that different pathogenic factors lead firstly to hepatic steatosis and secondly to hepatic damage (“the second hit”).4 Accumulation of hepatic fat is closely linked to insulin resistance, which increases lipolysis of peripheral adipose tissue with resultant increased fat influx into the liver in the form of free fatty acids. Furthermore, insulin resistance promotes de novo triglyceride synthesis within the liver and inhibits fatty acid oxidation thereby promoting triglyceride accumulation.5 Therefore, improving insulin sensitivity has been a key strategy in the treatment of NAFLD.
It is unknown what “second hit” leads to the development of liver damage, although several factors have been implicated including oxidative stress, mitochondrial abnormalities, and hormonal disturbances involving leptin and adiponectin.6 In particular, oxidative stress with subsequent lipid peroxidation and generation of reactive oxygen species seems to be prominent in NAFLD and has been identified as a therapeutic target for antioxidants. Injury by secondary insults leads to the generation of pro‐inflammatory cytokines such as tumour necrosis factor α, which are targeted by hepatoprotective agents such as pentoxifylline. Hyperinsulinaemia and hyperglycaemia may also upregulate pro‐fibrogenic cytokines and thus provide a rational for insulin sensitising agents such as metformin and the thiozoladinediones to prevent progressive liver damage.7
Natural history
NAFLD exists as a histological spectrum of changes; simple steatosis refers to >5% hepatic steatosis in the absence of significant inflammation and hepatocellular damage whereas non‐alcoholic steatohepatitis (NASH) demonstrates inflammation and hepatocellular damage and sometimes fibrosis.8 NAFLD may be progressive resulting in cirrhosis that may be complicated by hepatocellular carcinoma and liver failure. Overall, about 5% of patients with NAFLD develop cirrhosis over an average of a seven year period with 1.7% dying from complications of liver cirrhosis.9 The high prevalence and chronic nature NAFLD subsequently translates to a significant health burden for the general community. In addition, subjects with a diagnosis of NAFLD have a higher risk of all cause mortality than the general population.9 This may be partly related to an increased risk of liver related death, but may also be related to death from vascular disease as a result of underlying metabolic abnormalities and insulin resistance. Thus treatment of patients with NAFLD should aim to identify and treat associated metabolic factors such as obesity, glucose intolerance, dyslipidaemia, and hypertension. Secondly, treatment aimed at preventing progressive liver injury should be offered to those considered to be at risk. Diabetes mellitus and obesity are risk factors for progressive hepatic fibrosis,10,11 and diabetes is also a risk factor for death in patients with NAFLD.9,12 Histological features also assist in stratifying patient risk of progressive liver disease. Simple steatosis is comparatively benign with a 0%–4% risk of developing cirrhosis over a one to two decade period.13,14,15 In contrast, 5%–8% of patients with NASH may develop cirrhosis over approximately five years.16,17,18 Assessment of fibrosis stage is also valuable in prognosticating risk of developing liver related morbidity, with patients with advanced fibrosis (bridging fibrosis and cirrhosis) at most risk. Although these features aid in stratifying patients at risk, a significant proportion of patients will have all of these adverse prognostic markers but will not develop liver related morbidity or mortality. Thus accurate prediction of those patients who will benefit most from treatment is difficult.
Diagnosis
The diagnosis of NAFLD requires confirmation of hepatic steatosis by imaging or liver biopsy with clinical exclusion of excessive (>20 g/day) alcohol ingestion.8 Ultrasound, computed tomography, or magnetic resonance studies can confirm the presence of hepatic steatosis with a comparatively high degree of accuracy.19 Ultrasound is comparatively cheap and readily available but is less sensitive at detecting minimal (<30%) steatosis or among obese patients.20 Thus a negative ultrasound does not necessarily exclude NAFLD. Liver biopsy is the gold standard for diagnosis and is the only investigation able to distinguish between simple steatosis and NASH or stage the degree of fibrosis.21 The decision to perform a liver biopsy must be individualised and may be useful when there is diagnostic uncertainty (for example, in the presence of raised iron parameters, auto‐antibodies, or suspected coexisting drug toxicity) or to provide prognositication regarding outcome. Liver biopsy may also be performed in patients with risk factors of advanced fibrosis (diabetes, obesity, age >45, AST:ALT>1)22 where a diagnosis of cirrhosis has implications for screening for varices and hepatocellular carcinoma.
Treatment
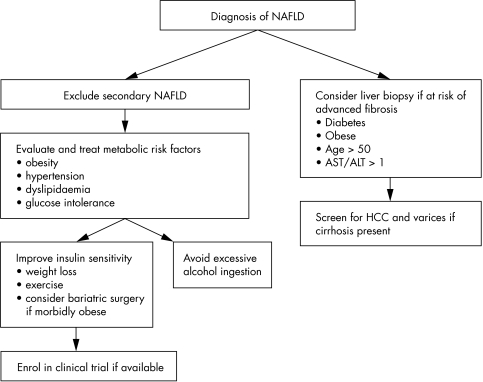
Treatment strategies for NAFLD have revolved around (1) identification and treatment of associated metabolic conditions such as diabetes and hyperlipidaemia; (2) improving insulin resistance by weight loss, exercise, or pharmacotherapy; (3) using hepato‐protective agents such as antioxidants to protect the liver from secondary insults (fig 1). Many agents have shown promising results in preliminary pilot trials, however, there have been few treatment modalities examined in rigorous randomised double blind placebo controlled trials with adequate statistical power. Furthermore, interpretation of trials using biochemical markers of liver injury (for example, hepatic aminotransaminases) as treatment end points needs to be done cautiously, particularly in the absence of a control group. The natural history of patients with NAFLD and raised aminotransaminases is characterised by improvement of aminotransferases regardless of whether hepatic fibrosis improves or worsens.10,11
Figure 1 Treatment algorithm for NAFLD.
Treatment of associated metabolic conditions
The metabolic syndrome and its features of central obesity (waist circumference ⩾94 cm for men, ⩾80 cm for women), glucose intolerance (fasting glucose ⩾6.10 mmol/l), hypertriglyceridaemia (>1.70 mmol/l), low HDL cholesterol (<1.30 mmol/l in women, <1.03 mmol/l in men), and hypertension (⩾135/80 mm Hg) are associated with cardiovascular morbidity and mortality.23,24 These features are commonly present in subjects with NAFLD, with 67%–71% being obese, 12%–37% having impaired fasting glycaemia, 57%–68% having disturbed lipid profiles, and 36%–70% being hypertensive.1,9,25 Therefore, patients with newly diagnosed NAFLD should be screened for these conditions and appropriate treatment instituted in an effort to ameliorate the vascular risk as well as to improve NAFLD.
Weight loss and exercise
Moderate amounts of weight loss as well as exercise are associated with improvement in insulin sensitivity and thus are logical treatment modalities for patients with NAFLD who are overweight or obese.26,27 Weight reduction may be achieved by caloric restriction from dieting, physical exercise, and/or pharmacotherapeutic agents as well as bariatric surgery in those patients with morbid obesity who are candidates for bariatric surgery.
Trials examining the effect of diet and exercise are non‐randomised, of short duration with limited numbers of participants (table 2).28,29,30,31,32 Liver biochemistry and hepatic steatosis seem to improve, however, improvement in hepatic inflammation and fibrosis has not been seen, although this may be attributable to the lack of statistical power and inadequate treatment duration. It should be noted however, that rapid weight loss induced by very low energy diet (388 kcal/day) is associated with increased portal inflammation and serum bilirubin levels and thus should be avoided.28 Energy restriction of about 25–30 kcal/kg/day seems reasonable with a target weight loss of about 10% of bodyweight over six months.30,32
Table 2 Effect of weight loss in non‐alcoholic fatty liver disease in adults.
| Author | Intervention | Number | Study Type | Comparison | Duration (months) | ALT | Histology | ||
|---|---|---|---|---|---|---|---|---|---|
| Steatosis | Inflammation | Fibrosis | |||||||
| Ueno32 | Diet/exercise | 25 | Open label, non‐randomised | No treatment | 3 | Improved | Improved | No change | No change |
| Huang29 | Diet/exercise | 23 | Pilot trial | Baseline | 12 | No change | No change | No change | No change |
| Andersen28 | Diet | 41 | Case series | Baseline | 6 | Improved | Improved | No change* | No change |
| Okita30 | Diet | 14 | Pilot trial | Baseline | 6 | Improved | NA | NA | NA |
| Palmer31 | Diet | 39 | Case series | Baseline | Improved | NA | NA | NA | |
| Harrison38 | Orlistat | 10 | Pilot trial | Baseline | 6 | Improved | No change | No change | No change |
| HatzitoliosA35 | Orlistat | 21 | Pilot trial | Baseline | 6 | Improved | NA | NA | NA |
| Sabuncu40 | Orlistat | 12 | Pilot trial | Baseline | 6 | Improved | Improved† | NA | NA |
| Sibutramine | 13 | Pilot trial | Baseline | 6 | Improved | Improved† | NA | NA | |
| Dixon41 | Gastric banding | 36 | Pilot trial | Baseline | 26 | Improved | Improved | Improved | Improved |
| Silverman44 | Gastro‐jejenostomy | 91 | Case series | Baseline | 18 | No change | Improved | No change | Improved |
| Luyckx43 | Gastric banding | 69 | Case series | Baseline | 27 | Improved | Improved | Worse | No change |
| Kral42 | Biliopancreatic diversion | 104 | Case series | Baseline | 74 | Improved | Improved | No change | Worse‡ |
| Clark JM92 | Reux‐en Y gastric bypass | 16 | Case series | Baseline | 10 | No change | Improved | Improved | Improved |
NA, not assessed. *No change in lobular inflammation, but significant worsening of portal inflammation; †as assessed by ultrasound; ‡significance level p = 0.053.
The optimal diet to treat NAFLD is not known. Patients with NAFLD seem more likely to have a diet high in saturated fats and cholesterol and low in fibre and antioxidants.33 Mono and poly‐unsaturated fats may potentially improve insulin resistance and may be beneficial in improving hepatic steatosis.34 One small pilot trial of 23 NAFLD patients with hypertriglyceridaemia noted improvement of ALT levels with omega‐3 fatty acid supplementation over six months, although effect on histology was not assessed.35 Most trials have used a diet similar to that recommended by the American Heart Association with energy restriction and energy intake composed of 40%–50% carbohydrates, 15%–20% protein, and 25%–40% predominately unsaturated fats.29,30,32 The effect of low (5%–10%) carbohydrate (Atkins diet) compared with standard (40%–60%) carbohydrate diet on NAFLD is unknown. Degree of weight loss is similar between diets after 12 months, although the low carbohydrate diet is associated with lower serum levels of triglyceride and higher HDL cholesterol levels.36,37 It should be noted that even under trial conditions and with frequent dietary assessments, compliance is poor with 30%–41% of participants dropping out emphasising the difficulty of maintaining weight loss through lifestyle change.29,36,37
In an effort to assist weight loss, various pharmacotherapeutic agents have been evaluated. Orlistat is a lipase inhibitor that reduces fat absorption and promotes weight loss. A small pilot study showed improvement in aminotransaminases with a mean 10 kg weight loss after six months of orlistat.38 A non‐significant reduction in steatosis was seen. Anorectic drugs such as fenfluramine and phentermine in addition to dietary and behavioural modifications were reported to improve aminotransaminase levels in 11 obese patients,39 but these drugs may induce cardiovascular and lung toxicity and they have been withdrawn from the market. More recently, sibutramine was associated with weight loss and improvement in alanine aminotransferase (ALT) and aspartate aminotransferase (AST) over six months in 13 patients.40 Histology was not assessed, however, regression of hepatic steatosis as determined by ultrasound, occurred in 11 patients. The same series found similar improvements in 12 patients who were assigned to orlistat.
Among morbidly obese patients, several observational studies have shown consistent improvement in aminotransaminase levels and degree of hepatic steatosis after bariatric surgery.41,42,43,44 The effect on hepatic inflammation and fibrosis has been more variable. Malabsorptive bariatric procedures such as biliopancreatic diversion and jejenoileal bypass are associated with an increase in hepatic fibrosis with cases of cirrhosis and liver related death reported after the latter procedure.42,45,46 In addition, rapid weight loss associated with gastric banding has been associated with an increase in lobular hepatitis, although this has not been a universal experience.43,47
Several studies have examined weight loss in obese children with NAFLD. Diet and exercise leading to roughly 500 g/week weight loss in nine children, led to improvement in aminotransaminases and hepatic steatosis as determined by ultrasound.48 Similarly, weight loss from diet (1200–1400 calories/day) and exercise (at least six hours/week) was evaluated in 33 obese children aged between 4 and 16 years.49 Weight loss was associated with normalisation of liver tests and improvement or normalisation of hepatic steatosis on ultrasound. Improvement in aminotransaminases has also been reported in a series of six children with NAFLD, with fluctuating liver enzymes reported in those unable to lose weight.50
In summary, the evidence of efficacy of diet and exercise in patients with NAFLD is surprisingly scant. However, as it is comparatively safe, inexpensive, and has other health benefits, it should remain the first line among patients with NAFLD and increased BMI. Patients with NAFLD and normal BMI still have some degree of insulin resistance; physical exercise in itself improves insulin resistance and thus NAFLD among subjects who are not obese or overweight based on BMI measurements. Some patients with normal BMI and NAFLD meet criteria for central obesity, and thus, waist circumference needs to be recorded in all patients with NAFLD regardless of BMI. Rapid weight loss attributable to very low calorie dieting or bariatric surgery risks exacerbation of liver injury and should be avoided. The risk/benefit of long term drugs for weight loss has not been clarified.
Insulin sensitising drugs
It is well established that insulin resistance is a common association with patients with NAFLD and plays an important part in lipid accumulation within the liver and perhaps its progression to NASH.7,51 In keeping with this, insulin resistance is predictive of the necroinflammatory form of NAFLD and conditions associated with insulin resistance such as obesity and diabetes are associated with the presence of advanced fibrosis among subjects with NASH.22,47 This had provided the impetus to trial insulin sensitising drugs such as metformin and the thiozoladinediones in NAFLD.
Metformin is a biguanide antihyperglycaemic agent whose mechanism of action is not well understood.52 In animal models of fatty liver, metformin improved hepatic steatosis, which was accompanied by down‐regulation of TNFα and lipid transcription factors.53 Several small pilot trials of four to six months' duration using doses of 1–1.5 g/day, have showed improvement in ALT levels compared with baseline (table 3).54,55,56 Interestingly, a longer pilot trial using up to 2 g/day found no difference in ALT levels after 12 months' treatment despite initial improvement at three months.57 Ten patients in this trial underwent biopsies at the end of treatment; improvement in steatosis was seen in one third of patients, inflammation in 20%, and fibrosis in 10%. A larger open label study from Italy randomised non‐diabetic subjects to 2 g/day metformin (n = 55), diet (n = 27), or 800 IU/day vitamin E (n = 28).58 Significantly more subjects taking metformin had normalisation of ALT levels compared with those taking vitamin E or diet treatment. Follow up liver biopsy was performed in 17 of the 55 subjects assigned to metformin therapy; significant improvements were seen in steatosis, inflammation, and fibrosis compared with baseline. Although encouraging, these results need to be reproduced in larger and well controlled clinical trials before assuming metformin is an effective and safe treatment for patients with NAFLD.
Table 3 Insulin sensitising agents in the treatment non‐alcoholic fatty liver disease.
| Author | Intervention | Number | Study type | Comparison | Duration (months) | ALT | Histology | ||
|---|---|---|---|---|---|---|---|---|---|
| Steatosis | Inflammation | Fibrosis | |||||||
| Magalotti55 | Metformin | 11 | Pilot trial | Baseline | 6 | Improved | NA | NA | NA |
| Schwimmer56 | Metformin | 10 | Pilot trial | Baseline | 6 | Improved | Improved* | NA | NA |
| Nair57 | Metformin | 15 | Pilot trial | Baseline | 12 | No change | No change | No change | No change |
| Marchesini54 | Metformin | 14 | Pilot trial | Baseline | 4 | Improved | NA | NA | NA |
| Bugianesis58 | Metformin | 110 | Open label, randomised | Vitamin E or diet | 12 | Improved | NA | NA | NA |
| Promrat67 | Pioglitazone | 18 | Pilot trial | Baseline | 11 | Improved | Improved | Improved | Improved |
| Bajaj93 | Pioglitazone | 11 | Pilot trial | Baseline | 4 | NA | Improved* | NA | NA |
| Shadid94 | Pioglitazone | 5 | Pilot trial | Baseline | 4 | Improved | NA | NA | NA |
| Neuschwander‐Tetri66 | Rosiglitazone | 30 | Pilot trial | Baseline | 11 | Improved | Improved | Improved | No change† |
| Sanyal68 | Pioglitazone + vitamin E | 20 | Open label, randomised | Vitamin E | 6 | No difference | No difference | Improved | No difference |
*Determined by magnetic resonance spectroscopy; †improvement seen in zone 3 fibrosis but not overall fibrosis score.
Lactic acidosis is a feared complication of metformin therapy, although it is rare and primarily seen among patients with renal or cardiac failure.59 However, the risk among patients with advanced liver fibrosis has not been well studied. In the few studies to date, 0%–7% of patients taking metformin therapy had increased lactate levels but not acidosis.54,56,57,58 Very few patients taking metformin had cirrhosis and thus it remains unclear whether it is safe to prescribe metformin in these patients.
The thiozoladinediones bind to the peroxisome proliferator activated receptor γ (PPAR) resulting in improved insulin sensitivity and redistribution of adipose tissue.60 In animal models, PPARγ agonists also have a protective effect against liver fibrosis by inhibiting activation of hepatic stellate cells.61,62 Troglitazone showed promising results in a pilot trial63 before being removed from the market because of idiosyncratic liver toxicity.64 The second generation “glitazones” rosiglitazone and pioglitazone are structurally different to troglitazone and seem to be safer.65
Two well designed pilot trials using pioglitazone (30 mg daily) and rosiglitazone (4 mg twice daily) showed improvement in ALT, hepatic steatosis, and features of hepatic inflammation compared with baseline.66,67 Pioglitazone but not rosiglitazone was associated with improvement in the overall fibrosis stage. A randomised trial of 20 non‐diabetic patients with NASH comparing pioglitazone (30 mg/day) plus vitamin E (400 IU/day) with vitamin E (400 IU/day) alone, found both groups improved hepatic steatosis grade compared with baseline, although the degree of improvement with pioglitazone was greater; features of hepatic inflammation also improved in the pioglitazone group compared with baseline.68 Comparing treatments at the end of the study however, found no difference in ALT, steatosis grade, or fibrosis stage between groups, although hepatic inflammation were significantly less in the pioglitazone group. Interpretation of these studies without a placebo group is difficult, as ALT levels, hepatic steatosis, and inflammation tend to improve over time as fibrosis progresses in NAFLD.10,11 Weight gain with fat redistribution from the central/truncal area to the lower body was the most common side effect occurring in 67%–72% of subjects taking pioglitazone or roziglitazone.66,67 Of concern, 1 of 30 subjects in the rosiglitazone trial and 1 of 10 patients taking pioglitazone were withdrawn because of hepatotoxicity.66,68 Although definitive cause‐effect was not proved, potential hepatotoxicity in the setting of liver disease remains a concern.
Antioxidants
Subjects with NAFLD exhibit increased levels of oxidative stress and lipid peroxidation that may play a part in disease progression.69,70 Vitamin E is a potent antioxidant and has been evaluated among paediatric and adult patients with NAFLD (table 4). Two small pilot trials have shown reduction of ALT levels among adult and paediatric patients with NASH. Subsequently, two small randomised controlled trials have failed to show any benefit of vitamin E on ALT levels; one study randomised 16 adult subjects to vitamin E (800 IU/day) or no treatment over three months71; the other trial consisted of 28 obese children taking vitamin E (400 mg/daily for two months, 100 mg/daily for three months) or placebo.72 In the only randomised study assessing histology, Harrison and colleagues randomised 45 patients to vitamins E (1000 IU/day) and C (1000 mg/day) or placebo for six months.73 Vitamin treatment significantly improved hepatic inflammation and fibrosis compared with baseline. However, the comparison of changes between placebo and vitamin E/C groups occurring with treatment at the end of the study showed no differences in ALT, hepatic inflammation or fibrosis. Recent evidence has also suggested that vitamin E supplementation may not be innocuous but may be associated with an increased risk of death and heart failure.74 Therefore in the absence of convincing evidence of benefit and the possible spectre of harm, vitamin E cannot be recommended for treatment of NAFLD outside of clinical trials.
Table 4 Hepatoprotective agents in the treatment of non‐alcoholic fatty liver disease.
| Author | Intervention | Number | Study type | Comparison | Duration (months) | ALT | Histology | ||
|---|---|---|---|---|---|---|---|---|---|
| Steatosis | Inflammation | Fibrosis | |||||||
| Laurin82 | UDCA | 24 | Pilot trial | Baseline | 12 | Improved | Improved | No change | No change |
| Kiyici81 | UCDA | 17 | Pilot trial | Baseline | 6 | Improved | NA | NA | NA |
| Vajro95 | UCDA | 31 | Open label, randomised | Diet | 6 | No difference | NA | NA | NA |
| Lindor83 | UDCA | 166 | Blinded RCT | Placebo | 24 | No difference | No difference | No difference | No difference |
| Abdelmalek79 | Betaine | 10 | Pilot trial | Baseline | 12 | Improved | Improved | No difference | Improved |
| Merat75 | Probucol | 17 | Pilot trial | Baseline | 6 | Improved | NA | NA | NA |
| Merat76 | Probucol | 30 | Blinded RCT | Placebo | 6 | Improved | NA | NA | NA |
| Adams78 | Pentoxifylline | 20 | Pilot trial | Baseline | 12 | Improved | NA | NA | NA |
| Satapathy77 | Pentoxifylline | 18 | Pilot trial | Baseline | 6 | Improved | NA | NA | NA |
| Loguercio86 | VSL no 3 | 22 | Pilot trial | Baseline | 3 | Improved | NA | NA | NA |
| Yokohama80 | Losartan | 7 | Pilot trial | Baseline | 11 | Improved | No change | No change | No change |
| Hasegawa96 | Vitamin E | 22 | Pilot trial | Baseline | 12 | Improved | No change | No change | No change |
| Kugelmas71 | Vitamin E + diet and exercise | 16 | Open label, randomised | Diet and exercise | 3 | No difference | NA | NA | NA |
| Vajro72 | Vitamin E | 28 | Blinded RCT | Placebo | 5 | No difference | No difference* | NA | NA |
| Harrison73 | Vitamin E + C | 45 | Blinded RCT | Placebo | 6 | No difference | NA | No difference | No difference† |
| Lavine97 | Vitamin E | 11 | Pilot trial | Baseline | 5 | Improved | NA | NA | NA |
“No change” if no statistically significant change reported. *Assessed by ultrasound; †fibrosis score improved compared with baseline but not in comparison with placebo at end of treatment.
Probucol is a lipid lowering antioxidant, which after showing promise in a pilot trial,75 improved ALT levels compared with placebo in a six month randomised trial.76 However, probucol is not universally available and has been withdrawn from Australia and the USA after concern regarding its pro‐arrhythmic potential.
Other hepato‐protective agents
A variety of hepato‐protective agents used in other liver disease have been evaluated in patients with NAFLD (table 5). Pentoxifylline inhibits TNFα and has been shown to improve short term survival in severe alcoholic hepatitis. Early pilot trials have shown improvement in aminotransaminases in NAFLD patients with 1200–1600 mg/daily of pentoxifylline.77,78 Similarly, betaine, a methyl donor that protects against hepatic lipid accumulation, lowered aminotransaminase levels and also improved steatosis, inflammation, and liver fibrosis in a pilot trial of 10 patients.79
Table 5 Lipid lowering drugs in the treatment of non‐alcoholic fatty liver disease.
| Author | Intervention | Number | Study type | Comparison | Duration (months) | ALT | Histology | ||
|---|---|---|---|---|---|---|---|---|---|
| Steatosis | Inflammation | Fibrosis | |||||||
| Rallidis88 | Pravistatin | 5 | Pilot trial | Baseline | 6 | Improved | No change | Improved | No change |
| Kiyici81 | Atorvastatin | 27 | Pilot trial | Baseline | 6 | Improved | NA | NA | NA |
| Hatzitolios35 | Atorvastatin | 28 | Pilot trial | Baseline | 6 | Improved | NA | NA | NA |
| Omega‐3 fatty acids | 23 | Pilot trial | Baseline | 6 | Improved | NA | NA | NA | |
| Laurin82 | Clofibrate | 16 | Pilot trial | Baseline | 12 | No change | No change | No change | No change |
| Basaranoglu89 | Gemfibrozil | 46 | RCT | Control group | 1 | Improved | NA | NA | NA |
Angiotensin II promotes insulin resistance and hepatic fibrosis in animal models. Losartan is an antagonist against the angiotensin II receptor that improved aminotransaminases, serum markers of fibrosis, and levels of profibrotic cytokine transforming growth factor β1 in a pilot trial of seven subjects with NASH.80 Significant histological improvement was not seen, although this may have been because of lack of power.
Ursodeoxycholic acid (UDCA) has anti‐inflammatory, immune modulating, and antiapoptotic properties and is widely used in chronic cholestatic liver diseases. After promising results from several pilot studies, a large randomised placebo controlled study recently found no effect on liver biochemistry or histology.81,82,83 In that study81 a significant improvement in the liver enzymes and degree of steatosis was found at two years of treatment as compared with baseline; this significant improvement in liver enzymes and steatosis was also seen in the placebo group. The improvement seen with UDCA treatment was not significantly better than that seen in the placebo group.81 Based on this study, UDCA is not recommended for the treatment of NAFLD.
Intestinal derived bacterial endotoxin seems to sensitise animal model fatty livers to the effects of TNFα with subsequent liver damage.84 Consequently, probiotics have been shown to ameliorate liver injury in these models.85 Administration of the probiotic VSL no 3 to 22 NAFLD patients over three months improved ALT levels as well as markers of lipid peroxidation.86 Effects on histology are unknown.
Lipid lowering drugs
As hypertriglyceridaemia and low HDL cholesterol levels are a manifestation of insulin resistance and common among subjects with NAFLD, several investigators have used lipid lowering drugs to treat NAFLD (table 5). The use of statin drugs is currently contraindicated in the presence of active liver disease or persistent unexplained increases of aminotransaminases. Recent evidence, however, shows that patients with raised liver enzymes may not be at increased risk of serious hepatotoxicity with standard doses of these drugs.87 Subsequently two small pilot trials have shown improvement of liver enzymes with atorvastatin.35,81 In addition, pravistatin 20 mg given for six months normalised liver enzymes and improved hepatic inflammation among five patients with NASH.88 Two small trials have also examined the fenofibrates; one 12 month trial of clofibrate 2 g/day showed no improvement in liver enzymes or histology,82 whereas gemfibrozil 600 mg/day improved ALT levels compared with no treatment over four weeks of treatment.89
Future directions
Increased understanding of the pathogenesis of NAFLD and particularly the factors responsible for progressive liver injury, will permit better targeting of therapeutic agents. Adiponectin is a hormone secreted by adipose tissue that has insulin sensitising as well as apparent hepatoprotective effects and thus may play a part in hepatic fat accumulation as well as liver injury in patients with NAFLD. Supplementation of adiponectin led to improvement in hepatic steatosis and ALT levels to animal models of NAFLD.90 Human studies have not been performed.
Agonists of PPARγ (thioglitazones) and PPARα (fibrates) act to improve insulin sensitivity and up‐regulate hepatic FFA oxidation thus decreasing hepatic steatosis. Both types of agonists have shown promising results in pilot trials in NAFLD. Combination dual PPAR γ and α agonists (muraglitazar, tesaglitazar) would therefore seem to be attractive candidates for treatment of NAFLD. Phase 2 clinical trials are currently underway examining the influence of these agents on cardiovascular risk factors.91
Conclusions
NAFLD is now acknowledged to be the commonest liver condition in the western world, largely because of the considerable increase in metabolic diseases such as obesity and diabetes. It is clear that NAFLD leads to liver related morbidity and mortality in a subset of people, particularly those who are obese, diabetic, and who have NASH. However, a better understanding of the natural history of NAFLD will permit better identification of at risk patients who should be targeted for long term and potentially expensive treatment.
Learning points
Metabolic risk factors for NAFLD
Central obesity (waist circumference ⩾94 cm for men, ⩾80 cm for women)
Impaired fasting glycaemia (⩾6.1 mmol/l)
Hypertriglyceridaemia (>1.70 mmol/l),
Low HDL‐cholesterol (<1.30 mmol/l in women, <1.03 mmol/l in men)
Hypertension (⩾135/⩾80 mm Hg)
Treatment recommendations for NAFLD
Exclude secondary causes of hepatic steatosis
Screen for metabolic risk factors
Avoid hepatotoxins such as alcohol excess
Regular exercise
Weight loss if centrally obese
Consider bariatric surgery if morbidly obese
Treatment of NAFLD should begin with screening and managing metabolic risk factors that may modify the risk of liver disease as well as non‐liver related disease such as ischaemic heart disease. First line treatment should consist of lifestyle change with weight loss and exercise to improve insulin sensitivity. However, because of long term compliance difficulties, pharmaceutical agents aimed at reducing insulin resistance or protecting the liver from additional insults are needed.
Many pilot trials have shown promising initial results in improving liver enzymes or features of liver histology. However, the efficacy of these agents still remains in question, and none of them can yet be recommended outside of clinical trials. Furthermore, the cost effectiveness of pharmacological therapy of NAFLD has to be defined. Some randomised, double blind, placebo controlled trials evaluating pioglitazone, metformin, vitamin E, betaine, and silymarin are currently in progress, in both adults and children. These trials will hopefully provide new therapeutic options for the clinician in the near future.
Multiple choice questions (true (T)/false (F); answers at end of references)
-
Treatment of nonalcoholic fatty liver disease (NALFD) should be aimed at preventing its progression to the following complications;
cirrhosis
hepatocellular carcinoma
liver failure
liver abscess
-
The following are adverse prognostic indicators among patients with NAFLD;
obesity
cirrhosis
raised aminotransaminases
diabetes
-
The following treatments should be routinely recommended for patients with NAFLD;
weight loss if obese
exercise
reduction of excessive alcohol ingestion
thiozoladinediones
-
With regard to weight loss in obese patients with NAFLD:
it should be gradual and medically supervised
total starvation or very low energy diets are safe and effective
it is difficult to achieve and maintain by most obese patients
the available antiobesity drugs have shown to prevent progression to cirrhosis
-
The following drugs have been conclusively shown to improve liver histology among patients with NAFLD;
metformin
pioglitazone
vitamin E
ursodeoxycholic acid
Abbreviations
NAFLD - non‐alcoholic fatty liver disease
ALT - alanine aminotransferase
AST - aspartate aminotransferase
NASH - non‐alcoholic steatohepatitis
Answers
1. (A) T, (B) T, (C) T, (D) F; 2. (A) T, (B) T, (C) F, (D) T; 3. (A) T, (B) T, (C) T, (D) F; 4. (A) T, (B) F, (C) T, (D) F; 5. (A) F, (B) F, (C) F, (D) F.
Footnotes
Funding: LA is sponsored by a Postgraduate Medical Research Scholarship (no 353710) from the National Health and Medical Research Council as well as by the Athelstan and Amy Saw Postgraduate Medical Scholarship from The University of Western Australia.
Conflicts of interest: none declared.
References
- 1.Browning J, Szczepaniak L, Dobbins R.et al Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology 2004401387–1395. [DOI] [PubMed] [Google Scholar]
- 2.Nomura H, Kashiwagi S, Hayashi J.et al Prevalence of fatty liver in a general population of Okinawa, Japan. Jpn J Med 198827142–149. [DOI] [PubMed] [Google Scholar]
- 3.Angulo P. Nonalcoholic fatty liver disease. N Engl J Med 20023461221–1231. [DOI] [PubMed] [Google Scholar]
- 4.Day C P, James O F. Steatohepatitis: a tale of two “hits”? Gastroenterology 1998114842–845. [DOI] [PubMed] [Google Scholar]
- 5.Browning J D, Horton J D. Molecular mediators of hepatic steatosis and liver injury. J Clin Invest 2004114147–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haque M, Sanyal A J. The metabolic abnormalities associated with non‐alcoholic fatty liver disease. Best Pract Res Clin Gastroenterol 200216709–731. [DOI] [PubMed] [Google Scholar]
- 7.Paradis V, Perlemuter G, Bonvoust F.et al High glucose and hyperinsulinemia stimulate connective tissue growth factor expression: a potential mechanism involved in progression to fibrosis in nonalcoholic steatohepatitis. Hepatology 200134738–744. [DOI] [PubMed] [Google Scholar]
- 8.Neuschwander‐Tetri B A, Caldwell S H. Nonalcoholic steatohepatitis: summary of an AASLD single topic conference. Hepatology 2003371202–1219. [DOI] [PubMed] [Google Scholar]
- 9.Adams L A, Lymp J, St Sauver J.et al The natural history of nonalcoholic fatty liver disease: a population based cohort study. Gastroenterology 2005129113–121. [DOI] [PubMed] [Google Scholar]
- 10.Fassio E, Alvarez E, Dominguez N.et al Natural history of nonalcoholic steatohepatitis: a longitudinal study of repeat liver biopsies. Hepatology 200440820–826. [DOI] [PubMed] [Google Scholar]
- 11.Adams L A, Sanderson S, Lindor K D.et al The histological course of nonalcoholic fatty liver disease: a longitudinal study of 103 patients with sequential liver biopsies. J Hepatol 200542132–138. [DOI] [PubMed] [Google Scholar]
- 12.Younossi Z M, Gramlich T, Matteoni C A.et al Nonalcoholic fatty liver disease in patients with type 2 diabetes. Clin Gastroenterol Hepatol 20042262–265. [DOI] [PubMed] [Google Scholar]
- 13.Matteoni C A, Younossi Z M, Gramlich T.et al Nonalcoholic fatty liver disease: a spectrum of clinical and pathological severity. Gastroenterology 19991161413–1419. [DOI] [PubMed] [Google Scholar]
- 14.Dam‐Larsen S, Franzmann M, Andersen I B.et al Long term prognosis of fatty liver: risk of chronic liver disease and death. Gut 200453750–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Teli M R, James O F, Burt A D.et al The natural history of nonalcoholic fatty liver: a follow‐up study. Hepatology 1995221714–1719. [PubMed] [Google Scholar]
- 16.Lee R G. Nonalcoholic steatohepatitis: a study of 49 patients. Hum Pathol 198920594–598. [DOI] [PubMed] [Google Scholar]
- 17.Cortez‐Pinto H, Baptista A, Camilo M E.et al Nonalcoholic steatohepatitis‐‐a long‐term follow‐up study: comparison with alcoholic hepatitis in ambulatory and hospitalized patients. Dig Dis Sci 2003481909–1913. [DOI] [PubMed] [Google Scholar]
- 18.Powell E E, Cooksley W G, Hanson R.et al The natural history of nonalcoholic steatohepatitis: a follow‐up study of forty‐two patients for up to 21 years. Hepatology 19901174–80. [DOI] [PubMed] [Google Scholar]
- 19.Joy D, Thava V R, Scott B B. Diagnosis of fatty liver disease: is biopsy necessary? Eur J Gastroenterol Hepatol 200315539–543. [DOI] [PubMed] [Google Scholar]
- 20.Mottin C C, Moretto M, Padoin A V.et al The role of ultrasound in the diagnosis of hepatic steatosis in morbidly obese patients. Obes Surg 200414635–637. [DOI] [PubMed] [Google Scholar]
- 21.Saadeh S, Younossi Z M, Remer E M.et al The utility of radiological imaging in nonalcoholic fatty liver disease. Gastroenterology 2002123745–750. [DOI] [PubMed] [Google Scholar]
- 22.Angulo P, Keach J C, Batts K P.et al Independent predictors of liver fibrosis in patients with nonalcoholic steatohepatitis. Hepatology 1999301356–1362. [DOI] [PubMed] [Google Scholar]
- 23.Hunt K J, Resendez R G, Williams K.et al National Cholesterol Education Program versus World Health Organization metabolic syndrome in relation to all‐cause and cardiovascular mortality in the San Antonio heart study. Circulation 20041101251–1257. [DOI] [PubMed] [Google Scholar]
- 24.Malik S, Wong N D, Franklin S S.et al Impact of the metabolic syndrome on mortality from coronary heart disease, cardiovascular disease, and all causes in United States adults. Circulation 20041101245–1250. [DOI] [PubMed] [Google Scholar]
- 25.Fan J G, Zhu G, Li X J.et al Prevalence of and risk factors for fatty liver in a general population of Shanghai China. J Hepatol 200543508–513. [DOI] [PubMed] [Google Scholar]
- 26.Petersen K F, Dufour S, Befroy D.et al Reversal of nonalcoholic hepatic steatosis, hepatic insulin resistance, and hyperglycemia by moderate weight reduction in patients with type 2 diabetes. Diabetes 200554603–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Houmard J A, Tanner C J, Slentz C A.et al Effect of the volume and intensity of exercise training on insulin sensitivity. J Appl Physiol 200496101–106. [DOI] [PubMed] [Google Scholar]
- 28.Andersen T, Gluud C, Franzmann M B.et al Hepatic effects of dietary weight loss in morbidly obese subjects. J Hepatol 199112224–229. [DOI] [PubMed] [Google Scholar]
- 29.Huang M A, Greenson J K, Chao C.et al One‐year intense nutritional counseling results in histological improvement in patients with non‐alcoholic steatohepatitis: a pilot study. Am J Gastroenterol 20051001072–1081. [DOI] [PubMed] [Google Scholar]
- 30.Okita M, Hayashi M, Sasagawa T.et al Effect of a moderately energy‐restricted diet on obese patients with fatty liver. Nutrition 200117542–547. [DOI] [PubMed] [Google Scholar]
- 31.Palmer M, Schaffner F. Effect of weight reduction on hepatic abnormalities in overweight patients. Gastroenterology 1990991408–1413. [DOI] [PubMed] [Google Scholar]
- 32.Ueno T, Sugawara H, Sujaku K.et al Therapeutic effects of restricted diet and exercise in obese patients with fatty liver. J Hepatol 199727103–107. [DOI] [PubMed] [Google Scholar]
- 33.Musso G, Gambino R, De Michieli F.et al Dietary habits and their relations to insulin resistance and postprandial lipemia in nonalcoholic steatohepatitis. Hepatology 200337909–916. [DOI] [PubMed] [Google Scholar]
- 34.Fernandez M I, Torres M I, Gil A.et al Steatosis and collagen content in experimental liver cirrhosis are affected by dietary monounsaturated and polyunsaturated fatty acids. Scand J Gastroenterol 199732350–356. [DOI] [PubMed] [Google Scholar]
- 35.Hatzitolios A, Savopoulos C, Lazaraki G.et al Efficacy of omega‐3 fatty acids, atorvastatin and orlistat in non‐alcoholic fatty liver disease with dyslipidemia. Indian J Gastroenterol 200423131–134. [PubMed] [Google Scholar]
- 36.Foster G D, Wyatt H R, Hill J O.et al A randomized trial of a low‐carbohydrate diet for obesity. N Engl J Med 20033482082–2090. [DOI] [PubMed] [Google Scholar]
- 37.Stern L, Iqbal N, Seshadri P.et al The effects of low‐carbohydrate versus conventional weight loss diets in severely obese adults: one‐year follow‐up of a randomized trial. Ann Intern Med 2004140778–785. [DOI] [PubMed] [Google Scholar]
- 38.Harrison S A, Fincke C, Helinski D.et al A pilot study of orlistat treatment in obese, non‐alcoholic steatohepatitis patients. Aliment Pharmacol Ther 200420623–628. [DOI] [PubMed] [Google Scholar]
- 39.Kazi N, DeMeo M, Mikolaitis S. Effects of weight loss on abnormal liver function test (LFT) in obese patients. FASEB J 1997A6003466 [Google Scholar]
- 40.Sabuncu T, Nazligul Y, Karaoglanoglu M.et al The effects of sibutramine and orlistat on the ultrasonographic findings, insulin resistance and liver enzyme levels in obese patients with non‐alcoholic steatohepatitis. Rom J Gastroenterol 200312189–192. [PubMed] [Google Scholar]
- 41.Dixon J B, Bhathal P S, Hughes N R.et al Nonalcoholic fatty liver disease: Improvement in liver histological analysis with weight loss. Hepatology 2004391647–1654. [DOI] [PubMed] [Google Scholar]
- 42.Kral J G, Thung S N, Biron S.et al Effects of surgical treatment of the metabolic syndrome on liver fibrosis and cirrhosis. Surgery 200413548–58. [DOI] [PubMed] [Google Scholar]
- 43.Luyckx F H, Desaive C, Thiry A.et al Liver abnormalities in severely obese subjects: effect of drastic weight loss after gastroplasty. Int J Obes Relat Metab Disord 199822222–226. [DOI] [PubMed] [Google Scholar]
- 44.Silverman E M, Sapala J A, Appelman H D. Regression of hepatic steatosis in morbidly obese persons after gastric bypass. Am J Clin Pathol 199510423–31. [DOI] [PubMed] [Google Scholar]
- 45.Campbell J M, Hunt T K, Karam J H.et al Jejunoileal bypass as a treatment of morbid obesity. Arch Intern Med 1977137602–610. [PubMed] [Google Scholar]
- 46.Marubbio A T, Jr, Buchwald H, Schwartz M Z.et al Hepatic lesions of central pericellular fibrosis in morbid obesity, and after jejunoileal bypass. Am J Clin Pathol 197666684–691. [DOI] [PubMed] [Google Scholar]
- 47.Dixon J B, Bhathal P S, O'Brien P E. Nonalcoholic fatty liver disease: predictors of nonalcoholic steatohepatitis and liver fibrosis in the severely obese. Gastroenterology 200112191–100. [DOI] [PubMed] [Google Scholar]
- 48.Vajro P, Fontanella A, Perna C.et al Persistent hyperaminotransferasemia resolving after weight reduction in obese children. J Pediatr 1994125239–241. [DOI] [PubMed] [Google Scholar]
- 49.Franzese A, Vajro P, Argenziano A.et al Liver involvement in obese children. Ultrasonography and liver enzyme levels at diagnosis and during follow‐up in an Italian population. Dig Dis Sci 1997421428–1432. [DOI] [PubMed] [Google Scholar]
- 50.Rashid M, Roberts E A. Nonalcoholic steatohepatitis in children. J Pediatr Gastroenterol Nutr 20003048–53. [DOI] [PubMed] [Google Scholar]
- 51.Marchesini G, Brizi M, Morselli‐Labate A M.et al Association of nonalcoholic fatty liver disease with insulin resistance. Am J Med 1999107450–455. [DOI] [PubMed] [Google Scholar]
- 52.Hundal R S, Inzucchi S E. Metformin: new understandings, new uses. Drugs 2003631879–1894. [DOI] [PubMed] [Google Scholar]
- 53.Lin H Z, Yang S Q, Chuckaree C.et al Metformin reverses fatty liver disease in obese, leptin‐deficient mice. Nat Med 20006998–1003. [DOI] [PubMed] [Google Scholar]
- 54.Marchesini G, Brizi M, Bianchi G.et al Metformin in non‐alcoholic steatohepatitis. Lancet 2001358893–894. [DOI] [PubMed] [Google Scholar]
- 55.Magalotti D, Marchesini G, Ramilli S.et al Splanchnic haemodynamics in non‐alcoholic fatty liver disease: effect of a dietary/pharmacological treatment. A pilot study. Dig Liver Dis 200436406–411. [DOI] [PubMed] [Google Scholar]
- 56.Schwimmer J B, Middleton M S, Deutsch R.et al A phase 2 clinical trial of metformin as a treatment for non‐diabetic paediatric non‐alcoholic steatohepatitis. Aliment Pharmacol Ther 200521871–879. [DOI] [PubMed] [Google Scholar]
- 57.Nair S, Diehl A M, Wiseman M.et al Metformin in the treatment of non‐alcoholic steatohepatitis: a pilot open label trial. Aliment Pharmacol Ther 20042023–28. [DOI] [PubMed] [Google Scholar]
- 58.Bugianesi E, Gentilcore E, Manini R.et al A randomized controlled trial of metformin versus vitamin E or prescriptive diet in nonalcoholic fatty liver disease. Am J Gastroenterol 20051001082–1090. [DOI] [PubMed] [Google Scholar]
- 59.Misbin R I. The phantom of lactic acidosis due to metformin in patients with diabetes. Diabetes Care 2004271791–1793. [DOI] [PubMed] [Google Scholar]
- 60.Shadid S, Jensen M D. Effects of pioglitazone versus diet and exercise on metabolic health and fat distribution in upper body obesity. Diabetes Care 2003263148–3152. [DOI] [PubMed] [Google Scholar]
- 61.Kawaguchi K, Sakaida I, Tsuchiya M.et al Pioglitazone prevents hepatic steatosis, fibrosis, and enzyme‐altered lesions in rat liver cirrhosis induced by a choline‐deficient L‐amino acid‐defined diet. Biochem Biophys Res Commun 2004315187–195. [DOI] [PubMed] [Google Scholar]
- 62.Yuan G J, Zhang M L, Gong Z J. Effects of PPARg agonist pioglitazone on rat hepatic fibrosis. World J Gastroenterol 2004101047–1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Caldwell S H, Hespenheide E E, Redick J A.et al A pilot study of a thiazolidinedione, troglitazone, in nonalcoholic steatohepatitis. Am J Gastroenterol 200196519–525. [DOI] [PubMed] [Google Scholar]
- 64.Menon K V N, Angulo P, Lindor K D. Severe cholestatic hepatitis from troglitazone in a patient with nonalcoholic steatohepatitis and diabetes mellitus. Am J Gastroenterol 2001961631–1634. [DOI] [PubMed] [Google Scholar]
- 65.Marcy T R, Britton M L, Blevins S M. Second‐generation thiazolidinediones and hepatotoxicity. Ann Pharmacother 2004381419–1423. [DOI] [PubMed] [Google Scholar]
- 66.Neuschwander‐Tetri B A, Brunt E M, Wehmeier K R.et al Improved nonalcoholic steatohepatitis after 48 weeks of treatment with the PPAR‐gamma ligand rosiglitazone. Hepatology 2003381008–1017. [DOI] [PubMed] [Google Scholar]
- 67.Promrat K, Lutchman G, Uwaifo G I.et al A pilot study of pioglitazone treatment for nonalcoholic steatohepatitis. Hepatology 200439188–196. [DOI] [PubMed] [Google Scholar]
- 68.Sanyal A J, Mofrad P S, Contos M J.et al A pilot study of vitamin E versus vitamin E and pioglitazone for the treatment of nonalcoholic steatohepatitis. Clin Gastroenterol Hepatol 200421107–1115. [DOI] [PubMed] [Google Scholar]
- 69.Yesilova Z, Yaman H, Oktenli C.et al Systemic markers of lipid peroxidation and antioxidants in patients with nonalcoholic Fatty liver disease. Am J Gastroenterol 2005100850–855. [DOI] [PubMed] [Google Scholar]
- 70.Sanyal A J, Campbell‐Sargent C, Mirshahi F.et al Nonalcoholic steatohepatitis: association of insulin resistance and mitochondrial abnormalities. Gastroenterology 20011201183–1192. [DOI] [PubMed] [Google Scholar]
- 71.Kugelmas M, Hill D B, Vivian B.et al Cytokines and NASH: a pilot study of the effects of lifestyle modification and vitamin E. Hepatology 200338413–419. [DOI] [PubMed] [Google Scholar]
- 72.Vajro P, Mandato C, Franzese A.et al Vitamin E treatment in pediatric obesity‐related liver disease: a randomized study. J Pediatr Gastroenterol Nutr 20043848–55. [DOI] [PubMed] [Google Scholar]
- 73.Harrison S A, Torgerson S, Hayashi P.et al Vitamin E and vitamin C treatment improves fibrosis in patients with nonalcoholic steatohepatitis. Am J Gastroenterol 2003982485–2490. [DOI] [PubMed] [Google Scholar]
- 74.Guallar E, Hanley D F, Miller E R., 3rd n editorial update: Annus horribilis for vitamin E. Ann Intern Med 2005143143–145. [DOI] [PubMed] [Google Scholar]
- 75.Merat S, Malekzadeh R, Sohrabi M R.et al Probucol in the treatment of nonalcoholic steatohepatitis: an open‐labeled study. J Clin Gastroenterol 200336266–268. [DOI] [PubMed] [Google Scholar]
- 76.Merat S, Malekzadeh R, Sohrabi M R.et al Probucol in the treatment of non‐alcoholic steatohepatitis: a double‐blind randomized controlled study. J Hepatol 200338414–418. [DOI] [PubMed] [Google Scholar]
- 77.Satapathy S K, Garg S, Chauhan R.et al Beneficial effects of tumor necrosis factor‐alpha inhibition by pentoxifylline on clinical, biochemical, and metabolic parameters of patients with nonalcoholic steatohepatitis. Am J Gastroenterol 2004991946–1952. [DOI] [PubMed] [Google Scholar]
- 78.Adams L A, Zein C O, Angulo P.et al A pilot trial of pentoxifylline in nonalcoholic steatohepatitis. Am J Gastroenterol 2004992365–2368. [DOI] [PubMed] [Google Scholar]
- 79.Abdelmalek M F, Angulo P, Jorgensen R A.et al Betaine, a promising new agent for patients with nonalcoholic steatohepatitis: results of a pilot study. Am J Gastroenterol 2001962711–2717. [DOI] [PubMed] [Google Scholar]
- 80.Yokohama S, Yoneda M, Haneda M.et al Therapeutic efficacy of an angiotensin II receptor antagonist in patients with nonalcoholic steatohepatitis. Hepatology 2004401222–1225. [DOI] [PubMed] [Google Scholar]
- 81.Kiyici M, Gulten M, Gurel S.et al Ursodeoxycholic acid and atorvastatin in the treatment of nonalcoholic steatohepatitis. Can J Gastroenterol 200317713–718. [DOI] [PubMed] [Google Scholar]
- 82.Laurin J, Lindor K D, Crippin J S.et al Ursodeoxycholic acid or clofibrate in the treatment of non‐alcohol‐induced steatohepatitis: a pilot study. Hepatology 1996231464–1467. [DOI] [PubMed] [Google Scholar]
- 83.Lindor K D, Kowdley K V, Heathcote E J.et al Ursodeoxycholic acid for treatment of nonalcoholic steatohepatitis: results of a randomized trial. Hepatology 200439770–778. [DOI] [PubMed] [Google Scholar]
- 84.Yang S Q, Lin H Z, Lane M D.et al Obesity increases sensitivity to endotoxin liver injury: implications for the pathogenesis of steatohepatitis. Proc Natl Acad Sci U S A 1997942557–2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Li Z, Yang S, Lin H.et al Probiotics and antibodies to TNF inhibit inflammatory activity and improve nonalcoholic fatty liver disease. Hepatology 200337343–350. [DOI] [PubMed] [Google Scholar]
- 86.Loguercio C, Federico A, Tuccillo C.et al Beneficial effects of a probiotic VSL#3 on parameters of liver dysfunction in chronic liver diseases. J Clin Gastroenterol 200539540–543. [DOI] [PubMed] [Google Scholar]
- 87.Chalasani N. Statins and hepatotoxicity: focus on patients with fatty liver. Hepatology 200541690–695. [DOI] [PubMed] [Google Scholar]
- 88.Rallidis L S, Drakoulis C K, Parasi A S. Pravastatin in patients with nonalcoholic steatohepatitis: results of a pilot study. Atherosclerosis 2004174193–196. [DOI] [PubMed] [Google Scholar]
- 89.Basaranoglu M, Acbay O, Sonsuz A. A controlled trial of gemfibrozil in the treatment of patients with nonalcoholic steatohepatitis. J Hepatol 199931384. [DOI] [PubMed] [Google Scholar]
- 90.Xu A, Wang Y, Keshaw H.et al The fat‐derived hormone adiponectin alleviates alcoholic and nonalcoholic fatty liver diseases in mice. J Clin Invest 200311291–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Tenenbaum A, Motro M, Fisman E Z. Dual and pan‐peroxisome proliferator‐activated receptors (PPAR) co‐agonism: the bezafibrate lessons. Cardiovasc Diabetol 2005414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Clark J M, Alkhuraishi A R, Solga S F.et al Roux‐en‐Y gastric bypass improves liver histology in patients with non‐alcoholic fatty liver disease. Obes Res 2005131180–1186. [DOI] [PubMed] [Google Scholar]
- 93.Bajaj M, Suraamornkul S, Pratipanawatr T.et al Pioglitazone reduces hepatic fat content and augments splanchnic glucose uptake in patients with type 2 diabetes. Diabetes 2003521364–1370. [DOI] [PubMed] [Google Scholar]
- 94.Shadid S, Jensen M D. Effect of pioglitazone on biochemical indices of non‐alcoholic fatty liver disease in upper body obesity. Clin Gastroenterol Hepatol 20031384–387. [DOI] [PubMed] [Google Scholar]
- 95.Vajro P, Franzese A, Valerio G.et al Lack of efficacy of ursodeoxycholic acid for the treatment of liver abnormalities in obese children. J Pediatr 2000136739–743. [PubMed] [Google Scholar]
- 96.Hasegawa T, Yoneda M, Nakamura K.et al Plasma transforming growth factor‐beta1 level and efficacy of alpha‐ tocopherol in patients with non‐alcoholic steatohepatitis: a pilot study. Aliment Pharmacol Ther 2001151667–1672. [DOI] [PubMed] [Google Scholar]
- 97.Lavine J E. Vitamin E treatment of nonalcoholic steatohepatitis in children: a pilot study. J Pediatr 2000136734–738. [PubMed] [Google Scholar]