Abstract
Patients with systemic lupus erythematosus (SLE) and rheumatoid arthritis (RA) have increased risk for atherosclerotic cardiovascular disease. We compared the presence of coronary artery calcium (CAC) in age- and race-matched women with SLE, RA, and healthy controls without diabetes mellitus or history of myocardial infarct, angina pectoris or stroke, and investigated its relation with traditional risk factors, inflammation, and endothelial activation. Study subjects completed cardiovascular risk factor assessment and electron-beam computed tomography that measured CAC. Both female patient groups had similar prevalence and extent of CAC as well as a significant increased odds of having any CAC (odds ratio 1.87, 95% CI 1.09-3.21) and more extensive CAC (odds ratio 4.04, 95% CI 1.42-11.56 for CAC score>100) when compared to healthy controls. After controlling for differences in cardiovascular risk factors, including insulin resistance and hypertension, the results remained statistically significant. After adjustment for differences in levels of C-reactive protein and/or soluble intercellular adhesion molecule-1, however, women with chronic inflammatory diseases no longer had significantly increased odds of having any CAC or more extensive CAC when compared to controls. In conclusion, asymptomatic and non-diabetic women with chronic inflammatory diseases had significantly increased odds of having CAC and more extensive CAC when compared to age- and race-matched healthy controls. The increased odds for coronary artery calcium may in part result from higher levels of inflammation and endothelial activation in these patients.
Introduction
Studies from the general population implicate systemic inflammation as a risk for atherosclerotic cardiovascular disease (ASCVD). In SLE and RA, inflammation characteristically is marked and sustained. Furthermore, inflammatory cytokines and chemokines may not only predict ASCVD risk, but also participate in atherogenesis and promote acute cardiovascular events. Inflammatory markers such as C-reactive protein (CRP) have been implicated in initiating, advancing, and destabilizing human atherosclerotic plaques.1-3 Furthermore, levels of CRP have been demonstrated to independently predict the risk of future myocardial infarction and stroke in non-SLE and non-RA populations.4-6 Similar to CRP, soluble intercellular adhesion molecule-1 (sICAM-1) has been detected in atherosclerotic lesions and is a predictor of CHD risk.7-10 These findings suggest that chronic inflammation and immune activation in SLE and RA may in part explain the accelerated atherosclerosis common to both disorders. This study examines whether the subclinical CAC in non-diabetic SLE women and RA women is higher compared to that of healthy controls and investigates its relation with CHD factors including inflammation and endothelial activation.
Methods
We enrolled 157 women with SLE, 181 women with RA, and 157 healthy controls, who had no history of cardiovascular event (myocardial infarct, angina, or stroke). These women were participating in two studies: the “Heart Effects on Atherosclerosis and Risk of Thrombosis in SLE” study and the “Cardiovascular Disease in RA” study. Women with RA diagnosed after age 16 according to the American College of Rheumatology Criteria11 were non-selectively recruited from the University of Pittsburgh Medical Center (UPMC) Arthritis Network. The primary objective of the RA study was to determine the prevalence of arterial calcification and associated CHD risk factors in women with RA. Similarly, women who fulfilled the 1987 revised American College of Rheumatology criteria of SLE12 but with no prior history of cardiovascular event were non-selectively recruited from the Pittsburgh Lupus Registry to participate in the SLE study, which was designed to compare the prevalence and risk factors of coronary artery and aortic calcification in SLE women and healthy controls. All RA and SLE women had at least 2 years of disease duration. This lupus registry includes women diagnosed with SLE who have been seen either at the UPMC inpatient and outpatient facilities or by practicing rheumatologists in the Pittsburgh metropolitan area. In this study, the healthy women (controls) were matched to the SLE women by age (+/- 5 years) and race. The recruitment methods for healthy controls were based on the following: 1) Voters registration list or Motor Vehicle License list depending on where the case (SLE woman) was found in these lists; 2) direct sample neighborhood control if the case was not in the previous lists. We excluded subjects with diabetes mellitus, as defined by history of diabetes or fasting glucose ≥7 mmol/L (≥126 mg/dL) or hypoglycemic therapy, and then matched all 3 groups of women by age (+/- 5 years) and race.
Information on patient demographics and potential risk factors was collected at the time of Electron Beam Computed Tomography (EBT) scan. The protocols for data collection, EBT scanning and reading were similar for the SLE and RA studies. The University of Pittsburgh Institutional Review Board approved these studies; all patients gave written informed consent.
The study visit included anthropomorphic measurements (height, weight, and waist and hip circumferences), two consecutive blood pressure readings (with patients seated), and a blood draw after a required fast, which was uniform between groups. Blood samples were used to measure total cholesterol, triglycerides, and high-density lipoprotein (HDL) cholesterol at the Lipid Laboratory in the University of Pittsburgh Graduate School of Public Health, which has been certified by the Centers for Disease Control and Prevention. Low-density lipoprotein (LDL) cholesterol was calculated from measured total cholesterol, HDL and triglycerides (Friedewald equation).13 Metabolic syndrome was defined by the National Cholesterol Education Program Adults Treatment Panel III guidelines.14 The homeostatic model assessment of insulin resistance (HOMA-IR) was calculated by [insulin (mU/liter) × glucose (mmoles/Liter)] ÷22.5.15, 16 Hypertension was defined by physician diagnosis, measured mean blood pressure ≥140/90 mm Hg or antihypertensive medication use. Other information was collected on family history of ASCVD (first-degree relative having myocardial infarction or stroke before age 60), cigarette smoking (current, past, or never), and menopausal status.
Information was recorded on corticosteroid treatment (current use, ever used, and daily dosage) and current use of hydroxychloroquine, immunosuppressants for SLE or other disease-modifying anti-rheumatic drugs or biologic therapies for RA, non-steroidal anti-inflammatory drugs, antihypertensives, hormonal therapy, and lipid-lowering medications.
Fibrinogen was measured using a modified clot-rate assay. An enzyme-linked immunosorbent assay was used for determination of high sensitivity CRP (hsCRP). sICAM-1 was measured using commercial assays (Parameter Human sICAM-1 Immunoassay; R&D Systems, Minneapolis, MN) at the Laboratory for Clinical Biochemistry Research (University of Vermont, Burlington, VT).
The EBT scans were performed using an Imatron C-150 scanner (Imatron, San Francisco, CA) using standard imaging procedures in all participants. The same scanner and methodology was used in the SLE, RA, and healthy controls. For CAC, 3-mm slices were scanned at the same point in diastole (at 80% of the patient's RR interval in electrocardiogram) during a single breath hold, starting at the aortic root to the apex of the heart. All EBT scans were read by the same cardiologist (DE). The individual region of interest scores were then summed for a total Agatston calcification score.17 We based our analysis on the total Agatston calcification scores as previously described.18
All subjects who were matched by age (+/- 5 years) and race and had no diabetes mellitus were included in the analysis. We first did a three way comparison: SLE vs. RA, SLE vs. controls, and RA vs. controls. Since we did not find significant differences in CAC or more extensive CAC between the SLE and RA groups, we combined the two rheumatic disease groups. The differences in clinical variables in the SLE and RA combined group vs. the control group were determined by univariate conditional logistic regression. Pair-wise comparison (matched analysis) using conditional logistic regression was done with control as the reference group. Waist-hip ratio, insulin, HOMA-IR, hsCRP, and sICAM-1 were log-transformed to normality. The distribution of CAC scores was highly skewed and could not be normalized by standard transformations. CAC score was categorized based on the published guidelines that corresponded to probability of significant CHD: 0 (very low probability of CHD), 1 to 10 (very unlikely CHD), 11 to 100 (likely mild to minimal coronary stenosis), >100 to 400 (non-obstructive CHD), and >400 (high likelihood of > 1 “significant” coronary stenosis).19 Since very few subjects have CAC score > 400 (6 SLE, 3 RA, and 2 control women), we modified the guidelines by combining the two higher CAC groups as one with CAC score >100. Univariate conditional and multivariable conditional logistic regressions were used to determine the significance and strength of association between the presence of CAC and more extensive CAC in study groups. Differences in factors known to be associated with CHD were adjusted in the multivariable conditional logistic models. In a matched case-control power analysis, 210 cases (SLE or RA) and 105 controls will provide 80% power to detect a minimum odds ratio of 1.5 for presence of CAC assuming the baseline prevalence of CAC in controls was 0.35 and correlation between the cases and control risks was 0.20. All tests used a two-tailed significance level of 0.05. Analyses were performed using the Stata SE 9.0 (Stata Corporation, College Station, TX) and SAS 9.1 (SAS, Gary, NC) for Windows.
Results
Based on matching for age and race, 105 women from each group were included in the analysis. The excluded RA women were significantly older and more likely to have higher prevalence of hypertension and higher levels of sICAM compared to the RA women who remained in the study group. Otherwise, there were no significant differences in CHD risk factors such as cholesterol, and hsCRP between the excluded and study groups. The prevalence of any CAC and CAC >100 was significantly higher in the excluded RA women compared to the RA women in the study (70% vs. 48%, p=0.003; 32% vs. 15%, p=0.009, respectively). No differences in prevalence of CAC and CAC >100 were observed between the excluded and study groups of the SLE women and controls.
The characteristics of the individuals in the three study groups are summarized in Table 1. Women with SLE or RA were more likely to be postmenopausal than healthy controls (p=0.002). SLE women were also more likely to have hypertension compared to controls. RA women had significantly higher levels of hsCRP and homocysteine compared to controls. Women with SLE or RA were more likely to have higher sICAM-1 when compared with controls (p <0.0001). Body mass index, waist-hip ratio, smoking history, family history of ASCVD, mean systolic or diastolic blood pressures, total cholesterol, HDL and LDL cholesterol were comparable among the three groups.
Table 1.
Demographic, clinical, and laboratory characteristics of the study subjects
| Characteristics* | SLE
(n=105) |
RA
(n=105) |
Controls
(n=105) |
P† |
|---|---|---|---|---|
| Demographics | ||||
| Age (years) | 51.1 (9.3) | 52.4 (7.8) | 51.6 (8.6) | 0.51 |
| Race (Caucasian) | 101 (96.2%) | 101 (96.2%) | 101 (96.2%) | 0.99 |
| Age at diagnosis (years) | 34.4 (11.3) | 37 (11.3) | - | 0.04 |
| Disease duration (years) | 16.5 (7.2) | 15.7(10.4) | - | 0.27 |
| Postmenopausal | 64 (61%)‡ | 69 (65.7%)‡ | 52 (49.5%) | 0.002 |
| Hypertension | 56 (53.3%)‡ | 29 (27.6%) | 31 (30.1%) | 0.06 |
| Body mass index (kg/m2) | 28.2 (6.4) | 27.9 (5.9) | 28.4(6.7) | 0.63 |
| The metabolic syndrome | 28 (26.7%) | 24 (22.9%) | 22 (21%) | 0.47 |
| Waist-hip ratio | 0.83 (IQR 0.78-0.87) | 0.84 (IQR 0.79-0.91) | 0.80 (IQR 0.77-0.84) | 0.05 |
| Systolic blood pressure (mmHg) | 121.2 (18.6) | 119.9 (17.3) | 123.1 (17.6) | 0.22 |
| Diastolic blood pressure (mmHg) | 76.9 (10.3) | 76.6 (9.6) | 77 (10.1) | 0.84 |
| Glucose (mmol/L) | 4.9 (IQR 4.6-5.3) | 4.8 (IQR 4.4-5.1)‡ | 5.2 (IQR 4.9-5.6) | <0.0001 |
| Insulin (pmol/L) | 82.6 (IQR 63.2-120.8) | 74.3 (IQR 55.6-91) | 79.2(IQR 64.6-102.8) | 0.27 |
| HOMA-IR | 2.6 (IQR 1.9-3.9) | 2.2 (IQR 1.7-2.9) | 2.6 (IQR 2.1-3.6) | 0.04 |
| Total cholesterol (mmol/L) | 5.1 (1.1) | 5.4 (0.9) | 5.3 (0.9) | 0.80 |
| Total cholesterol (mg/dL) | 195.1(43.5) | 209.8 (34.5) | 203.6 (36.6) | |
| LDL cholesterol (mmol/L) | 2.9 (0.9) | 3.2 (0.9) | 3.2 (0.9) | 0.26 |
| LDL cholesterol (mg/dL) | 113.7 (34.8) | 123 (34.9) | 122.9 (33.3) | |
| HDL cholesterol (mmol/L) | 1.4 (0.4) | 1.6 (0.4) | 1.5 (0.4) | 0.80 |
| HDL cholesterol (mg/dL) | 55.5 (16.7) | 60.8 (14.3) | 58.6(15.5) | |
| Triglycerides (mmol/L) | 1.5 (0.9) | 1.5(0.9) | 1.3 (0.5) | 0.02 |
| Triglycerides (mg/dL) | 134.8 (81.6) | 130.3 (79.8) | 112.7 (48.6) | |
| Current smoker | 8 (7.6%) | 13 (12.4%) | 7 (6.7%) | 0.34 |
| Ever smokers | 37 (35.2%) | 46 (43.8%) | 39 (37.1%) | 0.65 |
| Current medications | ||||
| Corticosteroids | 39 (37.1%) | 46 (43.8%) | - | 0.32 |
| Dosage (mg/day) | 5 (3.1-7.8) | 5 (3-5) | - | 0.87 |
| Hydroxychloroquine | 52 (49.5%) | 23 (21.9%) | - | 0.0002 |
| Immunosuppressives | 14 (13.3%) | 63 (60%) | - | <0.0001 |
| Anti-tumor necrosis factor-α | 0 | 41 (39.1%) | - | - |
| Non-steroidal anti-inflammatory drugs | 39 (37.1%)‡ | 75 (71.4%)‡ | 13 (12.4%) | <0.0001 |
| Aspirin | 11 (10.5%) | 2 (1.9%) | 8 (7.6%) | 0.64 |
| Hormonal therapy | 13 (12.4%) | 37(35.2%)‡ | 16 (15.2%) | 0.09 |
| Lipid-lowering drug | 9 (8.6%) | 9 (8.6%) | 4 (3.8%) | 0.12 |
| hsCRP (mg/L) | 2.3 (IQR 1.0-5.6) | 5.2 (IQR 1.9-11.5) ‡ | 1.6 (IQR 0.6-3.7) | <0.0001 |
| Fibrinogen (μmol/L) | 10 (IQR 7.8-11.7) | 9.1 (IQR 7.3-10.9) | 9.7 (IQR 8.7-11.1) | 0.08 |
| Soluble intercellular adhesion molecule-1 (ng/mL) | 268.7 (IQR 242.9-318.6)‡ | 271.4 (IQR 231.5-338.1)‡ | 249.5 (IQR 219.7-276.1) | <0.0001 |
| Homocysteine (μmol/L) | 69.5 (IQR 59.9-85.8) | 79.1 (IQR 66.6-95.4) ‡ | 65.8 (IQR 54.7-75.4) | 0.002 |
Values are mean (SD) or median (interquartile range/IQR: 25th-75th)
Comparison between SLE and RA vs. control groups; or comparison between SLE and RA groups when control group is not present
p<0.02 vs. control
There was no significant difference in current or ever steroid use between women with SLE and RA, and the median daily dose was also similar. However, cumulative exposure to corticosteroids could not be compared directly for SLE and RA. Hydroxychloroquine was more commonly used in the SLE women (49.5%) than those with RA (21.9%). More RA women were taking steroid-sparing agents compared to those with SLE (p<0.0001) at time of study. One third of the 63 RA women taking disease modifying drugs were also on concomitant anti-tumor necrosis factor-α therapy.
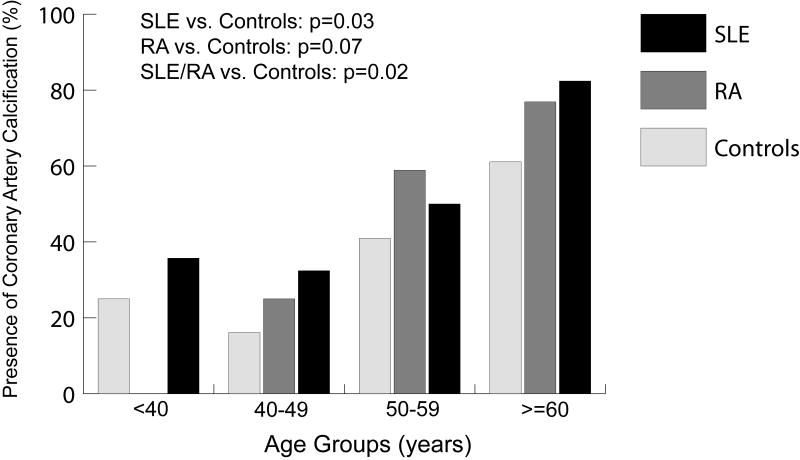
Median CAC scores were 0 (IQR: 0-22.7) in SLE, 0.5 (IQR: 0-38.2) in RA, and 0 (IQR: 0-3.1) in controls. The overall prevalence of CAC appeared to be higher in asymptomatic women with either SLE (47.6%) or RA (47.6%) compared to controls (35.2%). Figure 1 demonstrates the higher prevalence of CAC in SLE and RA women by age groups compared to controls (p=0.02). Furthermore, among those less than 40 years of age, 36% (5/14) of the SLE women and 25% (3/12) of the controls had any CAC whereas none of the 8 RA women had any CAC. After adjusting for differences in CHD risk factors, women with SLE or RA continued to have a 2-fold increase in odds of having any CAC and nearly 4-fold increase in odds of having more extensive CAC, as shown in Table 2, when compared to healthy controls. However, having either SLE or RA was no longer associated with the presence of any CAC or more extensive CAC after adjusting for differences in markers of inflammation and endothelial activation (hsCRP and sICAM-1). The loss of significant association with CAC outcomes occurred even if hsCRP and sICAM-1 were added separately to the multivariable model with and without CHD risk factors.
Figure 1.
Prevalence of coronary artery calcification in women with SLE, RA, and healthy controls by age group.
Table 2.
Unadjusted and multivariable-adjusted associations of coronary heart disease (CHD) risk factors, inflammatory factors with presence of coronary artery calcium and more extensive coronary artery calcium in women with systemic lupus erythematosus or rheumatoid arthritis compared to age- and race-matched controls
| Outcome variables* | Unadjusted OR
(95% CI) |
CHD risk factors
Adjusted OR (95% CI) |
CHD risk factors and inflammatory factors
Adjusted OR (95% CI) |
|---|---|---|---|
| Presence of CAC | 1.87 (1.09-3.21) | 2.16 (1.15-4.06) | 1.53 (0.74-3.14) |
| More extensive CAC | |||
| 1-10 | 1.69 (0.85-3.35) | 2.15 (0.99-4.67) | 1.45 (0.60-3.54) |
| 11-100 | 1.44 (0.68-3.07) | 1.69 (0.72-3.95) | 1.19 (0.46-3.11) |
| >100 | 4.04 (1.42-11.56) | 3.83 (1.23-11.89) | 2.89 (0.85-9.89) |
CAC=coronary artery calcium; CHD risk factors: history of smoking, postmenopausal status, hypertension, HOMA-IR (log) and total cholesterol/high density lipoprotein cholesterol ratio, homocysteine; inflammatory features: hsC-reactive protein (log), sICAM-1 (log); OR=odds ratio. 95% CI=95% confidence interval
To further investigate the influence of hsCRP and sICAM-1 in CAC outcomes in women with rheumatic disease, we analyzed our data in those with SLE or RA only. In addition to moderate correlation between hsCRP and BMI (Spearman rank correlation/rs=0.41, p<0.0001), there was a significant interaction between hsCRP and BMI in the regression model for any CAC (p=0.02) and more extensive CAC (p=0.006). We thus used waist-hip ratio rather than BMI as the measure of adiposity since it was not highly correlated with other predictors in these multivariable models. Among women with SLE or RA, those in the highest quartile of hsCRP were more likely to have any CAC (OR 5.10; 95% CI: 2.12-12.28) and more extensive CAC (OR 3.54; 95% CI: 1.64-7.65) compared to the referent group as shown in Table 3. Women with SLE or RA in the second lower quartile of hsCRP (1.4-3.2) appeared to have increase in odds for having more extensive CAC (OR 2.27; 95% CI: 1.01-5.03) after adjusting for age. However, only the association between the CAC outcomes and the top quartile of hsCRP persisted after controlling for CHD risk factors and SLE/RA duration. Similar to hsCRP, the top quartile of sICAM-1 was associated with any CAC (OR 3.51; 95% CI 1.46-8.46) and more extensive CAC (OR 2.65; 95% CI: 1.21-5.84) in the age-adjusted logistic regression models; this association was no longer significant after adjusting for CHD risk factors as listed in Table 3, and SLE or RA duration. Levels of hsCRP and sICAM were mildly correlated with each other (rs: 0.26, p=0.0001). There was no interaction between hsCRP and sICAM. When quartiles of both hsCRP and sICAM-1 were included in the same regression model, the top quartile of hsCRP remained significantly associated with any CAC and more extensive CAC whereas the association between sICAM-1 and CAC outcomes was attenuated. Additional adjustment for CAC measures with race, family history of ASCVD, and medications such as aspirin, lipid-lowering agents or hydroxychloroquine, had minimal impact on the association found in these multivariable models.
Table 3.
Unadjusted and multivariable-adjusted associations of quartiles of C-reactive protein with presence of coronary artery calcium and more extensive coronary artery calcium in women with systemic lupus erythematosus or rheumatoid arthritis
| Quartile of hsCRP levels | Presence of CAC* | More Extensive CAC | ||
|---|---|---|---|---|
| Age-adjusted OR
(95% CI) |
CHD risk factors and disease duration
Adjusted OR (95% CI) |
Age-adjusted OR
(95% CI) |
CHD risk factors and disease duration
Adjusted OR (95% CI) |
|
| <1.4 | Referent | Referent | Referent | Referent |
| 1.4-3.3 | 2.26 (0.97-5.29) | 1.91 (0.74-4.92) | 2.27 (1.01-5.03) | 1.81 (0.79-4.16) |
| 3.4-8.1 | 1.75 (0.73-4.17) | 1.11 (0.40-3.08) | 1.56 (0.69-3.54) | 0.99 (0.40-2.45) |
| >8.1 | 5.10 (2.12-12.28) | 4.15 (1.56-11.08) | 3.54 (1.64-7.65) | 2.79 (1.24-6.28) |
CAC=coronary artery calcium; CHD risk factors: age, history of smoking, hypertension, waist-hip ratio (every 0.2 units), HOMA-IR (log) and total cholesterol/high density lipoprotein cholesterol ratio: OR=odds ratio. 95% CI=95% confidence interval
Addition of race, menopausal status, family history of CHD, homocysteine, current aspirin use, lipid-lowering medication or hydroxychloroquine use did not change the multivariate model.
Discussion
To our knowledge, this is the first study to compare the prevalence of coronary artery calcification among non-diabetic and age-matched female patients with SLE, RA, and healthy controls. Our study found a higher prevalence of asymptomatic CAC in SLE women (48%) and RA women (48%) compared to healthy controls (35%). Our reported prevalence of CAC in the SLE women is higher than the reported frequency of 20% by Asanuma et al and 30% by von Feldt et al.20, 21 The difference in prevalence may be explained in part by the older age of our SLE women (mean age: 51 vs. 40 and 43 years respectively). Asanuma et al also reported CAC in 7% (2/30) patients with SLE less than 40 years of age but none in controls of the same age group. Interestingly, we found that one third of our SLE women and a quarter of our healthy controls under the age of 40 had premature CAC, while none of the RA women in this age group had any CAC. Our control group has a similar metabolic profile as the patient group, which may explain the high prevalence of CAC in these control women. The prevalence of CAC in RA was similar to that reported by Chung et al.22 These findings further substantiate the greater burden of atherosclerosis in patients with chronic inflammatory diseases.
Women with SLE or RA were more likely to have any CAC and more extensive CAC when compared to age- and race-matched controls, independent of traditional CHD risk factors. The significant association between SLE or RA and CAC disappeared after controlling for hsCRP and sICAM. This may be due to the much higher levels of inflammation in patients with SLE or RA compared to those without rheumatic disease. HsCRP and sICAM-1 were associated with increased CAC in nondiabetic women with SLE or RA. Similar to our findings, hsCRP has previously been shown to be independently associated with arterial stiffness, another noninvasive measure of atherosclerosis, in patients with SLE or RA after adjusting for age at diagnosis, disease duration, and cholesterol levels.23 In several population-based epidemiologic studies,24-26 hsCRP was not independently associated with atherosclerotic burden by CAC outcomes after adjusting for traditional CHD risk factors. On the contrary, the top quartile of hsCRP remained independently associated with a significant increase in atherosclerotic burden as measured by EBT in women with SLE or RA after adjusting for CHD risk factors.
High levels of inflammation or CRP in the SLE or RA patients may be independently contributing to atherosclerosis. There is evidence that CRP may participate in atherogenesis beginning with increased leukocyte adhesion and migration,27, 28 and vascular endothelial dysfunction.29 CRP has also been identified in atherosclerotic plaques of patients with unstable angina30 and co-localized with complement in the infarcted myocardium.31 In women without SLE or RA, sICAM-1 predicted cardiovascular events that reflect progression of coronary atherosclerotic disease and luminal narrowing but not acute vaso-occlusive events such as myocardial infarction or stroke.10 sICAM-1 also has been associated with disease activity in patients with SLE and RA32 and with insulin resistance in patients with SLE.33 Unlike the potential pathogenic role of CRP in atherogenesis, the association of endothelial activation with atherosclerosis may be more related to the CHD risk factors in women with SLE or RA.
Our study has several limitations. First, this is a cross-sectional study. A prospective study is under way to determine the prognostic significance of CRP and presence of CAC in patients with SLE or RA. Second, EBT measures calcified atherosclerotic plaque but does not assess the presence of non-calcified plaque or plaque stability, both of which are also important determinants of future CHD events. Third, the risk factors including hsCRP and sICAM-1 were calculated at one point in time for this study and may not reflect the cumulative burden of inflammation in these patients. Fourth, the majority of our study subjects were Caucasian and therefore, we were unable to detect differences in race when we analyzed the relationship of hsCRP and sICAM-1 in patients with chronic inflammatory diseases. Finally, we controlled for known CHD risk factors in multivariable regression analyses. It is possible that there may be other CHD risks which we have not taken into account.
This is the first study to show that nondiabetic women with SLE or RA have more CAC than their matched controls and that this risk may be linked to CRP. This is consistent with the exceedingly high risk of myocardial infarction in young women with SLE and RA. Our study also supports the notion that inflammation and endothelial activation may play the most significant roles in accounting for this excess risk.
Acknowledgments
The authors would like to thank the women who participated in our studies and Dr. Kathleen McKinnon for review of our manuscript.
Grant support: the Arthritis Foundation, Western PA chapter, NIH/MAC grant 1-P60-AR-44811 01; NIH R01 AR46588-01; and K24 AR02213-01; NIH/NCRR/GCRC Grant MO1-RR000056; NIH K23 AR47571; NIH K23 AR51044; American Heart Association Established Investigator Award 0040149N; American College of Rheumatology/REF Physician Scientist Development Award.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Maseri A. Inflammation, atherosclerosis, and ischemic events – exploring the hidden side of the moon. N Engl J Med. 1997;336(14):1014–1016. doi: 10.1056/NEJM199704033361409. [DOI] [PubMed] [Google Scholar]
- 2.Watanabe T, Haraoka S, Shimokama T. Inflammatory and immunological nature of atherosclerosis. Int J Cardiol. 1996;54(suppl):S25–34. doi: 10.1016/s0167-5273(96)88773-0. [DOI] [PubMed] [Google Scholar]
- 3.Zhang Y, Cliff W, Schoefl G, Higgins G. Coronary C-reactive protein distribution: its relation to development of atherosclerosis. Atherosclerosis. 1999;145:375–379. doi: 10.1016/s0021-9150(99)00105-7. [DOI] [PubMed] [Google Scholar]
- 4.Ridker P. C-reactive protein and risks of future myocardial infarction and thrombotic stroke. Eur Heart J. 1998;19:1–3. doi: 10.1053/euhj.1997.0604. [DOI] [PubMed] [Google Scholar]
- 5.Ridker P, Buring J, Shih J, Matias M, Hennekens C. Prospective study of C reactive protein and risk of cardiovascular disease in apparently healthy women. Circulation. 1998;98:731–733. doi: 10.1161/01.cir.98.8.731. [DOI] [PubMed] [Google Scholar]
- 6.Bogaty P, Robitaille N, Solymoss S, Boyer L, Auger D, Labbe L, Simard S, Rail J, Genest JJ, Turgeon J. Atherogenic, hemostatic, and other potential risk markers in subjects with previous isolated myocardial infarction compared with long-standing uncomplicated stable angina. Am Heart J. 1998;136(5):884–893. doi: 10.1016/s0002-8703(98)70135-8. [DOI] [PubMed] [Google Scholar]
- 7.O'Malley T, Ludlam CA, Riemermsa RA, Fox KAA. Early increase in levels of soluble inter-cellular adhesion molecule-1 (sICAM-1). Potential risk factor for the acute coronary syndromes. Eur Heart J. 2001;22(14):1226–1234. doi: 10.1053/euhj.2000.2480. [DOI] [PubMed] [Google Scholar]
- 8.Hwang SJ, Ballantyne CM, Sharrett AR, Smith LC, Davis CE, Gotto AM, Jr, Boerwinkle E. Circulating Adhesion Molecules VCAM-1, ICAM-1, and E-selectin in Carotid Atherosclerosis and Incident Coronary Heart Disease Cases : The Atherosclerosis Risk In Communities (ARIC) Study. Circulation. 1997;96(12):4219–4225. doi: 10.1161/01.cir.96.12.4219. [DOI] [PubMed] [Google Scholar]
- 9.Ridker PM, Hennekens CH, Roitman-Johnson B, Stampfer MJ, Allen J. Plasma concentration of soluble intercellular adhesion molecule 1 and risks of future myocardial infarction in apparently healthy men. The Lancet. 1998;351(9096):88–92. doi: 10.1016/S0140-6736(97)09032-6. [DOI] [PubMed] [Google Scholar]
- 10.Albert MA, Glynn RJ, Buring JE, Ridker PM. Differential effect of soluble intercellular adhesion molecule-1 on the progression of atherosclerosis as compared to arterial thrombosis: A prospective analysis of the Women's Health Study. Atherosclerosis. 2007 doi: 10.1016/j.atherosclerosis.2007.04.051. Published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arnett F, Edworthy S, Bloch D, McShane D, Fries J, Cooper N, Healey L, Kaplan S, Liang M, Luthra H, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31(3):315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 12.Petri M, Perez-Gutthann S, Spence D, Hochberg M. Risk factors for coronary artery disease in patients with systemic lupus erythematosus. Am J Med. 1992;93:513–519. doi: 10.1016/0002-9343(92)90578-y. [DOI] [PubMed] [Google Scholar]
- 13.Friedewald W, Levy R, Fredrickson D. Estimation of the concentration of low-density lipoprotein cholesterol in plasma without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 14.NIH. Third Report of the National Cholesterol Education Program Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adults Treatment Panel III) Bethesda, MD: National Institutes of Health; 2001. [Google Scholar]
- 15.Matthews D, Hosker J, Rudenski A, Naylor B, Treacher D, Turner R. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 16.Dessein PH, Joffe BI. Insulin resistance and impaired beta cell function in rheumatoid arthritis. Arthritis Rheum. 2006;54(9):2765–2775. doi: 10.1002/art.22053. [DOI] [PubMed] [Google Scholar]
- 17.Agatston A, Janowitz W, Hildner F, Zusmer N, Viamonte MJ. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15(4):827–832. doi: 10.1016/0735-1097(90)90282-t. [DOI] [PubMed] [Google Scholar]
- 18.Newman A, Naydeck B, Sutton-Tyrrell K, Edmundowicz D, Gottdiener J, Kuller L. Coronary artery calcification in older adults with minimal clinical or subclinical cardiovascular disease. J Am Geriatr Soc. 2000;48(3):342–343. doi: 10.1111/j.1532-5415.2000.tb02643.x. [DOI] [PubMed] [Google Scholar]
- 19.Rumberger J, Brundage B, Rader D, Kondos G. Electron beam computed tomographic coronary calcium scanning: a review and guidelines for use in asymptomatic persons. Mayo Clin Proc. 1999;74(3):243–252. doi: 10.4065/74.3.243. [DOI] [PubMed] [Google Scholar]
- 20.Asanuma Y, Oeser A, Shintai A, Turner E, Olsen N, Fazio S, Linton M, Raggi P, Stein C. Premature Coronary-artery atherosclerosis in systemic lupus erythematosus. N Engl J Med. 2003;349:2407–2415. doi: 10.1056/NEJMoa035611. [DOI] [PubMed] [Google Scholar]
- 21.von Feldt J, Scalzi L, Cucchiara A, Morthala S, Kealey C, Flagg S, Genin A, van Dyke A, Nackos E, Chander A, Gehrie E, Cron R, Whitehead A. Homocysteine levels and disease duration independently correlate with coronary artery calcification in patients with systemic lupus erythematosus. Arthritis Rheum. 2006;54(7):2220–2227. doi: 10.1002/art.21967. [DOI] [PubMed] [Google Scholar]
- 22.Chung C, Oeser A, Raggi P, Gebretsadik T, Shintani A, Sokka T, Pincus T, Avalos I, Stein C. Increased coronary-artery atherosclerosis in rheumatoid arthritis: relationship to disease duration and cardiovascular risk factors. Arthritis Rheum. 2005;52(10):3045–3053. doi: 10.1002/art.21288. [DOI] [PubMed] [Google Scholar]
- 23.Roman M, Devereux R, Schwartz J, Lockshin M, Paget S, Davis A, Crow M, Sammaritano L, Levine D, Shankar B, Moeller E, Salmon J. Arterial stiffness in chronic inflammatory diseases. Hypertension. 2005;46(1):194–199. doi: 10.1161/01.HYP.0000168055.89955.db. [DOI] [PubMed] [Google Scholar]
- 24.Khera A, de Lemos JA, Peshock RM, Lo HS, Stanek HG, Murphy SA, Wians FH, Jr, Grundy SM, McGuire DK. Relationship Between C-Reactive Protein and Subclinical Atherosclerosis: The Dallas Heart Study. Circulation. 2006;113(1):38–43. doi: 10.1161/CIRCULATIONAHA.105.575241. [DOI] [PubMed] [Google Scholar]
- 25.Wang TJ, Larson MG, Levy D, Benjamin EJ, Kupka MJ, Manning WJ, Clouse ME, D'Agostino RB, Wilson PWF, O'Donnell CJ. C-Reactive Protein Is Associated With Subclinical Epicardial Coronary Calcification in Men and Women: The Framingham Heart Study. Circulation. 2002;106(10):1189–1191. doi: 10.1161/01.cir.0000032135.98011.c4. [DOI] [PubMed] [Google Scholar]
- 26.van der Meer IM, Oei HHS, Hofman A, Pols HAP, de Jong FH, Witteman JCM. Soluble Fas, a mediator of apoptosis, C-reactive protein, and coronary and extracoronary atherosclerosis: The Rotterdam Coronary Calcification Study. Atherosclerosis. 2006;189(2):464–469. doi: 10.1016/j.atherosclerosis.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 27.Ballou S, Lozanski G. Induction of inflammatory cytokine release from cultured human monocytes by C-reactive protein. Cytokine. 1992;4(5):361–368. doi: 10.1016/1043-4666(92)90079-7. [DOI] [PubMed] [Google Scholar]
- 28.Pasceri V, Willerson JT, Yeh ETH. Direct Proinflammatory Effect of C-Reactive Protein on Human Endothelial Cells. Circulation. 2000;102(18):2165–2168. doi: 10.1161/01.cir.102.18.2165. [DOI] [PubMed] [Google Scholar]
- 29.Venugopal SK, Devaraj S, Jialal I. C-Reactive Protein Decreases Prostacyclin Release From Human Aortic Endothelial Cells. Circulation. 2003;108(14):1676–1678. doi: 10.1161/01.CIR.0000094736.10595.A1. [DOI] [PubMed] [Google Scholar]
- 30.Ishikawa T, Hatakeyama K, Imamura T, Date H, Shibata Y, Hikichi Y, Asada Y, Eto T. Involvement of C-reactive protein obtained by directional coronary atherectomy in plaque instability and developing restenosis in patients with stable or unstable angina pectoris. Am J Cardiol. 2003;91(3):287–292. doi: 10.1016/s0002-9149(02)03156-9. [DOI] [PubMed] [Google Scholar]
- 31.Nijmeijer R, Krijnen PAJ, Assink J, Klaarenbeek MAR, Lagrand WK, Veerhuis R, Visser CA, Meijer CJLM, Niessen HWM, Hack CE. C-reactive protein and complement depositions in human infarcted myocardium are more extensive in patients with reinfarction or upon treatment with reperfusion. Eur J Clin Invest. 2004;34(12):803–810. doi: 10.1111/j.1365-2362.2004.01425.x. [DOI] [PubMed] [Google Scholar]
- 32.MacHold K, Keiner H, Graninger W, Granninger W. Soluble intercellular adhesion molecule-1 (sICAM-1) in patients with rheumatoid arthritis and systemic lupus erythematosus. Clin Immunol Immunopath. 1993;68(1):74–78. doi: 10.1006/clin.1993.1098. [DOI] [PubMed] [Google Scholar]
- 33.Tso T, Huang W. Elevated soluble intercellular adhesion molecule-1 levels in patients with systemic lupus erythematosus: relation to insulin resistance. J Rheumatol. 2007;34(4):726–730. [PubMed] [Google Scholar]