Abstract
During oocyte maturation in Xenopus, previously quiescent maternal mRNAs are translationally activated at specific times. We hypothesized that the translational recruitment of individual messages is triggered by particular cellular events and investigated the potential for known effectors of the meiotic cell cycle to activate the translation of the FGF receptor-1 (XFGFR) maternal mRNA. We found that both c-mos and cdc2 activate the translation of XFGFR. However, although oocytes matured by injection of recombinant cdc2/cyclin B translate normal levels of XFGFR protein, c-mos depletion reduces the level of XFGFR protein induced by cdc2/cyclin B injection. In oocytes blocked for cdc2 activity, injection of mos RNA induced low levels of XFGFR protein, independent of MAPK activity. Through the use of injected reporter RNAs, we show that the XFGFR 3′ untranslated region inhibitory element is completely derepressed by cdc2 alone. In addition, we identified a new inhibitory element through which both mos and cdc2 activate translation. We found that cdc2 derepresses translation in the absence of polyadenylation, whereas mos requires poly(A) extension to activate XFGFR translation. Our results demonstrate that mos and cdc2, in addition to functioning as key regulators of the meiotic cell cycle, cooperate in the translational activation of a specific maternal mRNA during oocyte maturation.
INTRODUCTION
During meiosis and the early stages of embryonic development in many metazoans, gene expression is regulated posttranscriptionally. Thus, during these important stages of development, mRNA localization, changes in mRNA stability, translational repression and regulated activation, and posttranslational modification of protein products are critical for driving the cell through the cell cycle, preparing the germ cells for fertilization, and establishing the embryonic body plan. During oogenesis in Xenopus laevis, maternal mRNAs accumulate in the growing oocyte, but a large number of these mRNAs are translationally repressed during this time. Such “masked” maternal messages are recruited for translation at various times after oocyte maturation is initiated, during which time the cell undergoes meiosis. How this complex regulation is achieved is not well understood.
Much of what is known about the specificity of translational activation has been found through analysis of the sequences within the mRNA responsible for translational recruitment. One conserved sequence near the 3′ end of most mRNAs is the cytoplasmic polyadenylation element (CPE), with the general sequence of U4–6AU (Fox et al., 1989; McGrew et al., 1989). The CPE regulates elongation of the poly(A) tail in the cytoplasm, a process that has been shown to be critical for the translational activation of a number of mRNAs during Xenopus oocyte maturation (Richter, 1996; Wickens et al., 1996). CPEs can be classified according to their exact sequence, and the type of CPE contained within an mRNA dictates not only the timing but also the cell cycle regulation of polyadenylation (Sheets et al., 1994; Ballantyne et al., 1997; de Moor and Richter, 1997). A protein that binds to the CPE, the cytoplasmic polyadenylation element–binding protein (CPEB), has been identified, and depletion of this protein ablates cytoplasmic polyadenylation both in vitro and in vivo (Paris et al., 1991; Hake and Richter, 1994; Stebbins-Boaz et al., 1996). However, it is not yet clear how CPEB regulates adenylation through different CPEs with the required level of specificity.
Other sequences that regulate translation are inhibitory elements contained within 3′ untranslated regions (3′UTRs). For a number of RNAs in a variety of species, specific interactions have been demonstrated between cellular proteins and 3′UTR regulatory sequences, including clam cyclin A and ribonucleotide reductase, rabbit erythroid 15-lipoxygenase, Drosophila oskar and hunchback, and Caenorhabditis elegans fem-3 and tra-2 mRNAs (Goodwin et al., 1993; Ostareck-Lederer et al., 1994; Kim-Ha et al., 1995; Murata and Wharton, 1995; Walker et al., 1996; Zhang et al., 1997). Such RNA-protein interactions are postulated to repress translation, as has been shown for both lipoxygenase and tra-2. The 48-kDa cellular factor that binds the lipoxygenase 3′UTR has been shown to repress translation of this RNA in vitro (Ostareck-Lederer et al., 1994). Additionally, the GLD-1 protein is a component of the direct repeat factor, which interacts with the tra-2 3′UTR element, and GLD-1 inhibits translation of this RNA both in vivo and in vitro (Jan et al., 1999). Translational activation of mRNAs containing regulatory elements must accompany derepression of these sequences at the appropriate time and is thought to be achieved through modification, release, or degradation of the inhibitory proteins with which they interact. In support of this model, phosphorylation of the 82-kDa factor that binds the masking element in the clam ribonucleotide reductase and cyclin A RNAs is correlated with and may be important for translational activation of this RNA (Walker et al., 1996; Minshall et al., 1999). Because a number of different inhibitory elements thus far identified do not contain obvious homology to each other, these unique sequences can easily be regulated independently through the proteins that bind them and thus may serve as a source of specificity for translational regulation.
For an RNA element to dictate the timing of translation, however, these RNA sequences, or the proteins with which they interact, must be recognized by cellular factors that can either promote or derepress translation at the appropriate time. These cellular factors, then, must themselves be activated at particular times. One way that proper timing could be achieved is via an intrinsic clock that is set upon reentry into the cell cycle. In this situation, activation would proceed independently of cell cycle effectors. An alternative mechanism by which the cell could achieve the proper timing of translational recruitment is through its assessment of the extent of cell cycle progression. In this case, the activation of a specific cell cycle effector would serve to signal that a particular milestone had been met. Cell cycle regulators then would not only drive the cell through meiosis but would also serve as cellular signals to initiate specific molecular events, such as the translational activation of a subset of mRNAs. Thus, the cellular factors that recruit specific mRNAs for translation during meiosis may be activated by, or may even be composed of, key components of the cell cycle machinery.
Early in progesterone-stimulated meiosis of Xenopus oocytes, c-mos, which is a quiescent mRNA in the immature oocyte, is translated (Sagata et al., 1988). After the translation of low levels of mos protein, a serine/threonine kinase, a protein kinase cascade is activated, all members of which are present in the immature oocyte in an inactive form. Mos activates the MAPK pathway by directly phosphorylating mek, a MAPK kinase, which then phosphorylates and activates MAPK (Nebreda et al., 1993; Posada et al., 1993; Shibuya and Ruderman, 1993). MAPK, then, is thought to activate cdc2/cyclin B indirectly by down-regulating its inhibitory kinase Myt1 (Haccard et al., 1995; Huang et al., 1995; Huang and Ferrell, 1996; Palmer et al., 1998). cdc2 is a serine/threonine kinase that requires interaction with cyclin B1 or B2 for its activity. Through a positive feedback loop involving mos, cdc2/cyclin B, and members of the MAPK pathway, the translation, stability, and activity of mos protein increases, as shown by the fact that oocytes that lack functional cdc2 accumulate very little mos protein (Nebreda et al., 1995). This positive feedback loop also contributes to higher levels of activated cdc2/cyclin B, which ultimately drives the cell into meiosis and germinal vesicle breakdown (GVBD). Oocytes exit from meiosis I after the degradation of cyclin B protein, with a corresponding decrease in cdc2 activity (Huchon et al., 1993). Entry into meiosis II accompanies the reactivation of cdc2/cyclin B and requires new translation of mos (Daar et al., 1991; Kanki and Donoghue, 1991). Oocytes ultimately arrest in metaphase of meiosis II, at which time the mature oocyte is ready for fertilization.
Recently, there has been increased interest in discovering the roles that key components of the Xenopus meiotic cell cycle might play in initiating specific molecular events within the cell. Several reports have focused on the roles that two kinases, c-mos and cdc2, might play in activating the cytoplasmic polyadenylation of a number of maternal mRNAs (Ballantyne et al., 1997; de Moor and Richter, 1997). The polyadenylation and translation of several mRNAs, including D7, cyclin B1, and histone B4, has been shown to be dependent on c-mos translation (Ballantyne et al., 1997; de Moor and Richter, 1997), but for at least some of these RNAs, including cyclin B1 and histone B4, cdc2 may be the more proximal effector (de Moor and Richter, 1997).
Our laboratory has focused on the translational regulation of FGF receptor-1 mRNA (XFGFR), a maternal message translationally activated during oocyte maturation (Robbie et al., 1995). Because FGF signaling is essential in the early embryo, the regulated expression of XFGFR protein during oocyte maturation is critical for normal development (Amaya et al., 1991, 1993). Thus, XFGFR mRNA is one of many maternal messages that must be appropriately translated to prepare the cell for postfertilization events. We have previously identified a 180-nucleotide translation inhibitory element (TIE) contained within the XFGFR 3′UTR (Robbie et al., 1995). This element, which interacts specifically with a 43-kDa cytoplasmic protein, represses translation in the immature oocyte and can direct the timing and regulation of translational activation of this mRNA during oocyte maturation (Robbie et al., 1995; Culp and Musci, 1998).
In this report, we have explored the potential for known cell cycle effectors to function in the translational recruitment of XFGFR during Xenopus oocyte maturation. We found that both c-mos and cdc2 initiate events leading to the translation of XFGFR and that these two effectors cooperate in this process through distinct cis elements in the XFGFR mRNA by means of different mechanisms of activation.
MATERIALS AND METHODS
Isolation of XFGFR 5′UTR
The XFGFR 5′UTR was isolated by rapid amplification of cDNA ends from stage VI Xenopus oocyte total RNA with a XFGFR-specific primer, termed 6187, encoding bases 256–235 of the coding region (5′-GGATCTCCTCCCCTGTTATGCG-3′). A 0.4-kilobase (kb) fragment was amplified during the PCR step and blunt-end subcloned into the EcoRV site of Bluescript KS. Sequence analysis of this clone, R6, identified a 151-nucleotide region upstream of the XFGFR start of translation. We confirmed that this clone contains most if not all of the XFGFR 5′UTR by primer extension analysis (our unpublished results).
The nucleotide sequence of the XFGFR 5′UTR, with the initiating ATG underlined, is as follows: ACCCTACTAA TATAAGGAAT ATAAGTTGGA CGTGCGGAGA CAAAAAGAGA TCAAGAAAAA AAAACCTGGA AACAGGAGCC GTGCAAAGCT TGTCTCGTGC CTGACACTGG AGGTCTCATG GATTCGGGCA GTGTGCACTA GCCAACTTGG GATG.
Plasmid Construction
The construct βgalUTR contains the XFGFR 3′UTR downstream of β-galactosidase, as described previously (Culp and Musci, 1998).
The XFGFR 5′UTR was placed upstream of the β-galactosidase reporter by PCR amplification of the 5′UTR from R6 with a primer containing a BamHI site immediately adjacent to the start of the 5′UTR, xfr5utrbam (5′-CAGTGGATCCACCCTACTAATATAAGGAAT-3′), and a downstream primer that places the translation-initiating ATG in the context of an NcoI site, xfr5utrnco (5′-GAGACCATGGCAAGTTGGCTAGTGC-3′). The amplification product was digested with BamHI and NcoI and filled in with Klenow, and the fragment was subcloned into CScytobgal (Rupp et al., 1994) that had been digested with HindIII/BamHI and filled in with Klenow. This construct, CS5βgal, places the XFGFR 5′UTR and β-galactosidase gene downstream of the cytomegalovirus (CMV) IE94 promoter and removes the SP6 promoter and β-globin 5′UTR. The 1.2-kb BglII/XbaI fragment of βgalUTR, which contains the XFGFR 3′UTR, was then subcloned into BglII/XbaI-digested CS5βgal to generate CS5βgalUTR. To enable the generation of RNA in vitro containing the XFGFR 5′UTR, we subcloned the SP6 promoter from SP64pA (Promega, Madison, WI) on a 0.2-kb NheI/HindIII fragment into the BamHI site of CS5βgalUTR, creating 5βgalUTR. RNA generated from this plasmid contains 14 nucleotides of polylinker before the start of the XFGFR 5′UTR.
βgalCPE and 5βgalCPE were generated by partially digesting βgalUTR and 5βgalUTR, respectively, with BamHI and AflIII, filling in the sites with Klenow, and reclosing the plasmid, thereby removing all but the last 193 nucleotides of the XFGFR 3′UTR, which contains the functional CPE of XFGFR.
The XFGFR 5′UTR was placed upstream of the XFGFR coding region by PCR amplification from R6 with the use of a T7 downstream primer located in the Bluescript vector and the xfr5utrbam primer described above. The 0.45-kb amplified fragment was digested with BamHI and XhoI and subcloned into CS2+, which contains the CMV IE94 promoter, followed by an SP6 promoter, a leader sequence derived from Xenopus β-globin 5′UTR, a polylinker, and SV40 polyadenylation sequences (Rupp et al., 1994). The XFGFR coding region and 3′UTR were placed downstream of the 5′UTR by subcloning the XmnI/XbaI fragment of XFR3′UTR (Robbie et al., 1995) into the intermediate described above, which had been digested with XmnI and XbaI. A polylinker containing an EcoRI restriction site, followed by 30 adenosine residues, an NsiI restriction site, an XhoI site, and an XbaI site, was then subcloned into the EcoRI/XbaI sites of the cloning intermediate. We then replaced the 1.1-kb HindIII/SalI fragment (containing the SP6 promoter and β-globin 5′UTR) of this construct with the 1.3-kb HindIII/SalI fragment (containing the SP6 promoter 14 nucleotides upstream of the XFGFR 5′UTR start) from 5βgalUTR. This plasmid, 5XFRUTR, allows the generation of an RNA in vitro that does not contain β-globin 5′UTR sequences.
XFRCPE was generated by subcloning the 250-base pair (bp) BglII/XbaI fragment of 5βgalCPE containing the XFGFR CPE into XbaI-digested XFR plasmid. 5XFRCPE was generated by subcloning the 1-kb BglII/XhoI fragment of XFRCPE into BglII/XhoI-digested 5XFRUTR. XFRΔUTR and 5XFRΔUTR were generated by subcloning the 1-kb BamHI/XhoI fragment of βgalΔUTR (Culp and Musci, 1998) into the BamHI/XhoI sites of XFRCPE and 5XFRCPE, respectively.
CS2Δ90 was generated by cloning the 965-bp HincII/SpeI fragment of cycΔ90 (provided by A. Murray, University of California, San Francisco, CA) into the XhoI/XbaI sites of CS2+6MT (Rupp et al., 1994), placing the N-terminal truncated sea urchin cyclin B gene downstream of the CMV IE94 promoter and six myc tags (with which the cyclin gene is in frame).
RNA Synthesis
In vitro transcription was performed with the use of Message Machine kits (Ambion, Austin, TX). mos RNA was generated from the plasmid TZKA+ (a gift of M. Murakami and G. Vande Woude, National Cancer Institute–Frederick Cancer Research and Development Center, Frederick, MD) by digesting the plasmid with HindIII and transcribing with T7 polymerase.
Wild-type (p34cdc2) and dominant-negative (p34cdc2 K33R) cdc2 RNAs were transcribed with T7 polymerase from the BamHI-digested plasmids, pET8c-Xlcdc2 and pET8c-Xlcdc2 K33R (a kind gift of T. Hunt, Imperial Cancer Research Fund).
MAPK phosphatase RNA (MKP-1) was synthesized with T7 polymerase from HindIII-digested MKP-1 plasmid (provided by M. Whitman, Harvard Medical School, Boston, MA).
XFR RNA was transcribed with SP6 polymerase from the XFR plasmid (Amaya et al., 1991) linearized at the SacI site immediately downstream of the Xenopus β-globin 3′UTR sequences.
All templates for in vitro RNA synthesis containing the XFGFR 3′UTR or CPE are followed by 30 adenosine residues in the midst of several restriction sites. Digestion of these plasmids with NsiI and transcription in vitro with SP6 yields RNAs that terminate with 30 adenosine residues. The poly(A) tail can be further elongated in vivo, as directed by the XFGFR cytoplasmic polyadenylation sequences. These synthetic RNAs are named for the plasmids from which they were generated. To test for the requirement for polyadenylation, some of these same constructs were digested with XbaI and transcribed in vitro, yielding an RNA that contains the 30 adenosines followed by 20 nonadenosine nucleotides. These additional 20 nucleotides prevent the RNA from serving as a substrate for polyadenylation in vivo (our unpublished results). The names for these RNAs are the same as the names for the templates from which they were derived, with a terminal X.
Oocyte Injections
Ovarian tissue was obtained from adult X. laevis that had been injected with 25 U of PMSG 24–48 h previously. Stage VI oocytes were manually defolliculated, and all experiments were performed in modified Barth’s solution at 16°C.
To assay the ability of cdc2 to activate endogenous XFGFR translation in intact versus c-mos–depleted or MAPK-blocked oocytes, oocytes were first injected with 60 ng of an oligonucleotide antisense to c-mos, as described previously (Culp and Musci, 1998), or with ∼100 pg of in vitro transcribed RNA encoding MAPK phosphatase (MKP-1). In some experiments, ∼50 pg of reporter RNA was then injected into the cytoplasm. The oocytes were incubated for at least 16 h. Injected and control intact oocytes were then induced to mature by injection of 50 nl of recombinant cdc2/cyclin B1 protein (kindly provided by J. Gautier, College of Physicians and Surgeons of Columbia University, New York, NY) into the cytoplasm. In metabolically labeled oocytes, egg-derived cytostatic factor-arrested extract was used as a source of activated cdc2/cyclin B. Cytostatic factor-arrested extract was prepared as described by Murray (1991). Oocyte maturation was induced by injecting 3 nl of cytostatic factor-arrested extract, which is approximately 0.3% of the oocyte volume. In all experiments in which we used the extract, the results were identical to those of experiments in which recombinant cdc2/cyclin B was used. For the nondestructible cyclin experiment, 1 ng (in 5 nl) of CS2Δ90 plasmid DNA was injected into the nucleus to induce maturation. Oocytes were collected for analysis 16–20 h after the induction of maturation.
To assay the translation of endogenous XFGFR in the absence of endogenous cdc2 activity, oocytes were first injected with ∼50 ng of synthetic RNA encoding wild-type (p34cdc2) or dominant-negative cdc2 (p34cdc2 K33R). For experiments in which the involvement of MAPK was assessed, ∼100 pg of synthetic MKP-1 RNA was then injected. For experiments involving reporter RNAs, 50 pg of in vitro transcribed RNA was also injected. Oocytes were then incubated for at least 20 h. Injected and intact control oocytes were then incubated in 10 μM progesterone and/or were injected with 100 pg of synthetic mos RNA. In experiments in which the involvement of mek was assessed, 50 nl of recombinant activated mek (approximate activity of 9 pmol·min−1·μl−1) was injected (a kind gift of J. Maller, University of Colorado Health Sciences Center, Denver, CO). Oocytes were then incubated for 16–20 h before collecting for analysis.
Metabolic labeling of endogenous XFGFR was performed by injecting 50 nl of 5× concentrated [35S]methionine/cysteine (3.5 mCi, >1000 Ci/mmol; Promix, Amersham, Arlington Heights, IL) into groups of 60 intact, c-mos–depleted, or cdc2-blocked oocytes at various times after injection of cdc2/cyclin B or synthetic mos RNA. Oocytes were incubated for 1 h after injection and then collected for analysis.
XFGFR protein derived from synthetic XFR RNA was metabolically labeled by injecting XFR RNA into intact, c-mos–depleted, or cdc2-blocked oocytes. After incubation overnight in Promix at 0.5 mCi/ml, oocytes were washed five times and incubated in media containing 1 mM each cold l-methionine and l-cysteine for 3 h. Oocytes were then injected with cdc2/cyclin B or synthetic mos RNA and harvested at various times thereafter.
Protein Analyses
Western analysis of endogenous and injected XFGFR was performed as described previously (Robbie et al., 1995). Briefly, a pool of 20 oocytes was partially purified on wheat germ lectin Sepharose (Pharmacia, Piscataway, NJ). Bound proteins were electrophoresed on a 7.5% polyacrylamide gel and transferred to Immobilon P (Bio-Rad, Richmond, CA), and XFGFR protein was detected with a polyclonal (R#1) or a monoclonal (Ab50) antibody followed by a secondary antibody conjugated to HRP and detection with the use of Renaissance chemiluminescence reagents (New England Nuclear, Boston, MA).
Endogenous XFGFR protein was detected in metabolically labeled oocytes as described previously (Culp and Musci, 1998). Briefly, groups of 60 oocytes per time point were harvested, and XFGFR protein was partially purified on wheat germ lectin Sepharose. After the bound proteins were boiled off the Sepharose beads, XFGFR was immunoprecipitated with R#1 antiserum, separated by PAGE, and visualized by autoradiography. Radiolabeled XFGFR protein derived from injected synthetic RNA was immunoprecipitated directly with R#1 antiserum from extracts of 20 pooled oocytes and detected by PAGE and autoradiography.
Mos protein levels were assessed by immunoprecipitation followed by Western analysis. In most cases, the same oocyte extracts from which XFGFR protein had been isolated were then precleared with 1 μg of rabbit immunoglobulin G, and one-fourth volume of 5× immunoprecipitation buffer (250 mM Tris, pH 8.0, 50 mM EDTA, 5 mM EGTA, 5% Triton X-100, 2.5% deoxycholic acid, 0.5% SDS, 500 mM NaCl) was added. Mos protein was immunoprecipitated with 2 μl of a polyclonal antibody (c-mosXe; Santa Cruz Biotechnology, Santa Cruz, CA) and protein A–Sepharose (Pharmacia). The bound material was separated on a 10% polyacrylamide gel, and Western analysis was performed with a mAb (5S; courtesy of G. Vande Woude) at a 1:500 dilution.
β-Galactosidase protein levels were assessed by immunoprecipitation followed by Western analysis, similar to that for mos. Pools of 20 oocytes were immunoprecipitated with 0.3 μl of a polyclonal antibody (Cappel, Organon Teknika, Durham, NC), and Western analysis was performed with a mAb (Promega) at a 1:5000 dilution.
For MAPK protein analysis, pools of 10 oocytes were lysed in 100 μl of oocyte lysis buffer (Amaya et al., 1991), and 3 μl (0.3 oocyte equivalent) was electrophoresed on a 12.5% polyacrylamide gel (100:1 acrylamide/bisacrylamide). MAPK was detected with a polyclonal antibody (DC3; kindly provided by J. Ferrell, Stanford University School of Medicine, Stanford, CA).
H1 Kinase Assays
cdc2 activity was assayed by homogenizing groups of five oocytes in 50 μl of 100 mM KCl, 15 mM MgCl2, 5 mM EGTA, 80 mM β-glycerophosphate, pH 7.4. Five microliters of the lysate was incubated in a 20-μl reaction containing 20 mM HEPES, pH 7.8, 30 mM β-mercaptoethanol, 10 mM MgCl2, 100 μg/ml BSA, 125 μg/ml histone H1, 0.25 μCi of [γ-32P]ATP, 100 μM ATP for 15 min at 30°C, after which the reaction was electrophoresed on a 12.5% polyacrylamide gel and subjected to autoradiography.
RNA Analysis
Total RNA was isolated with the use of Trizol reagent (Life Technologies, Grand Island, NY). For RNase protection analysis, 5 μg of total RNA was hybridized to a 32P-labeled XFGFR coding region antisense probe, JM450, as described previously (Culp and Musci, 1998).
High-resolution Northern analysis was performed as described previously (Culp and Musci, 1998). Briefly, 40 μg (for analysis of endogenous XFGFR) or 20 μg (for analysis of injected RNA) of total RNA was hybridized to an oligonucleotide antisense to the XFGFR 3′UTR ∼500 nucleotides from the 3′ end. After digestion with RNase H, the RNA was electrophoresed on a 5% denaturing polyacrylamide gel and subjected to Northern analysis. The membrane was hybridized in Northern Max solution (Ambion) at 37°C for 16 h to a 32P-labeled 414-bp RsaI fragment derived from the 3′ end of the XFGFR 3′UTR.
RESULTS
c-mos Is Required for Normal Levels of XFGFR Protein
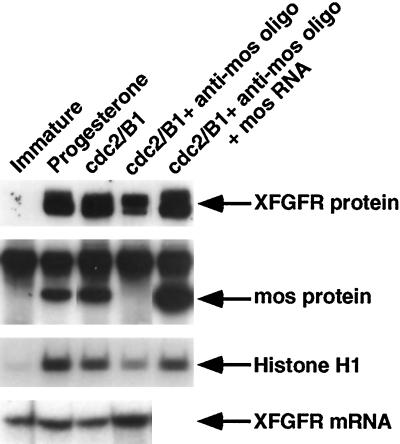
In previous work, we found that XFGFR protein first appears during oocyte maturation, just before GVBD, and is dependent on c-mos translation, which occurs early in progesterone-mediated oocyte maturation (Culp and Musci, 1998). This inability of progesterone alone to trigger the increase in XFGFR protein levels suggested that XFGFR protein appearance is likely not timed intrinsically but is dependent on cell cycle progression. However, this result did not necessarily imply that c-mos leads directly to the appearance of XFGFR protein, because c-mos is required for the activation of other known cell cycle regulators, including cdc2, mek, and MAPK, which may themselves regulate XFGFR protein levels. In fact, oocytes matured by injection of recombinant cdc2/cyclin B translate normal levels of XFGFR protein (Culp and Musci, 1998). However, in these oocytes, c-mos is translated to normal levels and mek and MAPK are also activated. Thus, we wondered whether the appearance of XFGFR protein after cdc2 injection was the result of a cdc2-activated pathway or whether XFGFR might be dependent on one of these other known kinases. To address this question, we tested whether cdc2, in the absence of c-mos, could induce the appearance of XFGFR protein. Oocytes were depleted of c-mos by antisense oligo injection and then injected with recombinant cdc2/cyclin B. Western analysis revealed that XFGFR protein accumulates in response to cdc2 in the absence of c-mos. However, the level of XFGFR protein produced is reduced in c-mos–depleted oocytes compared with progesterone-matured oocytes or intact cdc2-matured oocytes (Figure 1). These c-mos–depleted oocytes do not translate detectable levels of mos protein, and the reduction in XFGFR protein levels in c-mos–depleted oocytes is not due to reduced XFGFR mRNA levels in these oocytes (Figure 1). Normal XFGFR protein levels can be rescued by injection of in vitro synthesized mos RNA into c-mos–depleted oocytes just before cdc2 injection. These oocytes translate mos protein levels at least as high as that in intact mature oocytes. (The synthetic mos RNA was not cleaved in these oocytes because the RNA was injected >20 h after oligo injection, by which time the oligonucleotide is no longer intact.)
Figure 1.
Oocytes induced to mature with cdc2 exhibit reduced levels of XFGFR protein when depleted of c-mos. Intact or c-mos–depleted oocytes were induced to mature by injection of recombinant cdc2/cyclin B (cdc2/B1), and some oocytes were also injected with in vitro synthesized mos RNA. Immature and progesterone-matured oocytes are included for comparison. (Top panel) Extracts of oocytes were assessed for XFGFR translation by Western analysis with the Ab50 antibody. (Second panel) mos protein levels were determined in the same oocyte extracts as in the top panel by immunoprecipitation with a polyclonal anti-mosXe antibody (Santa Cruz Biotechnology), followed by Western analysis with a monoclonal anti-mos antibody (5S). The upper band in all lanes is the immunoglobulin G pulled down in the immunoprecipitation step. (Third panel) The stage of oocyte arrest was inferred from the level of cdc2 activity in oocyte extracts, as assayed in vitro with exogenously provided histone H1 as a substrate for cdc2. (Bottom panel) XFGFR mRNA levels were assessed by RNase protection analysis.
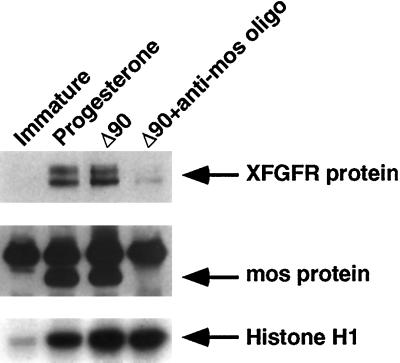
Translation of c-mos has been shown to be required for oocytes to enter meiosis II, whereupon the mature oocyte arrests at metaphase, when cdc2 kinase activity is high. In contrast, c-mos–depleted oocytes arrest in meiosis I, with reduced cdc2 activity levels (Figure 1) (Daar et al., 1991; Kanki and Donoghue, 1991). To determine whether a process activated during meiosis II or a continued high level of cdc2 activity might be more directly related to the c-mos dependence for normal levels of XFGFR protein, we investigated an alternative method to arrest the oocytes in meiosis I. Maturing oocytes exit from metaphase of meiosis I as a result of cyclin B protein degradation. Oocytes expressing a form of cyclin B protein (Δ90) that is unable to be degraded are unable to exit from meiosis I because of the continued presence of cyclin B protein complexed with cdc2, and thus they maintain a high level of cdc2 activity (Murray et al., 1989; Glotzer et al., 1991; Huchon et al., 1993). Consequently, oocytes expressing Δ90 undergo GVBD and arrest in metaphase of meiosis I. However, these oocytes translate levels of XFGFR protein equivalent to those of progesterone-matured oocytes that arrest in meiosis II (Figure 2). Thus, entry into meiosis II is not required for normal levels of XFGFR protein. Oocytes expressing Δ90 contain high levels of cdc2 activity as a result of the stable Δ90-cyclin B/cdc2 complex (Figure 2). To determine whether this high cdc2 activity is sufficient to achieve maximal XFGFR protein in the absence of c-mos, we expressed Δ90 in intact oocytes and in oocytes that were depleted of c-mos. Although cdc2 activity was equally high in both c-mos–depleted and intact oocytes, the amount of XFGFR protein was greatly reduced in the absence of c-mos. Thus, high cdc2 activity alone is insufficient to achieve maximal XFGFR protein levels, and although cdc2 can activate a pathway leading to an increase in XFGFR, c-mos is required for normal levels of XFGFR protein.
Figure 2.
c-mos depletion, and not meiosis I arrest or decreased cdc2 levels, is responsible for the reduced XFGFR protein levels in c-mos–depleted oocytes. Intact or c-mos–depleted oocytes were induced to mature and arrest in meiosis I by injection of an expression construct containing nondestructible cyclin B (Δ90). Immature and progesterone-matured oocytes are also shown. (Top panel) XFGFR protein levels, assayed by Western analysis with the Ab50 antibody. (Middle panel) mos protein levels were assessed in the same extracts by immunoprecipitation followed by Western analysis. (Bottom panel) cdc2 activity was assessed in vitro with histone H1 as substrate.
mos Effects an Increase in XFGFR Protein in the Absence of cdc2 Activity
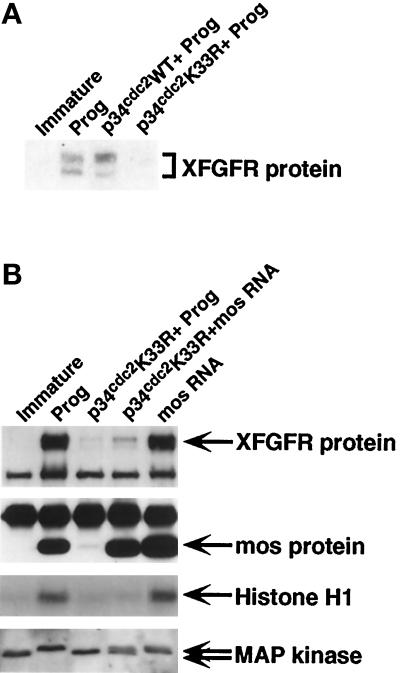
Because the results described above suggested that c-mos appears to be important for normal levels of XFGFR protein, we wished to determine whether c-mos alone can induce the appearance of XFGFR protein in the absence of cdc2 activity or whether c-mos simply enhances the effect of the cdc2-initiated pathway. To test this, we blocked cdc2 activity with a dominant-negative form of cdc2 (p34cdc2 K33R) (Nebreda et al., 1995). Although wild-type p34cdc2 has no effect on the ability of progesterone to induce oocyte maturation or XFGFR translation, oocytes expressing p34cdc2 K33R do not mature in response to progesterone, and very little XFGFR protein is detected (Figure 3). However, these oocytes also accumulate very little mos protein, as a result of the absence of the positive feedback loop with cdc2 (Figure 3B) (Nebreda et al., 1995). Thus, to test the ability of mos to activate XFGFR in the absence of cdc2, we increased the amount of mos protein in cdc2-blocked oocytes by injecting synthetic mos RNA. Although p34cdc2 K33R–expressing oocytes injected with mos RNA did not undergo GVBD or activate cdc2, they did accumulate mos protein to levels at least as high as those in progesterone-matured oocytes (Figure 3B). This increase in mos protein results in an increase in XFGFR protein as well; however, XFGFR levels are still reduced compared with the levels in intact oocytes matured by either progesterone or mos RNA injection. Thus, mos increases XFGFR protein levels in the absence of cdc2 activity and in the absence of cell cycle progression, but the amount of XFGFR protein that accumulates in response to mos is greatly reduced in the absence of functional cdc2.
Figure 3.
Oocytes blocked for cdc2 accumulate XFGFR protein in response to mos. (A) XFGFR Western analysis with Ab50 antibody on progesterone-matured intact oocytes and oocytes expressing p34cdc2 or p34cdc2 K33R treated with progesterone (Prog). Immature oocytes are also shown. (B) Intact or p34cdc2 K33R–expressing oocytes were treated with progesterone (Prog) or injected with synthetic mos RNA. (Top panel) XFGFR protein levels, as assayed by Western analysis with R#1 antibody. The lower band in all lanes is a nonspecific cross-reacting protein. (Second panel) mos protein levels were assayed in the same extracts that had been assayed for XFGFR by immunoprecipitation, followed by Western analysis for mos protein. The upper band in all lanes is the immunoglobulin G protein pulled down in the immunoprecipitation step. (Third panel) Histone H1 was used as an in vitro substrate to assess cdc2 activity levels. (Bottom panel) MAPK activation was assessed by the differential migration on Western blots of inactive and activated forms of MAPK (lower and upper arrow, respectively).
Data from other authors have suggested that functional cdc2 not only increases mos protein levels but that phosphorylated mos protein, the more stable and active form of the protein, accumulates only after GVBD and cdc2 activation (Watanabe et al., 1989; Nishizawa et al., 1992). Therefore, we wished to determine whether the lower XFGFR protein levels in the absence of cdc2 activity might be explained solely by a lower activity of mos protein. We assessed the activity of mos protein in cdc2-blocked oocytes by assaying MAPK activation because mos is an activator of mek, which in turn phosphorylates and activates MAPK (Nebreda et al., 1993; Posada et al., 1993; Shibuya and Ruderman, 1993). Although activation of MAPK within a single oocyte is an all-or-none event (Ferrell and Machleder, 1998), we used the proportion of oocytes with activated MAPK as an indicator of mos activity. In immature oocytes, inactive MAPK is hypophosphorylated and migrates at 42 kDa. Oocytes matured by progesterone or mos RNA injection contain activated MAPK, which is completely phosphorylated, and therefore exhibit a reduced mobility of ∼44 kDa (Figure 3B). Progesterone treatment of oocytes blocked for cdc2 activity revealed no detectable activated MAPK, reflecting the low mos activity in these oocytes. After mos RNA injection, however, MAPK was activated. Between different experiments, we observed variations in the proportion of oocytes containing activated MAPK in cdc2-blocked, mos-injected oocytes, ranging from 50 to 100% (our unpublished results). The levels of XFGFR protein in these oocytes also vary and correlate with MAPK activation, but they never equal the levels seen in intact mature oocytes, despite complete MAPK activation and high mos activity levels. We conclude, therefore, that although mos can effect an increase in XFGFR protein levels in the absence of functional cdc2, cdc2 is necessary for the normal complement of XFGFR protein.
mos Increases XFGFR Protein Levels Independently of the MAPK Pathway
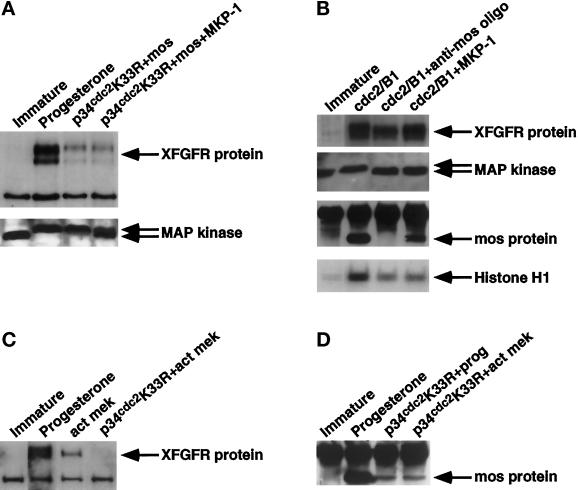
The experiments described above indicate that mos can direct an increase in XFGFR protein in the absence of cdc2 activity. However, because we observed that an increase in mos protein resulted in both a greater proportion of activated MAPK and increased levels of XFGFR protein, we wished to determine whether members of the MAPK pathway might be more proximal to XFGFR or whether the level of MAPK activation is simply an index of mos activity. We addressed this question in two ways, in both cases with the use of a MAPK phosphatase (MKP-1) (Sun et al., 1993), which dephosphorylates and inactivates MAPK. In the first approach, we expressed MKP-1 in oocytes lacking functional cdc2 (p34cdc2 K33R) and expressing exogenous mos RNA. Although a marked reduction in activated MAPK was observed in oocytes expressing MKP-1, the level of XFGFR protein was equivalent to that in cdc2-blocked oocytes injected with mos RNA alone (Figure 4A).
Figure 4.
Members of the MAPK pathway do not contribute to the accumulation of XFGFR protein. (A) XFGFR (top panel) and MAPK (bottom panel) Western analysis on oocytes injected with p34cdc2 K33R RNA, plus or minus MKP-1 RNA, followed by mos RNA injection. Immature and progesterone-matured oocytes are also shown. (B) Recombinant cdc2/cyclin B was injected into intact, c-mos–depleted, or MKP-1–expressing oocytes. Immature oocytes are also included. Extracts of oocytes were assayed for XFGFR translation (top panel), MAPK activation (second panel), mos protein levels (third panel), and cdc2 activity (bottom panel). (C) XFGFR Western analysis on extracts of oocytes injected with activated mek protein (act mek) in the presence or absence of p34cdc2 K33R. Immature and progesterone-matured oocytes are included for comparison. (D) mos protein levels assayed in immature oocytes, progesterone-matured oocytes, and oocytes injected with p34cdc2 K33R RNA and either stimulated with progesterone or injected with activated mek protein. XFGFR Western analysis was performed with R#1 antibody (A and C) or Ab50 antibody (B). MAPK Western blots show the locations of hyperphosphorylated, activated MAPK (top arrow) and the hypophosphorylated, inactive form (bottom arrow).
We then tested whether MAPK might be involved in the cdc2-directed increase in XFGFR protein. As other authors have shown, MAPK is not activated in c-mos–depleted oocytes induced to mature with cdc2 (Figure 4B) (Gotoh et al., 1995). Thus, we tested whether the reduced XFGFR protein levels in c-mos–depleted, cdc2/cyclin B–injected oocytes is a result of c-mos depletion itself or the inability to activate the MAPK pathway. Oocytes expressing MKP-1 were induced to mature by cdc2/cyclin B injection. We confirmed that although MKP-1–expressing oocytes contain no detectable activated MAPK and these oocytes arrest in meiosis I, as shown by the low histone H1 kinase activity, XFGFR protein levels are not reduced in these oocytes (Figure 4B). In fact, a slight increase in XFGFR protein levels was observed in the absence of active MAPK, suggesting a possible inhibitory function of MAPK on XFGFR protein levels. In these oocytes, the amount of mos protein produced is somewhat reduced, consistent with a break in the positive feedback loop between mos and the MAPK pathway. However, this level of mos protein produced contributes sufficiently to XFGFR protein levels, because oocytes depleted of both MAPK and c-mos activities have levels of XFGFR protein equivalent to those of oocytes depleted of just c-mos (our unpublished results). These data demonstrate that oocytes that have intact cdc2 and mos activities but no active MAPK exhibit no reduction in XFGFR protein.
To further explore the possibility that the MAPK pathway has a negative effect on XFGFR protein levels, we activated the MAPK pathway directly with a recombinant activated mek protein. We injected activated mek into intact oocytes or oocytes blocked for cdc2 activity. In intact oocytes, activated mek induced oocyte maturation and the appearance of XFGFR protein (Figure 4C). However, the level of XFGFR protein in mek-matured oocytes was reduced compared with that in progesterone-matured oocytes. In contrast, we detected no protein in cdc2-blocked oocytes injected with activated mek. We confirmed the findings of other authors that activation of the MAPK pathway induces mos translation (Figure 4D) (Matten et al., 1996; Roy et al., 1996). However, because of the lack of cdc2 activity, the level of mos protein in these oocytes was quite low and was similar to that in progesterone-stimulated, cdc2-blocked oocytes (Figure 4D). That some mos protein was present but no XFGFR protein was detected in mek-injected, cdc2-blocked oocytes suggests that the MAPK pathway does negatively regulate the appearance of XFGFR protein during oocyte maturation. Taken together, these data demonstrate that c-mos and cdc2 both contribute to the increase in XFGFR protein during meiosis independently of the MAPK pathway.
XFGFR Protein Accumulation Occurs with Neither a Delay Nor a Major Decrease in Protein Stability in Oocytes Depleted of c-mos or cdc2
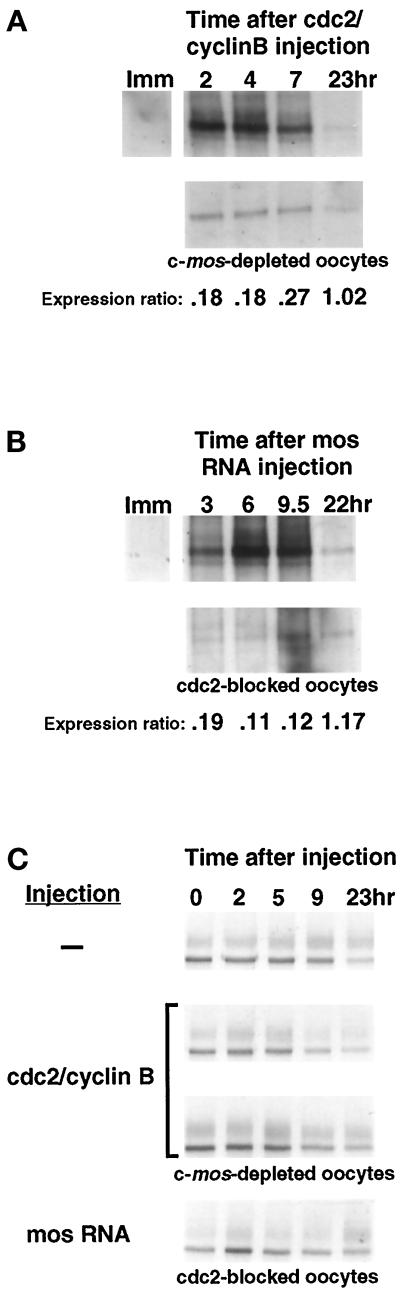
Our results demonstrate that c-mos and cdc2 each directs an increase in XFGFR protein levels, although both effectors are required for the full complement of XFGFR protein attained in intact mature oocytes. However, the submaximal protein levels in the absence of one of the two effectors might have several possible explanations: a reduced rate of translation, a decreased stability of the protein produced, or a delay in the normal increase in translation or protein stability. We first addressed whether the timing of translation is intact in the absence of either c-mos or cdc2. We examined XFGFR translation by pulse-label analysis after cdc2 injection into intact and c-mos–depleted oocytes. At various times after cdc2/cyclin B injection, oocytes were metabolically labeled for 1 h, the oocytes were collected, and XFGFR protein levels were assessed by immunoprecipitation. Although no labeled XFGFR protein was detected in immature oocytes, in both intact and c-mos–depleted oocytes injected with cdc2, labeled XFGFR protein was detected by the first time point after cdc2/cyclin B injection, and translation continued throughout the course of the experiment (Figure 5A). However, the amount of labeled protein in c-mos–depleted oocytes was reduced compared with that in intact oocytes.
Figure 5.
The timing of protein accumulation is intact and XFGFR protein is stable in the absence of either c-mos or cdc2 activities. (A) Immunoprecipitation of endogenous XFGFR protein from intact or c-mos–depleted oocytes metabolically labeled during the course of cdc2-induced meiosis. (B) Endogenous XFGFR protein immunoprecipitated from intact or p34cdc2 K33R–expressing (cdc2-blocked) oocytes metabolically labeled at various times after mos RNA injection. Oocytes were labeled by injecting [35S]methionine/cysteine at various times after cdc2/cyclin B or mos RNA injection (the times in hours are indicated above the lanes), incubating for 1 h, and then harvesting for protein analysis. The ratio of the intensities of thesignals in c-mos– or cdc2-depleted oocytes was compared with that of the cognate intact oocyte sample and is reported below each lane as the expression ratio. Imm, immature oocytes labeled similarly for 1 h. (C) Pulse-chase analysis of metabolically labeled intact, c-mos–depleted, or cdc2-blocked oocytes expressing synthetic XFR RNA. After incubation in [35S]methionine/cysteine and extensive washing in cold amino acids, immature oocytes were injected with either cdc2/cyclin B or synthetic mos RNA at time 0 and were collected at the indicated times thereafter for analysis by immunoprecipitation.
We also compared the timing of mos-induced XFGFR protein appearance in the presence and absence of cdc2 activity. We injected synthetic mos RNA into intact oocytes or oocytes blocked for cdc2 activity and metabolically labeled the oocytes for 1 h at various times after mos RNA injection. We detected labeled XFGFR protein in both intact and cdc2-blocked oocytes at the first time point after mos RNA injection (Figure 5B). Although the rate of XFGFR protein accumulation in intact oocytes initially increased and then subsequently decreased during mos-stimulated oocyte maturation, XFGFR protein accumulated in cdc2-blocked oocytes at a constant low level throughout the duration of the experiment. From this analysis, we conclude that although either c-mos or cdc2 alone is capable of effecting an increase in XFGFR protein levels with no discernible delay, the rate of protein accumulation is compromised in the absence of the other effector.
We then addressed by pulse-chase analysis whether the reduced levels of XFGFR protein in the absence of either c-mos or cdc2 might be the result of a decreased stability of the protein in the absence of one of the effectors. For this analysis, we used a synthetic RNA encoding the XFGFR coding region alone (XFR) (Robbie et al., 1995). This RNA is translated in immature oocytes, allowing us to compare directly the stability of XFGFR protein in immature oocytes with that in oocytes with intact mos and/or cdc2 activities. Immature intact oocytes, c-mos–depleted oocytes, or cdc2-blocked oocytes that had been injected with synthetic XFR RNA were metabolically labeled overnight. After extensive washing and incubation in cold amino acids, oocytes were injected with either cdc2/cyclin B or mos RNA and collected at various times for later immunoprecipitation. We found that XFGFR protein was quite stable in all oocytes (Figure 5B). Quantitation of several such experiments revealed that although XFGFR protein had a half-life of more than 5 h in immature oocytes, XFGFR protein appeared to be slightly more stable in oocytes that had intact cdc2 or c-mos activities (7 and 9 h, respectively; our unpublished results). However, this difference is not sufficient to account for the disparity between intact and c-mos–depleted oocytes in a 1-h pulse label of endogenous XFGFR protein (Figure 5C). To generate such a difference in a 1-h pulse, the half-life of the protein in the absence of one of the effectors would need to be <15 min. These results suggest that both mos and cdc2 contribute to an increase in the level of XFGFR protein, primarily by effecting an increase in translation, but that they also function to stabilize XFGFR protein.
cis Elements Involved in Activating XFGFR Translation
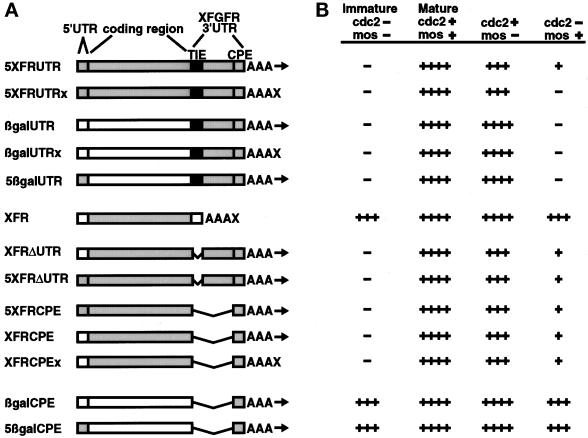
With our finding that both cdc2 and c-mos activate XFGFR translation, we then wished to distinguish the functions of these two effectors in this process. To identify potential regulatory elements within the XFGFR RNA and to determine whether they might respond differently to the two effectors, we generated synthetic RNAs that contained various portions of the XFGFR RNA. We especially wished to examine the activation of the 3′UTR, because our previous work had identified within this region a 180-nucleotide element, the TIE, which inhibits the translation of a heterologous reporter (Robbie et al., 1995). The TIE also mimics the timing of activation of endogenous XFGFR translation and thus appears to serve as a key element for the translational activation of this mRNA (Culp and Musci, 1998). However, to ensure that an intact synthetic RNA could recapitulate the translation profile of endogenous XFGFR mRNA, we first generated RNA in vitro that contained all of the elements of endogenous XFGFR: the 5′UTR, the coding region, and the 3′UTR. This RNA, 5XFRUTR, was injected into intact oocytes, c-mos–depleted oocytes, and oocytes blocked for cdc2 function. We then injected the oocytes with cdc2/cyclin B or mos RNA and assessed XFGFR protein levels by Western analysis. The results are shown schematically in Figure 6. As expected, 5XFRUTR RNA mimicked endogenous XFGFR mRNA, because this synthetic RNA was repressed in the immature oocyte, was activated during cdc2-induced oocyte maturation, and could be activated by mos in the absence of cdc2. Also, like endogenous XFGFR, 5XFRUTR was not completely activated by either mos or cdc2 in the absence of the other effector.
Figure 6.
c-mos and cdc2 function through different cis elements on the XFGFR RNA. (A) Scheme of synthetic RNAs tested. RNAs contain either the Xenopus β-globin (white) or XFGFR (gray) 5′UTR sequence and the β-galactosidase (white) or XFGFR (gray) coding region. 3′ untranslated sequences used include the 3′UTR from Xenopus β-globin (white), the entire XFGFR 3′UTR (gray), with or without the 180-nucleotide TIE (black), or just the last 193 nucleotides of the XFGFR 3′UTR containing the CPE. All of the RNAs featured a poly(A) tail containing 30 adenosine residues. The RNAs terminating with the poly(A) tail can be further adenylated in vivo (arrows). RNAs containing additional nonadenosine residues beyond the poly(A) tail are not substrates of adenylation in vivo (X). (B) Scheme of results of Western analyses. Immature oocytes were injected with the synthetic RNAs diagrammed in A, and protein levels were assayed under the following conditions: cdc2−/mos−, immature oocytes; cdc2+/mos+, intact oocytes matured by cdc2/cyclin B injection; cdc2+/mos−, c-mos–depleted oocytes injected with cdc2/cyclin B; cdc2−/mos+, p34cdc2 K33R–expressing oocytes injected with mos RNA. Oocytes injected with RNAs containing the XFGFR coding region were analyzed for XFGFR protein by Western analysis. Reporter protein levels in oocytes injected with RNAs containing the β-galactosidase coding region were analyzed by immunoprecipitation followed by Western analysis. Autoradiographs were subjected to scan analysis, and the levels of protein relative to those in intact mature oocytes are presented as follows: ++++, 80–100%; +++, 60–80%; ++, 40–60%; +, 20–40%; −, <20% of the amount in intact mature oocytes. Within a given experiment, translation in mature oocytes was similar in oocytes injected with different RNAs containing the same coding region.
We then tested whether the 3′UTR was sufficient to mimic the translational regulation by mos and cdc2 that we observed for full-length XFGFR RNA. We injected synthetic RNA, βgalUTR, which contains the β-galactosidase coding region and the entire XFGFR 3′UTR, into intact oocytes, c-mos–depleted oocytes, or cdc2-blocked oocytes, and β-galactosidase translation was assessed after cdc2 or mos RNA injection (Figure 6). As expected, translation of this RNA was repressed in immature oocytes as a result of the presence of the TIE within the XFGFR 3′UTR, and translation was activated during cdc2-induced oocyte maturation. However, we found that oocytes blocked for cdc2 activity and injected with synthetic mos RNA did not activate translation of the βgalUTR reporter RNA above that of the repressed state in the immature oocyte. In addition, oocytes induced to mature by cdc2 but lacking c-mos translated at least as much β-galactosidase protein as intact cdc2-matured oocytes (Figure 6). Thus, mos is neither capable of nor required for derepression of the XFGFR 3′UTR, and cdc2 alone is capable of fully activating RNA that contains the TIE. These observations are in contrast with the results observed for endogenous XFGFR, in which complete translational activation by cdc2 required c-mos, and mos was able to partially activate translation in the absence of cdc2 activity. This result demonstrates that cdc2 completely derepresses XFGFR translation through the 3′UTR but that mos cannot.
We then tested whether the XFGFR 5′UTR might contain an element through which mos might function. Translation of RNA containing the XFGFR 5′UTR and 3′UTR (5βgalUTR) was assayed as described above. Like βgalUTR, 5βgalUTR was repressed in the immature oocyte and was activated by cdc2-induced maturation, but it was neither activated by nor dependent on c-mos for complete activation by cdc2 (Figure 6). Thus, mos does not activate XFGFR translation through either the 5′UTR or the 3′UTR.
Because mos does not activate translation through the XFGFR untranslated regions, we hypothesized that the coding region might be involved in mos-dependent translation. However, in confirmation of previous results, XFR RNA, which contains the XFGFR coding region alone (with 5′ and 3′ untranslated region sequences derived from Xenopus β-globin), was not repressed in immature oocytes (Figure 6) (Robbie et al., 1995). The amount of XFGFR protein was not significantly altered by mos alone, which is further evidence that mos activity does not greatly affect the stability of the XFGFR protein. A slight increase in protein levels was observed during cdc2-induced oocyte maturation; however, this modest change was consistent with the global increase in translation during meiosis and likely was not due to cell cycle–regulated activation of this RNA specifically (Wasserman et al., 1982). We conclude from this result that although the coding region might contribute to the mos dependence of XFGFR RNA, on its own the coding region is not regulated by mos.
Because neither the coding region nor the untranslated regions in isolation exhibited the mos dependence observed for endogenous RNA, we considered the possibility that multiple regions of the XFGFR RNA in combination, as in the intact endogenous mRNA, form a regulatory element. Thus, we constructed four additional RNAs containing the XFGFR coding region and various portions of the untranslated regions, including the 5′UTR, the TIE-deleted 3′UTR, and the terminal 193 nucleotides of the 3′UTR (5XFRΔUTR, XFRΔUTR, 5XFRCPE, and XFRCPE). This latter region of the XFGFR 3′UTR, which we call the XFGFR CPE, contains U-rich sequences and directs cytoplasmic polyadenylation in vivo (our unpublished results), despite a lack of identity with the consensus CPE (Fox et al., 1989; McGrew et al., 1989). Unexpectedly, these four RNAs had the same translation profile (Figure 6). These RNAs were translationally repressed in the immature oocyte, despite the absence of the TIE; they were activated during cdc2-induced oocyte maturation; and either mos or cdc2 could partially activate them in the absence of the other effector (Figure 6). Because the presence or absence of either the 5′UTR or the majority of the 3′UTR had no effect on translation in this assay, we conclude that mos activates translation through sequences contained within the coding region and the CPE.
Our observation that the translation of XFRCPE RNA was inhibited in the immature oocyte, whereas the XFGFR coding region alone was not repressed, suggested that at least a portion of an inhibitory element is contained within the XFGFR CPE. However, RNA containing the β-galactosidase coding region and the XFGFR CPE (βgalCPE) was not repressed in immature oocytes, and translation was not appreciably altered by the addition of either mos or cdc2 activities (Figure 6). Thus, the XFGFR CPE alone cannot regulate the translation of a heterologous gene.
Finally, we wished to determine whether the 5′UTR sequences might contain any regulatory function. We constructed 5βgalCPE RNA, which contains the XFGFR 5′UTR, the β-galactosidase coding region, and the XFGFR CPE (to allow polyadenylation in vivo). Like βgalCPE, 5βgalCPE exhibited no translational repression in immature oocytes, and neither cdc2 nor mos greatly affected translation of this RNA (Figure 6). We conclude that the XFGFR 5′UTR does not contribute to either translational repression or activation of XFGFR during oocyte maturation.
These experiments demonstrate that two inhibitory elements are contained within the XFGFR mRNA. The first is the TIE, which resides within the 3′UTR and is completely derepressed by cdc2 during oocyte maturation; mos does not contribute to this process. The second is formed by a combination of sequences in the coding region and the CPE and is activated during meiosis by mos and cdc2 in a cooperative manner.
Requirement for Polyadenylation in Activating mos-dependent Translation
In the experiments described above, all RNAs containing XFGFR 3′UTR sequences terminated with short poly(A) tails (A30), which may be elongated in vivo as directed by the XFGFR CPE. We next examined whether mos and cdc2 require the process of polyadenylation to derepress XFGFR by assessing the translational activation of reporter RNAs that cannot be adenylated. These RNAs contain a poly(A) region (A30) that is followed by and terminates with 20 nonadenosine nucleotides, which prevents these RNAs from serving as substrates for adenylation in vivo (our unpublished results). Our previous work had shown that the poly(A) tail of XFGFR mRNA, which is normally extensively elongated during progesterone-mediated oocyte maturation, is not appreciably elongated when oocytes are induced to mature by cdc2 injection (Culp and Musci, 1998), suggesting little, if any, requirement for adenylation in cdc2-induced translational activation. We first tested whether cdc2 requires any poly(A) tail elongation to derepress the TIE. We injected nonadenylatable RNA containing the XFGFR 3′UTR (βgalUTRx) into intact, c-mos–depleted, or cdc2-blocked oocytes; subsequently, we injected either cdc2/cyclin B or mos RNA and assayed the amount of reporter protein produced. As expected, mos alone did not derepress this RNA. In contrast, the translational activation of βgalUTRx by cdc2 was equivalent to that of βgalUTR, despite the inability of βgalUTRx to be further adenylated (Figure 6). Thus, derepression of the TIE by cdc2 does not require polyadenylation.
We then tested whether poly(A) tail elongation is required for derepression of the XFGFR coding region/CPE by mos or cdc2. We found that, like XFRCPE, the nonadenylatable XFRCPEx was completely derepressed during cdc2-induced maturation and was activated by both mos and cdc2 in the absence of the other effector, suggesting that polyadenylation is not required to activate this element by either mos or cdc2 alone. However, in RNAs containing the complete 3′UTR (5XFRUTRx), the nonadenylatable form was not derepressed by mos alone. In contrast, these RNAs were completely derepressed during cdc2-induced maturation and were activated equally by cdc2 in the absence of c-mos, independent of their ability to be adenylated (Figure 6). From these data, we conclude that cdc2 does not require polyadenylation to activate the translation of XFGFR through the coding region/CPE, the TIE in the 3′UTR, or the full-length XFGFR RNA.
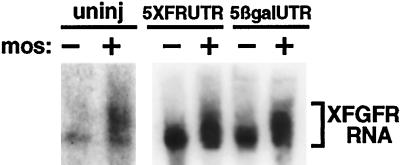
Our finding that mos requires poly(A) elongation to derepress the intact XFGFR RNA was a somewhat unexpected result, because in previous work we had shown that progesterone induces complete adenylation of endogenous XFGFR mRNA in the absence of c-mos (Culp and Musci, 1998). Thus, we wondered whether mos itself might induce XFGFR polyadenylation. Endogenous XFGFR in immature oocytes contains a discrete poly(A) tail of ∼30 adenosine residues (Culp and Musci, 1998). This short poly(A) tail was extended appreciably by either progesterone stimulation or mos injection (Figure 7) (Culp and Musci, 1998). Thus, XFGFR polyadenylation may be induced by either progesterone or mos activity.
Figure 7.
mos induces XFGFR polyadenylation independently of coding region sequences. Synthetic mos RNA was injected into intact oocytes or oocytes that had been injected with 5XFRUTR or 5βgalUTR RNA. Sixteen hours later, total RNA was extracted and subjected to high-resolution Northern analysis, with a probe derived from the 3′ terminus of the XFGFR 3′UTR. The extent of poly(A) elongation is indicated by the bracket.
Because mos functions through sequences in both the XFGFR coding region and the CPE to activate translation, we wished to determine whether these same sequences are required for mos to induce XFGFR polyadenylation. We expressed mos in oocytes previously injected with RNA that contained the XFGFR coding region and the CPE and that could be partially activated by mos alone (5XFRUTR) or with RNA that contained the XFGFR CPE but no coding region sequences and that were not derepressed at all by mos (βgalUTR). Northern analysis demonstrated that both RNAs were adenylated by mos (Figure 7). Thus, mos induced adenylation through the XFGFR CPE sequences alone and did not require the XFGFR coding region for this process. These experiments suggest that mos has distinct targets for translational activation and polyadenylation. Although mos activates translation through an inhibitory element formed by the coding region and the CPE, adenylation by mos uses only the CPE.
We conclude from these studies that although mos does not require polyadenylation to derepress translation through the coding region/CPE alone, in the presence of the TIE adenylation is required for mos to function as a translational activator. These experiments suggest that mos has two separate roles in activating XFGFR translation: polyadenylation through the CPE and derepression through the coding region/CPE.
DISCUSSION
Both c-mos and cdc2 Regulate XFGFR Translation
We initiated this study to determine the role of meiotic cell cycle activators in regulating XFGFR translation. We found that both cdc2 and c-mos, key components of the meiotic cell cycle, also serve to regulate XFGFR translation and protein levels during Xenopus oocyte maturation. These effectors, although both required for normal levels of XFGFR protein, do not function redundantly, and they cooperate in these processes. Two pieces of evidence indicate that both c-mos and cdc2 increase XFGFR protein levels primarily through translational activation of XFGFR mRNA: cdc2 derepresses the 3′UTR, therefore functioning at the level of translation (Figure 6); and in cdc2-blocked oocytes, polyadenylation of the full-length synthetic XFGFR RNA is required for mos to elicit its increase in XFGFR protein levels (Figure 6). In addition, mos activity does not greatly affect the steady-state levels of XFGFR protein generated from synthetic XFR RNA (Figure 6), and the stability of XFGFR protein generated from synthetic XFR RNA is quite high in immature oocytes and is only modestly increased by mos or cdc2 (Figure 5C). Although both mos and cdc2 confer stability to XFGFR protein, this activity cannot account for the much larger changes in XFGFR protein levels during oocyte maturation. We had wished to use polysome analysis to assay directly the abilities of mos and cdc2 to activate translation of XFGFR mRNA. However, in both immature and mature oocytes, XFGFR mRNA is associated with a large complex and is not released with reagents that dissociate ribosomes (our unpublished results). We conclude from these studies that both c-mos and cdc2 activate translation of XFGFR mRNA during oocyte maturation.
One target of cdc2 is the 3′UTR inhibitory element, the TIE. Because of the role this element plays in the timing and activation of translation, as elucidated in previous work, when we began this study the TIE seemed a likely target and perhaps the sole target of cellular activation pathways (Robbie et al., 1995; Culp and Musci, 1998). Surprisingly, however, we identified a second inhibitory element that is formed by a combination of sequences in the XFGFR coding region and the CPE. Complete activation of XFGFR translation through the coding region/CPE sequences, or this “second element,” requires the activities of both c-mos and cdc2. The XFGFR regulatory elements we have identified thus far and their known functions are summarized in Table 1. Through these experiments we show that two independent pathways, initiated by c-mos and cdc2, converge onto a single mRNA, XFGFR, to effect its complete translational activation.
Table 1.
RNA regulatory elements
| RNA element | Inhibitory in the immature oocyte? | Action during meiosis | Target of which effector(s) |
|---|---|---|---|
| TIE | Yes | Derepression | cdc2 |
| Coding region/CPE | Yes | Derepression, polyadenylation | c-mos/cdc2, c-mos |
| CPE (in isolation) | No | Polyadenylation | Progesterone/c-mos |
The potential for mos and cdc2 to effect changes in translation has been investigated in several recent studies. Poly(A) polymerase, a protein critical for poly(A) elongation, has been shown to be a direct target of cdc2 during Xenopus meiosis (Colgan et al., 1996). Although phosphorylation by cdc2 inhibits poly(A) polymerase activity, this inhibition is not achieved until poly(A) polymerase is completely phosphorylated late in oocyte maturation, when the translation of many mRNAs decreases (Colgan et al., 1998). In addition, cdc2 induces and is required for the phosphorylation of the CPEB, which is critical for the translational activation of a number of RNAs (Paris et al., 1991; Hake and Richter, 1994; Stebbins-Boaz et al., 1996). Direct phosphorylation of CPEB by cdc2 has not been shown, although CPEB does contain a cdc2 consensus phosphorylation site (Hake and Richter, 1994). A dependence on cdc2 activity for polyadenylation has been shown for some mRNAs, which suggests either an additional link between cdc2 and translation or the possibility that this dependence may be related to the phosphorylation of CPEB by cdc2 (Ballantyne et al., 1997; de Moor and Richter, 1997).
In contrast to the demonstrated connections between cdc2 and translation, the relationship between mos and translation is less well defined. Mos-dependent polyadenylation has been described for a number of Xenopus mRNAs, although cdc2 may be the more direct effector for at least some of them (Ballantyne et al., 1997; de Moor and Richter, 1997). At present, the only known physiological target of mos is mek, a member of the MAPK pathway (Nebreda and Hunt, 1993; Posada et al., 1993). However, we show here that the MAPK pathway is not involved in activating XFGFR translation, because inactivation of MAPK had no positive effect on either mos- or cdc2-activated XFGFR translation. Interestingly, MAPK may negatively regulate XFGFR translation or protein stability, because activation of the MAPK pathway reduced XFGFR protein levels, and depletion of activated MAPK somewhat increased the level of XFGFR protein induced by cdc2 (Figure 4, B and C). Our finding that the MAPK pathway does not positively regulate XFGFR translation suggests that mos functions through an as-yet-unknown pathway to derepress XFGFR mRNA.
Our studies show that neither mos nor cdc2 alone is sufficient to fully derepress the coding region/CPE. Thus, these two effectors may have distinct functions, although whether they act on the same or different RNA target(s) within the coding region/CPE is unclear at this time. Future experiments to define the coding region/CPE inhibitory element and the putative protein(s) that might bind this element will help elucidate whether c-mos and cdc2 function entirely separately to activate XFGFR translation or whether they converge on a common target.
The XFGFR Coding Region and CPE Together Inhibit Translation
Our current work has identified an inhibitory element within the XFGFR mRNA that requires both the XFGFR coding region and CPE to repress translation in the immature oocyte. Because these two regions are at least 1 kb apart on the full-length mRNA, the functioning of this inhibitory element must require the interaction of sequences over a significant distance. However, because deletion of the intervening 1 kb had no effect on the functioning of this element, the absolute distance between these sequences appears not to be critical. Interestingly, polyadenylation of histone B4 is dependent on c-mos, and the sequences that confer this c-mos dependence are contained in the coding region of the B4 RNA (de Moor and Richter, 1997). Because polyadenylation functions through and requires the CPE in the 3′UTR, the c-mos dependence of the B4 RNA must involve an interaction between the coding region and the CPE. Although the ultimate mos-dependent effects on XFGFR and histone B4 mRNAs are somewhat different, both involve a necessary interaction between the coding region and the CPE in their respective mRNAs. It will be of interest to determine if the coding region elements contained in these two RNAs exhibit sequence or structural similarities.
We observed that in intact RNA containing the TIE, derepression of the coding region/CPE by mos in the absence of cdc2 requires polyadenylation, a process directed by the CPE. This result suggests that the XFGFR coding region/CPE functions not only to inhibit translation in the immature oocyte but also to activate translation during oocyte maturation. Inhibition and activation within the same element has also been observed in other mRNAs. The clam cyclin A and ribonucleotide reductase mRNAs both contain masking elements within their 3′UTRs and bind a common inhibitory protein, p82 (Standart et al., 1990; Walker et al., 1996). However, it was recently discovered that p82 is a homologue of vertebrate CPEB and likewise is important for cytoplasmic polyadenylation of these clam RNAs during translational recruitment in oocyte maturation (Minshall et al., 1999; Walker et al., 1999). Similarly, the 3’ UTR element in C. elegans tra-2 RNA functions not only to repress translation but also to regulate polyadenylation, an activating function (Goodwin et al., 1993; Jan et al., 1997). The TIE within the XFGFR 3′UTR also has an inhibitory function in the immature oocyte, but once derepressed it appears to enhance translation during oocyte maturation through a mechanism distinct from polyadenylation (Culp and Musci, 1998). Thus, it is becoming clearer that some RNA elements and the proteins with which they interact have components of both inhibitory and activating functions. In contrast, the mouse prm-1 3′UTR contains at least two repressive elements, both of which are separable from the sequences required for translational activation (Fajardo et al., 1997). The 3′UTR inhibitory element in oskar mRNA is also distinct from the element required for derepression, which is located in the 5′UTR (Gunkel et al., 1998). In all of these RNAs, the opposing functions of translational repression and activation can be dissected with the use of translation extracts or chimeric reporter RNAs; we accede, however, that the processes of derepression and activation of masked messages may be mechanistically linked in vivo.
What Is the Role of Polyadenylation in Activating XFGFR Translation?
We reported previously that progesterone activates endogenous XFGFR polyadenylation during oocyte maturation, and we have demonstrated here that XFGFR polyadenylation is also activated by mos. In contrast, oocytes matured by injecting cdc2/cyclin B exhibit little to no XFGFR polyadenylation. However, these oocytes do translate mos protein. What does not logically follow then is why XFGFR mRNA is not polyadenylated by the mos activity that accumulates in these cells. This apparent paradox potentially provides clues regarding the role of XFGFR polyadenylation by mos or progesterone. We hypothesize that in progesterone- or mos-induced oocyte maturation, the initial event is elongation of the XFGFR poly(A) tail. Polyadenylation then allows mos to partially derepress the coding region/CPE. Upon later cdc2 activation, complete derepression through both the coding region/CPE and the TIE is achieved, resulting in maximal translation. Conversely, in oocytes matured by cdc2 injection, because cdc2 does not require polyadenylation for its activity, derepression of the cdc2-dependent elements in the XFGFR mRNA occurs first, followed by translation of c-mos. The previous activation of the TIE by cdc2 allows newly translated mos to access and derepress the coding region/CPE, obviating the need for polyadenylation. In this model, either poly(A) elongation or release of the TIE by cdc2 is the initial event required for mos to gain access to the coding region/CPE.
mRNA Recruitment through Modification of RNA-binding Proteins
Through these studies, we hope ultimately to understand how the specificity of translational activation is achieved. Does each repressed mRNA bind a unique inhibitory protein? Do classes of mRNAs defined by their timing of activation bind the same inhibitory protein? If cell cycle effectors regulate translation of individual RNAs by direct modification of RNA binding proteins, these effectors would likely need to interact with diverse targets to effect specific activation. If cell cycle regulators recruit mRNAs indirectly through intermediates, these key effectors would need to interact with only a few general “time-specific” activators, which might then activate specific classes of mRNAs.
In our experiments, we have not addressed the proximity of cdc2 and mos activities to XFGFR translational activation. We previously reported a specific interaction between the TIE and an oocyte cytoplasmic protein, through which we propose that translational repression of this element may be mediated (Robbie et al., 1995). We hypothesize that a similar RNA-protein interaction may also be responsible for inhibition of the XFGFR coding region/CPE. These RNA-binding proteins are then possible targets for posttranslational modification, which might result in derepression of XFGFR. Isolation and characterization of these proteins will allow us to assess whether these proteins are direct targets of mos and cdc2 and thus how these cell cycle regulators cooperate to activate XFGFR translation.
CPEB is a well-characterized example of an RNA-binding protein important for translational recruitment during meiosis. As might be expected of a protein whose function alters over time, CPEB is phosphorylated during oocyte maturation in response to cdc2 activity, and this phosphorylation correlates with the timing of polyadenylation of some mRNAs (Paris et al., 1991; Hake and Richter, 1994). Although polyadenylation of some RNAs is dependent on cdc2, other RNAs are adenylated independently of cdc2 activity (Ballantyne et al., 1997; de Moor and Richter, 1997). However, CPEB is thought to be required for cytoplasmic polyadenylation of all mRNAs, irrespective of their cdc2 dependence (Stebbins-Boaz et al., 1996). Consequently, this single protein is likely not sufficient to provide the specificity for this complex recruitment process. Other factors may associate with CPEB in vivo to specify the timing of adenylation and translational activation during oocyte maturation. The possible effect of phosphorylation on CPEB function has recently been suggested. Although in Xenopus extracts CPEB interacts with its RNA targets independently of phosphorylation status (Paris et al., 1991), in clam eggs, phosphorylated CPEB is not associated with mRNAs after they are recruited onto polysomes, suggesting an altered function for the phosphorylated form of this protein (Minshall et al., 1999).
Phosphorylation is a common mechanism of translational control in biological systems. Somatic cells that reenter the cell cycle after a period of quiescence imposed by nutritional starvation, serum deprivation, or some other mechanism experience an increase in global translation (Kaufman, 1994; Morris, 1995; Sonenberg, 1996). This is true as well in germ cells, including Xenopus oocytes, as they enter meiosis (Wasserman et al., 1982). This general increase in translation is correlated with, and is responsive to, changes in the phosphorylation status of a number of translation initiation factors, including eIF4E, eIF4G, eIF2B, and eIF2α, the PHAS/4eBP proteins that interact with eIF4E, and the ribosomal protein S6 (Morris, 1995; Sonenberg, 1996; Lawrence and Abraham, 1997). The effect of phosphorylating eIF4E, eIF4G, eIF2B, and S6 and of dephosphorylating eIF2α is to enhance the rate of initiation, generally thought to be the rate-limiting step in translation. In some cell systems, the mTOR/p70s6k kinase pathway is responsible for effecting these changes in phosphorylation, whereas in other cells, including Xenopus oocytes, the effectors responsible for this translational control are not known (Morley and Pain, 1995; Flynn and Proud, 1996; Sonenberg, 1996; Fraser et al., 1999). Intriguingly, phosphorylation enhances the interaction between eIF4E, the cap-binding protein, and eIF4G, which serves as a bridge between eIF4E and poly(A) polymerase (Bu et al., 1993; Morley et al., 1993; Tarun and Sachs, 1996; Fraser et al., 1999). In addition, recent experiments have demonstrated that eIF4G is critical for the polyadenylation-dependent translational recruitment of specific masked messages (Keiper and Rhoads, 1999). Thus, the translational activation of masked messages may be connected to changes in global translation during Xenopus meiosis. How intimately these two activities interlace remains to be discovered.
ACKNOWLEDGMENTS
We sincerely thank Jean Gautier, James Ferrell, Lewis T. Williams, Monica Murakami and the G. Vande Woude laboratory, James Maller, Scott Ballantyne, Andrew Murray, Ralph Rupp, Dave Turner, Tim Hunt, and Malcolm Whitman for providing invaluable reagents. We also thank Scott Ballantyne, Marvin Wickens, Monica Murakami, James Maller, and members of the Musci laboratory for many helpful discussions and insights. This work was supported by a grant from the National Institutes of Health (HD30431) and by a postdoctoral fellowship awarded to P.A.C. from the Cowell Foundation.
REFERENCES
- Amaya E, Musci TJ, Kirschner MW. Expression of a dominant negative mutant of the FGF receptor disrupts mesoderm formation in Xenopus embryos. Cell. 1991;66:257–270. doi: 10.1016/0092-8674(91)90616-7. [DOI] [PubMed] [Google Scholar]
- Amaya E, Stein PA, Musci TJ, Kirschner MW. FGF signaling in the early specification of mesoderm in Xenopus. Development. 1993;118:477–487. doi: 10.1242/dev.118.2.477. [DOI] [PubMed] [Google Scholar]
- Ballantyne S, Daniel DL, Jr, Wickens M. A dependent pathway of cytoplasmic polyadenylation reactions linked to cell cycle control by c-mos and CDK1 activation. Mol Biol Cell. 1997;8:1633–1648. doi: 10.1091/mbc.8.8.1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bu X, Haas DW, Hagedorn CH. Novel phosphorylation sites of eukaryotic initiation factor-4F and evidence that phosphorylation stabilizes interactions of the p25 and p220 subunits. J Biol Chem. 1993;268:4975–4978. [PubMed] [Google Scholar]
- Colgan DF, Murthy KG, Zhao W, Prives C, Manley JL. Inhibition of poly(A) polymerase requires p34cdc2/cyclin B phosphorylation of multiple consensus and nonconsensus sites. EMBO J. 1998;17:1053–1062. doi: 10.1093/emboj/17.4.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colgan DF, Murthy KGK, Prives C, Manley JL. Cell-cycle related regulation of poly(A) polymerase by phosphorylation. Nature. 1996;384:282–285. doi: 10.1038/384282a0. [DOI] [PubMed] [Google Scholar]
- Culp PA, Musci TJ. Translational activation and cytoplasmic polyadenylation of FGF receptor-1 are independently regulated during Xenopus oocyte maturation. Dev Biol. 1998;193:63–76. doi: 10.1006/dbio.1997.8785. [DOI] [PubMed] [Google Scholar]
- Daar I, Paules RS, Vande Woude GF. A characterization of cytostatic factor activity from Xenopus eggs and c-mos-transformed cells. J Cell Biol. 1991;114:329–335. doi: 10.1083/jcb.114.2.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Moor CH, Richter JD. The Mos pathway regulates cytoplasmic polyadenylation in Xenopus oocytes. Mol Cell Biol. 1997;17:6419–6426. doi: 10.1128/mcb.17.11.6419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fajardo MA, Haugen HS, Clegg CH, Braun RE. Separate elements in the 3′ untranslated region of the mouse protamine 1 mRNA regulate translational repression and activation during murine spermatogenesis. Dev Biol. 1997;191:42–52. doi: 10.1006/dbio.1997.8705. [DOI] [PubMed] [Google Scholar]
- Ferrell JE, Jr, Machleder EM. The biochemical basis of an all-or-none cell fate switch in Xenopus oocytes. Science. 1998;280:895–898. doi: 10.1126/science.280.5365.895. [DOI] [PubMed] [Google Scholar]
- Flynn A, Proud G. Insulin-stimulated phosphorylation of initiation factor 4E is mediated by the MAP kinase pathway. FEBS Lett. 1996;389:162–166. doi: 10.1016/0014-5793(96)00564-9. [DOI] [PubMed] [Google Scholar]
- Fox CA, Sheets MD, Wickens MP. Poly(A) addition during maturation of frog oocytes: distinct nuclear and cytoplasmic activities and regulation by the sequence UUUUUAU. Genes Dev. 1989;3:2151–2162. doi: 10.1101/gad.3.12b.2151. [DOI] [PubMed] [Google Scholar]
- Fraser CS, Pain VM, Morley SJ. The association of initiation factor 4F with poly(A)-binding protein is enhanced in serum-stimulated Xenopus kidney cells. J Biol Chem. 1999;274:196–204. doi: 10.1074/jbc.274.1.196. [DOI] [PubMed] [Google Scholar]
- Glotzer M, Murray AW, Kirschner MW. Cyclin is degraded by the ubiquitin pathway. Nature. 1991;349:132–138. doi: 10.1038/349132a0. [DOI] [PubMed] [Google Scholar]
- Goodwin EB, Okkema PG, Evans TC, Kimble J. Translational regulation of tra-2 by its 3′ untranslated region controls sexual identity in C. elegans. Cell. 1993;75:329–339. doi: 10.1016/0092-8674(93)80074-o. [DOI] [PubMed] [Google Scholar]
- Gotoh Y, Masuyama N, Dell K, Shirakabe K, Nishida E. Initiation of Xenopus oocyte maturation by activation of the mitogen-activated protein kinase cascade. J Biol Chem. 1995;270:25898–25904. doi: 10.1074/jbc.270.43.25898. [DOI] [PubMed] [Google Scholar]
- Gunkel N, Yano T, Markussen F-H, Olsen LC, Ephrussi A. Localization-dependent translation requires a functional interaction between the 5′ and 3′ ends of oskar mRNA. Genes Dev. 1998;12:1652–1664. doi: 10.1101/gad.12.11.1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haccard O, Lewellyn A, Hartley RS, Erikson E, Maller JL., Jr Induction of Xenopus oocyte meiotic maturation by MAP kinase. Dev Biol. 1995;168:677–682. doi: 10.1006/dbio.1995.1112. [DOI] [PubMed] [Google Scholar]
- Hake LE, Richter JD. CPEB is a specificity factor that mediates cytoplasmic polyadenylation during Xenopus oocyte maturation. Cell. 1994;79:617–627. doi: 10.1016/0092-8674(94)90547-9. [DOI] [PubMed] [Google Scholar]
- Huang C-YF, Ferrell JE. Dependence of Mos-induced Cdc2 activation on MAP kinase function in a cell-free system. EMBO J. 1996;15:2169–2173. [PMC free article] [PubMed] [Google Scholar]
- Huang W, Kessler DS, Erikson RL. Biochemical and biological analysis of Mek1 phosphorylation site mutants. Mol Biol Cell. 1995;6:237–245. doi: 10.1091/mbc.6.3.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huchon D, Rime H, Jessus C, Ozon R. Control of metaphase I formation in Xenopus oocyte: effects of an indestructible cyclin B and of protein synthesis. Biol Cell. 1993;77:133–141. doi: 10.1016/s0248-4900(05)80181-9. [DOI] [PubMed] [Google Scholar]
- Jan E, Motzny CK, Graves LE, Goodwin EB. The STAR protein, GLD-1, is a translational regulator of sexual identity in Caenorhabditis elegans. EMBO J. 1999;18:258–269. doi: 10.1093/emboj/18.1.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jan E, Yoon JW, Walterhouse D, Iannaccone P, Goodwin EB. Conservation of the C. elegans tra-2 3′UTR translational control. EMBO J. 1997;16:6301–6313. doi: 10.1093/emboj/16.20.6301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanki JP, Donoghue DJ. Progression from meiosis I to meiosis II in Xenopus oocytes requires de novo translation of the mosxe protooncogene. Proc Natl Acad Sci USA. 1991;88:5794–5798. doi: 10.1073/pnas.88.13.5794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman RJ. Control of gene expression at the level of translation initiation. Curr Opin Biotech. 1994;5:550–557. doi: 10.1016/0958-1669(94)90073-6. [DOI] [PubMed] [Google Scholar]
- Keiper BD, Rhoads RE. Translational recruitment of Xenopus maternal mRNAs in response to poly(A) elongation requires initiation factor eIF4G-1. Dev Biol. 1999;206:1–14. doi: 10.1006/dbio.1998.9131. [DOI] [PubMed] [Google Scholar]
- Kim-Ha J, Kerr K, Macdonald PM. Translational regulation of oskar mRNA by bruno, an ovarian RNA-binding protein, is essential. Cell. 1995;81:403–412. doi: 10.1016/0092-8674(95)90393-3. [DOI] [PubMed] [Google Scholar]
- Lawrence JC, Jr, Abraham RT. PHAS/4E-BPs as regulators of mRNA translation and cell proliferation. Trends Biochem Sci. 1997;22:345–349. doi: 10.1016/s0968-0004(97)01101-8. [DOI] [PubMed] [Google Scholar]
- Matten WT, Copeland TD, Ahn NG, Vande Woude GF. Positive feedback between MAP kinase and mos during Xenopus oocyte maturation. Dev Biol. 1996;179:485–492. doi: 10.1006/dbio.1996.0277. [DOI] [PubMed] [Google Scholar]
- McGrew LL, Dworkin-Rastl E, Dworkin MB, Richter JD. Poly(A) elongation during Xenopus oocyte maturation is required for translational recruitment and is mediated by a short sequence element. Genes Dev. 1989;3:803–815. doi: 10.1101/gad.3.6.803. [DOI] [PubMed] [Google Scholar]
- Minshall N, Walker J, Dale M, Standart N. Dual roles of p82, the clam CPEB homolog, in cytoplasmic polyadenylation and translational masking. RNA. 1999;5:27–38. doi: 10.1017/s1355838299981220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morley SJ, Pain VM. Hormone-induced meiotic maturation in Xenopus oocytes occurs independently of p70s6k activation and is associated with enhanced initiation factor (eIF)-4F phosphorylation and complex formation. J Cell Sci. 1995;108:1751–1760. doi: 10.1242/jcs.108.4.1751. [DOI] [PubMed] [Google Scholar]
- Morley SJ, Rau M, Kay JE, Pain VM. Increased phosphorylation of eukaryotic initiation factor 4 alpha during early activation of T lymphocytes correlates with increased initiation factor 4F complex formation. Eur J Biochem. 1993;218:39–48. doi: 10.1111/j.1432-1033.1993.tb18349.x. [DOI] [PubMed] [Google Scholar]
- Morris DR. Growth control of translation in mammalian cells. Prog Nucleic Acid Res Mol Biol. 1995;51:339–363. doi: 10.1016/s0079-6603(08)60883-1. [DOI] [PubMed] [Google Scholar]
- Murata Y, Wharton RP. Binding of pumilio to maternal hunchback mRNA is required for posterior patterning in Drosophila embryos. Cell. 1995;80:747–756. doi: 10.1016/0092-8674(95)90353-4. [DOI] [PubMed] [Google Scholar]
- Murray AW. Cell cycle extracts. In: Kay BK, Peng HB, editors. Methods in Cell Biology. San Diego, CA: Academic Press; 1991. pp. 581–605. [PubMed] [Google Scholar]
- Murray AW, Solomon MJ, Kirschner MW. The role of cyclin synthesis and degradation in the control of maturation promoting factor activity. Nature. 1989;339:280–286. doi: 10.1038/339280a0. [DOI] [PubMed] [Google Scholar]
- Nebreda AR, Gannon JV, Hunt T. Newly synthesized protein(s) must associate with p34cdc2 to activate MAP kinase and MPF during progesterone-induced maturation of Xenopus oocytes. EMBO J. 1995;14:5597–5607. doi: 10.1002/j.1460-2075.1995.tb00247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nebreda AR, Hill C, Gomez N, Cohen P, Hunt T. The protein kinase mos activates MAP kinase kinase in vitro and stimulates the MAP kinase pathway in mammalian somatic cells in vivo. FEBS Lett. 1993;333:183–187. doi: 10.1016/0014-5793(93)80401-f. [DOI] [PubMed] [Google Scholar]
- Nebreda AR, Hunt T. The c-mos proto-oncogene protein kinase turns on and maintains the activity of MAP kinase, but not MPF, in cell-free extracts of Xenopus oocytes and eggs. EMBO J. 1993;12:1979–1986. doi: 10.1002/j.1460-2075.1993.tb05847.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishizawa M, Okazaki K, Furuno N, Watanabe N, Sagata N. The ‘second-codon rule’ and autophosphorylation govern the stability and activity of Mos during the meiotic cell cycle in Xenopus oocytes. EMBO J. 1992;11:2433–2446. doi: 10.1002/j.1460-2075.1992.tb05308.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostareck-Lederer A, Ostareck DH, Standart N, Thiele BJ. Translation of 15-lipoxygenase mRNA is controlled by a protein that binds to a repeated sequence in the 3′ untranslated region. EMBO J. 1994;13:1476–1481. doi: 10.1002/j.1460-2075.1994.tb06402.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer A, Gavin AC, Nebreda AR. A link between MAP kinase and p34(cdc2)/cyclin B during oocyte maturation: p90(rsk) phosphorylates and inactivates the p34(cdc2) inhibitory kinase Myt1. EMBO J. 1998;17:5037–5047. doi: 10.1093/emboj/17.17.5037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paris J, Swenson K, Piwnica-Worms H, Richter JD. Maturation-specific polyadenylation: in vitro activation by p34cdc2 and phosphorylation of a 58-kDa CPE-binding protein. Genes Dev. 1991;5:1697–1708. doi: 10.1101/gad.5.9.1697. [DOI] [PubMed] [Google Scholar]
- Posada J, Yew N, Ahn NG, Vande Woude GF, Cooper JA. Mos stimulates MAP kinase in Xenopus oocytes and activates a MAP kinase kinase in vitro. Mol Cell Biol. 1993;13:2546–2553. doi: 10.1128/mcb.13.4.2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter JD. Dynamics of poly(A) addition and removal during development. In: Hershey JWB, Mathews MB, Sonenberg N, editors. Translational Control. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory; 1996. pp. 481–503. [Google Scholar]
- Robbie EP, Peterson M, Amaya E, Musci TJ. Temporal regulation of the Xenopus FGF receptor in development: a translational inhibitory element in the 3′ untranslated region. Development. 1995;121:1775–1785. doi: 10.1242/dev.121.6.1775. [DOI] [PubMed] [Google Scholar]
- Roy LM, Haccard O, Izumi T, Lattes BG, Lewellyn AL, Maller JL. Mos proto-oncogene function during oocyte maturation in Xenopus. Oncogene. 1996;12:2203–2211. [PubMed] [Google Scholar]
- Rupp RA, Snider L, Weintraub H. Xenopus embryos regulate the nuclear localization of XMyoD. Genes Dev. 1994;8:1311–1323. doi: 10.1101/gad.8.11.1311. [DOI] [PubMed] [Google Scholar]
- Sagata N, Oskarsson M, Copeland T, Brumbaugh J, Vande Woude GF. Function of c-mos proto-oncogene product in meiotic maturation in Xenopus oocytes. Nature. 1988;335:519–525. doi: 10.1038/335519a0. [DOI] [PubMed] [Google Scholar]
- Sheets MD, Fox CA, Hunt T, Vande Woude G, Wickens M. The 3′-untranslated regions of c-mos and cyclin mRNAs stimulate translation by regulating cytoplasmic polyadenylation. Genes Dev. 1994;8:926–938. doi: 10.1101/gad.8.8.926. [DOI] [PubMed] [Google Scholar]
- Shibuya EK, Ruderman JV. Mos induces the in vitro activation of mitogen-activated protein kinases in lysates of frog oocytes and mammalian somatic cells. Mol Biol Cell. 1993;4:781–790. doi: 10.1091/mbc.4.8.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonenberg N. mRNA 5′ Cap-binding protein eIF4E. In: Hershey JWB, Mathews MB, Sonenberg N, editors. Translational Control. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory; 1996. pp. 245–269. [Google Scholar]
- Standart N, Dale M, Stewart E, Hunt T. Maternal mRNA from clam oocytes can be specifically unmasked in vitro by antisense RNA complementary to the 3′-untranslated region. Genes Dev. 1990;4:2157–2168. doi: 10.1101/gad.4.12a.2157. [DOI] [PubMed] [Google Scholar]
- Stebbins-Boaz B, Hake LE, Richter JD. CPEB controls the cytoplasmic polyadenylation of cyclin, Cdk2 and c-mos mRNAs and is necessary for oocyte maturation in Xenopus. EMBO J. 1996;15:2582–2592. [PMC free article] [PubMed] [Google Scholar]
- Sun H, Charles CH, Lau LF, Tonks NK. MKP-1 (3CH134), an immediate early gene product, is a dual specificity phosphatase that dephosphorylates MAP kinase in vivo. Cell. 1993;75:487–493. doi: 10.1016/0092-8674(93)90383-2. [DOI] [PubMed] [Google Scholar]
- Tarun SZ, Jr, Sachs AB. Association of the yeast poly(A) tail binding protein with translation initiation factor eIF-4G. EMBO J. 1996;15:7168–7177. [PMC free article] [PubMed] [Google Scholar]
- Walker J, Dale M, Standart N. Unmasking mRNA in clam oocytes: role of phosphorylation of a 3′UTR masking element-binding protein at fertilization. Dev Biol. 1996;173:292–305. doi: 10.1006/dbio.1996.0024. [DOI] [PubMed] [Google Scholar]
- Walker J, Minshall N, Hake L, Richter J, Standart N. The clam 3′ UTR masking element-binding protein p82 is a member of the CPEB family. RNA. 1999;5:14–26. doi: 10.1017/s1355838299981219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasserman WJ, Richter JD, Smith LD. Protein synthesis during maturation promoting factor- and progesterone-induced maturation in Xenopus oocytes. Dev Biol. 1982;89:152–158. doi: 10.1016/0012-1606(82)90303-7. [DOI] [PubMed] [Google Scholar]
- Watanabe N, Vande Woude GF, Ikawa Y, Sagata N. Specific proteolysis of the c-mos proto-oncogene product by calpain on fertilization of Xenopus eggs. Nature. 1989;342:505–511. doi: 10.1038/342505a0. [DOI] [PubMed] [Google Scholar]
- Wickens M, Kimble J, Strickland S. Translational control of developmental decisions. In: Hershey JWB, Mathews MB, Sonenberg N, editors. Translational Control. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory; 1996. pp. 411–450. [Google Scholar]
- Zhang B, Gallegos M, Puoti A, Durkin E, Fields S, Kimble J, Wickens MP. A conserved RNA-binding protein that regulates sexual fates in the C. elegans hermaphrodite germ line. Nature. 1997;390:477–484. doi: 10.1038/37297. [DOI] [PubMed] [Google Scholar]