Abstract
Objective
To elucidate which anatomical sites need to be sampled to detect human papillomavirus (HPV) infection in the lower male genital tract.
Method
In an HPV survey of Mexican soldiers (median age 24 years; range 16–50 years), a cell sample from 2 cm deep into the distal urethra (group 1; n = 168 men), or 0.5 cm deep into the meatus urethralis (group 2; n = 414 men) was collected, along with a sample from the external genitalia. The different samples were tested for 27 HPV types using a polymerase chain reaction based strip assay.
Results
HPV DNA was detected more frequently in external genitalia samples (46.4%) than in the urethra (20.8%) or meatus samples (12.1%). Lack of samples from the urethra or meatus would have led to 5.1% and 1.5% false HPV negative results, respectively. The most frequently detected high risk HPV types (HPV 59, 52, 51, and 16) were similar in different sites, whereas low risk types were found rarely in urethra samples.
Conclusions
The addition of cell samples from the meatus to those from external genitalia contributed negligibly to the evaluation of the prevalence of HPV in men. HPV detection was slightly improved by the addition of urethra samples, but the gain may not justify the discomfort of the procedure in large epidemiological studies.
Keywords: human papillomavirus, penis, urethra, test sensitivity
The prevalence of human papillomavirus (HPV) in the genital tract of men tends to be similar to that in women (that is, between 3% and 40%, depending upon the population and age group considered).1,2,3,4,5,6,7,8 It is unclear, however, which genital sites need to be sampled to detect HPV in men. Exfoliated cells were collected from the meatus urethralis and from the distal urethra in certain studies,7 but urethral sampling is painful and can potentially decrease participation, especially in follow up studies.
We therefore compared the relative contribution of cell samples from the external genitalia and the distal urethra or meatus urethralis to the evaluation of HPV prevalence.
Methods
The present report deals with the first 820 men recruited between February 2001 and October 2002 in a larger study on HPV prevalence in 1612 Mexican soldiers. An age stratified random sample was drawn from a list of soldiers who were attending a 1 year minimum period of service in central Mexico. Overall, 7.5% of the men contacted refused to participate in the study and 1.4% could not attend because of concurrent illnesses. All study participants were fully informed of study aims and procedures and signed an informed consent form. The study was cleared by the ethics committee of the National Institute of Public Health of Mexico, and the International Agency for Research on Cancer.
Participants were instructed not to wash their genitalia 12 hours before the urological examination. Samples were collected using a cytobrush (Cytobrush Plus Sterile, Medscand Medical Inc, Hollywood, FL, USA), moistened in phosphate buffered saline (PBS), to brush the penis in a continuously rotational movement, from bottom to top, starting at the middle third of the scrotum. After retraction of the prepuce (for uncircumcised men), the coronal sulcus, the glans, and the tip of the penis were also brushed. The cytobrush was then cut, and placed in a tube containing 20 ml of PBS.
Among two consecutive groups of men in the first part of the study, a second cell sample was collected and placed in PBS.
Group 1: among the first 298 men recruited in the study, the second sample was taken from the distal urethra using a pre‐wetted Accellon Multi‐Biosampler Swab (Medscand Medical Inc, Hollywood, FL, USA), which was introduced 2 cm deep and rotated 360 degrees.
Group 2: among a subsequent group of 522 men, the second sample was obtained from the meatus urethralis by opening the tip of the penis and introducing a cotton swab 0.5 cm deep into the urethra.
All samples were stored at −20°C until shipment to the Department of HPV Typing of the National Institute of Public Health of Mexico, Cuernavaca, where the tubes were vigorously vortexed, released cells centrifuged to remove collection media, and the cell pellets re‐suspended in two aliquots of PBS solution and stored at −70°C.
HPV analysis
Testing for HPV in all samples was carried out by means of a strip assay, using the reverse line blot format.9 HPV DNA was amplified using biotinylated PGMY L1 consensus primers.10 To determine specimen adequacy, a fragment of the human β globin gene was co‐amplified with primers BGH20 and BPC04. For HPV typing, the PCR products were hybridised to probes immobilised on nylon strips. Each strip contained 29 probe lines, 27 type specific probes for HPV and two for high and low concentrations of the β globin genes. HPV 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 68, 73, and 82 were considered high risk types and HPV 6, 11, 26, 40, 42, 53, 54, 55, 57, 66, 83, and 84 were considered low risk types.11 Hybridisation signals were detected by colorimetric development. Strip interpretation was done using an acetate overlay indicating the position of each type specific probe. The hybridisation results were agreed upon independently by two reviewers. For each PCR run, positive controls (Siha cells, VPH 16 ATCC HTB‐35, C 4 II cells VPH 18 ATCC CRL 1595) and a negative control (K 562 cells, (ATCC) CCL‐243) were included. These PCR products were also tested by reverse line blot. After the exclusion of β globin negative samples (27.9% of samples from external genitalia, 0% of those from the urethra and 1.9% of those from the meatus), 168 men were left in group 1 and 414 in group 2.
Statistical analysis
To simulate findings from a study where only external genitalia samples were used, men who were HPV DNA positive at external genitalia or urethra (group 1), or at external genitalia or meatus (group 2), were considered true HPV positives, whereas those who were positive at urethra or meatus, but not at external genitalia, were considered false HPV negatives.
Results
The mean age of soldiers participating in the present study was 22.9 (range = 16–41 years) in group 1 and 24.4 (range 17–50 years) in group 2. Only 13.4% reported not to have had sexual intercourse and the mean number of lifetime sexual partners among sexually active soldiers was 5.1 (range 1–39) in group 1 and 4.1 (range 1–45) in group 2. Circumcision was reported by 14.7% of men in group 1 and 6.9% of men in group 2.
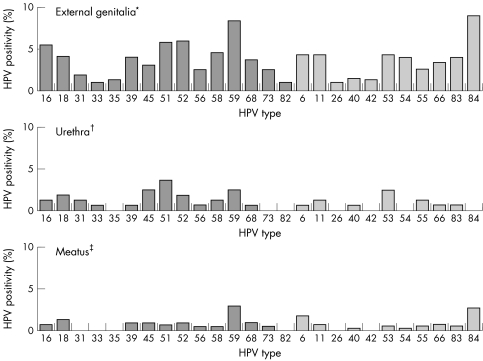
External genitalia samples were more frequently positive for HPV (46.4%) compared to urethra (20.8%) or meatus (12.1%) samples. Multiple type infections were found in 50.7% of the HPV positive samples from the external genitalia and in 23.5% of HPV positive samples from the urethra/meatus. High risk HPV types predominated in samples from all sites (fig 1). HPV 59 was the most frequently detected high risk type (8.4% of samples from the external genitalia and 2.7% of samples from the urethra/meatus). The next most frequent high risk types in the external genitalia were HPV 52 (6.0%), HPV 51 (5.8%), HPV 16 (5.5%), and HPV 58 (4.6%), whereas HPV 6, 11, 53 and, notably, HPV 84 were the low risk types most frequently detected. The range of HPV types found in urethra and meatus samples was similar to the range in external genitalia samples, although all types showed substantially lower prevalence (fig 1).
Figure 1 Prevalence of human papillomavirus (HPV) DNA by type and sample site among soldiers. Mexico, 2001–2. *HPV positivity, any type, among 582 samples = 46.4%; †HPV positivity, any type, among 168 samples = 20.8%; ‡HPV positivity, any type, among 414 samples = 12.1%. High risk types = dark bars; Low risk types = light bars.
The largest contribution to the evaluation of HPV positivity for all HPV types and for high risk and low risk types came from external genitalia samples (table 1). Among the 79 HPV positive men in group 1, four (5.1%) were HPV positive at the urethra sample only. Among the 198 HPV positive men in group 2, three (1.5%) were HPV positive at the meatus sample only.
Table 1 Detection of human papillomavirus (HPV) DNA by sample site among 277 HPV positive soldiers. Mexico, 2001–2.
| HPV positivity | No | % |
|---|---|---|
| Group 1 (n = 79) | ||
| All types | ||
| Both sites | 31 | 39.2 |
| External genitalia only | 44 | 55.7 |
| Urethra only | 4 | 5.1* |
| (1.4 to 12.5)† | ||
| High risk | ||
| Both sites | 26 | 42.6 |
| External genitalia only | 32 | 52.5 |
| Urethra only | 3 | 4.9* |
| (1.0 to 13.7)† | ||
| Low risk | ||
| Both sites | 7 | 17.1 |
| External genitalia only | 31 | 75.6 |
| Urethra only | 3 | 7.3* |
| (1.5 to 19.9)† | ||
| Group 2 (n = 198) | ||
| All types | ||
| Both sites | 47 | 23.7 |
| External genitalia only | 148 | 74.8 |
| Meatus only | 3 | 1.5* |
| (0.3 to 4.4)† | ||
| High risk | ||
| Both sites | 27 | 18.8 |
| External genitalia only | 114 | 79.2 |
| Meatus only | 3 | 2.1* |
| (0.4 to 6.0)† | ||
| Low risk | ||
| Both sites | 29 | 22.7 |
| External genitalia only | 98 | 76.6 |
| Meatus only | 1 | 0.8* |
| (0.0 to 4.3)† |
*Represents the proportion of false HPV negatives if only external genitalia sample had been used; †Corresponding 95% confidence intervals.
When the agreement between external genitalia and urethra or meatus samples (groups 1 and 2 combined) was evaluated for selected HPV types, 0/25 infections with HPV 6, 2/27 with HPV 11, 2/34 with HPV 16, 3/27 with HPV 18, and 1/50 with HPV 59 were detected exclusively from the urethra or meatus samples (data not shown).
Discussion
HPV DNA in men was found substantially more frequently on the skin of the external genitalia than in either the urethra or meatus. It is difficult to speak about the sensitivity of sampling different sites in men, as no recognised “gold standard” exists. However, if we consider those in whom HPV infection was detected in any of the examined sites (external genitalia, or urethra, or meatus) as true HPV positive men, sensitivity was quite high for the external genitalia sample alone, while for the other sites it was very low. The addition of the findings from the meatus would have avoided 1.5% of false HPV negatives, and the addition of urethra samples 5.1%. The passage of the sample from the urethra through the meatus could have produced some contamination, but this is unlikely to have substantially affected HPV prevalence in the urethra as HPV positivity in the meatus was shown to be rare.
In respect of individual HPV types, the frequent detection of HPV 59 and HPV 84 in the external genitalia samples is worth mentioning, as it had not been observed either in Mexican women12 or HPV surveys in non‐Mexican men.6,7,8
Major strengths of our study include the large size and the virtually complete participation of the Mexican soldiers who were invited. A limitation is represented by the difficulty of obtaining sufficient cellular DNA from the external genitalia, especially during the earliest study phase. The proportion of β globin positive samples increased during the study, from 56% in group 1 to 79% in group 2, possibly because of improvements in the quality of cell collection and processing. The difference was not the result of a difference in the number of circumcised men as their proportion was similar in groups 1 and 2 (15% and 7%, respectively) and their exclusion did not eliminate the difference in β globin positivity. A substantial increase in the proportion of β globin positive samples from male genitalia has recently been reported, by abrading the skin of external genitalia with emery paper before swabbing with a moistened swab.4
In light of the large number of HPV types found in men, it is reassuring that we did not find a substantial difference in the distribution of HPV types in different anatomical sites. Multiple type infections were twofold more frequent in HIV positive samples from the external genitalia than in those from the urethra or meatus, but high risk HPV types predominated in all genital sites examined, and the most common types were approximately the same.
The authors thank Mr M Plummer for statistical advice. This study was made possible by funding provided by the National Council of Science and Technology of Mexico, CONACYT (SALUD‐2002‐C01‐7800 and 34468‐M) and the International Agency for Research on Cancer (FIS/03/02). HPV DNA kits were generously donated by Roche Molecular Systems, Inc (Alameda, CA, USA).
Contributors
LVA, EL‐P, and AC conceived the study protocol and supervised the fieldwork; SV was responsible for statistical analysis; PH was responsible for HPV DNA testing and genotyping; JRK gave advice on HPV DNA testing; JSS, NM, and MH contributed to the study protocol; SF drafted the manuscript, which was critically revised by all authors.
Key messages
HPV DNA prevalence in the external genitalia is very high (46.4%) among young Mexican soldiers
HPV DNA prevalence is substantially lower in the urethra and meatus than in the external genitalia
The most commonly detected HPV types were HPV 59, 84, 52, and 51
Abbreviations
HPV - human papillomavirus
PBS - phosphate buffered saline
PCR - polymerase chain reaction
Footnotes
Conflict of interest: none declared.
References
- 1.Hippelainen M, Syrjanen S, Hippelainen M.et al Prevalence and risk factors of genital human papillomavirus (HPV) infections in healthy males: a study on Finnish conscripts. Sex Transm Dis 199320321–328. [PubMed] [Google Scholar]
- 2.Van Doornum G J, Prins M, Juffermans L H.et al Regional distribution and incidence of human papillomavirus infections among heterosexual men and women with multiple sexual partners: a prospective study. Genitourin Med 199470240–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lazcano‐Ponce E, Herrero R, Muñoz N.et al High prevalence of human papillomavirus infection in Mexican males: comparative study of penile‐urethral swabs and urine samples. Sex Transm Dis 200128277–280. [DOI] [PubMed] [Google Scholar]
- 4.Weaver B A, Feng Q, Holmes K K.et al Evaluation of genital sites and sampling techniques for detection of human papillomavirus DNA in men. J Infect Dis 2004189677–685. [DOI] [PubMed] [Google Scholar]
- 5.Svare E I, Kjaer S K, Worm A M.et al Risk factors for genital HPV DNA in men resemble those found in women: a study of male attendees at a Danish STD clinic. Sex Transm Infect 200278215–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shin H R, Franceschi S, Vaccarella S.et al Prevalence and determinants of genital infection with papillomavirus, in female and male university students in Busan, South Korea. J Infect Dis 2004190468–476. [DOI] [PubMed] [Google Scholar]
- 7.Franceschi S, Castellsagué X, Dal Maso L.et al Prevalence and determinants of human papillomavirus genital infection in men. Br J Cancer 200286705–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baldwin S B, Wallace D R, Papenfuss M R.et al Human papillomavirus infection in men attending a sexually transmitted disease clinic. J Infect Dis 20031871064–1067. [DOI] [PubMed] [Google Scholar]
- 9.Gravitt P E, Peyton C L, Apple R J.et al Genotyping of 27 human papillomavirus types by using L1 consensus PCR products by a single‐hybridization, reverse line blot detection method. J Clin Microbiol 1998363020–3027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gravitt P E, Peyton C L, Alessi T Q.et al Improved amplification of genital human papillomaviruses.J Clin Microbiol 200038357–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Muñoz N, Bosch F X, de Sanjosé S.et al Epidemiologic classification of human papillomavirus types associated with cervical cancer. N Engl J Med 2003348518–527. [DOI] [PubMed] [Google Scholar]
- 12.Lazcano‐Ponce E, Herrero R, Muñoz N.et al Epidemiology of HPV infection among Mexican women with normal cervical cytology. Int J Cancer 200191412–420. [DOI] [PubMed] [Google Scholar]