Abstract
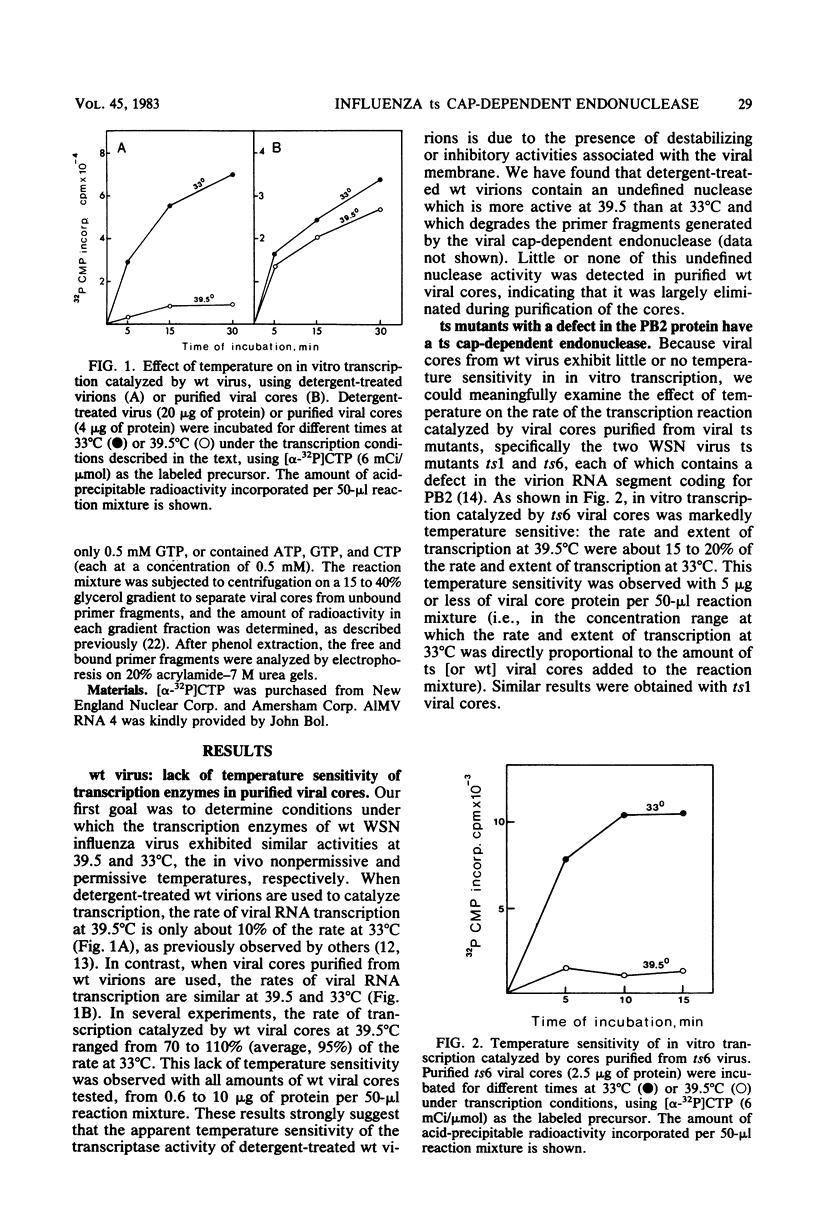
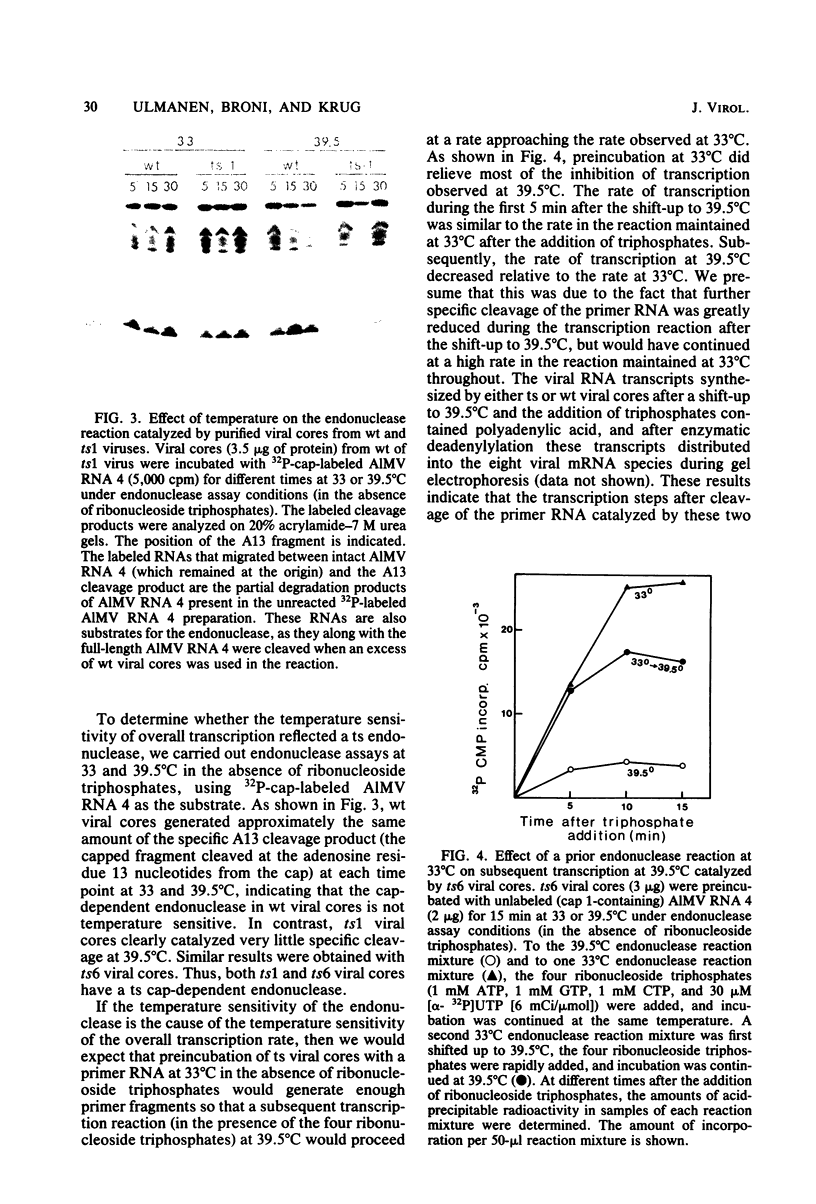
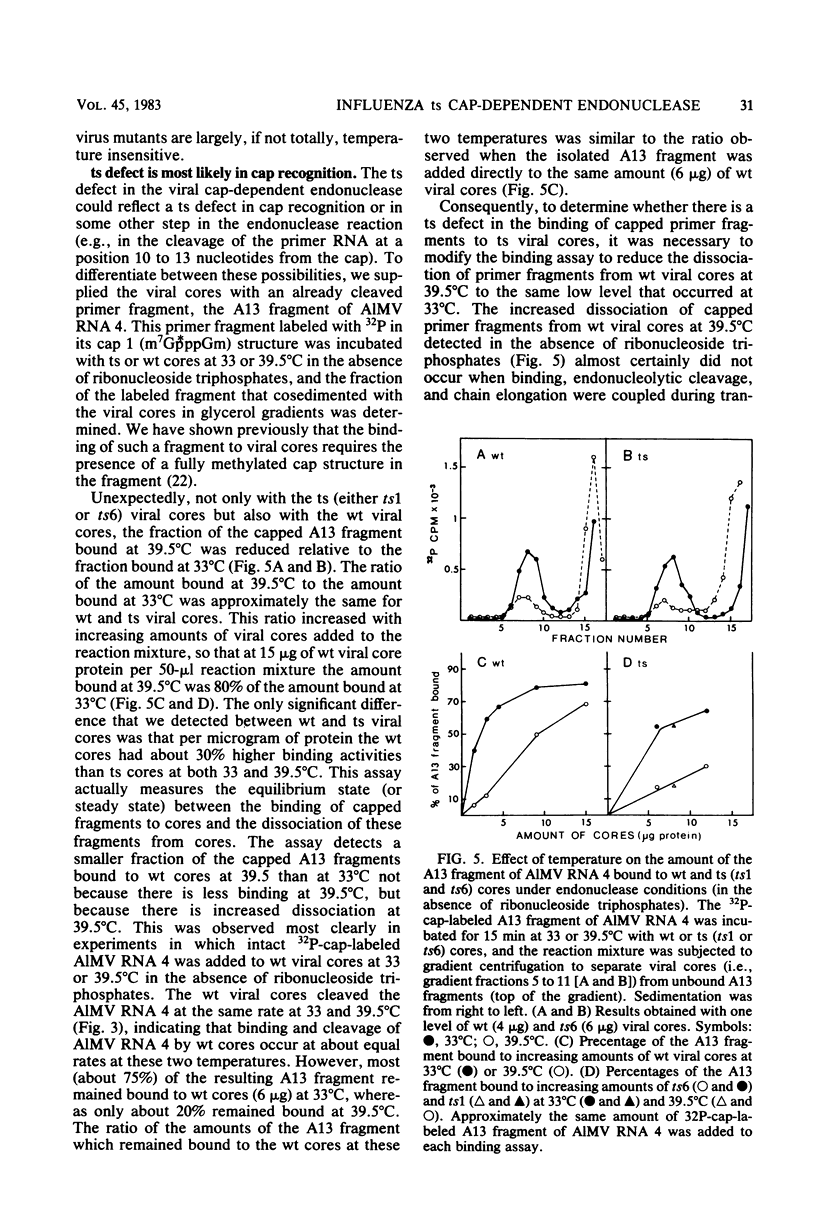
The first step in influenza viral mRNA synthesis is the endonucleolytic cleavage of heterologous RNAs containing cap 1 (m7GpppNm) structures to generate capped primers that are 10 to 13 nucleotides long, which are then elongated to form the viral mRNA chains. We examined the temperature sensitivity of these steps in vitro by using two WSN virus temperature-sensitive mutants, ts1 and ts6, which have a defect in the genome RNA segment coding for the viral PB2 protein. For these experiments, it was necessary to employ purified viral cores rather than detergent-treated virions to catalyze transcription, as preparations of detergent-treated virions contain destabilizing or inhibitory activities which render even the transcription catalyzed by wild-type virus temperature sensitive. Using purified wild-type viral cores, we found that the rates of endonucleolytic cleavage of capped primers and of overall transcription were similar at 39.5 and 33°C, the in vivo nonpermissive and permissive temperatures, respectively. In contrast, the activities of the cap-dependent endonucleases of ts1 and ts6 viral cores at 39.5°C were only about 15% of those at 33°C. The steps in transcription after endonucleolytic cleavage of the capped RNA primer were largely, if not totally, temperature insensitive, indicating that the mutations in the PB2 protein found in ts1 and ts6 virions affect only the endonuclease step. The temperature-sensitive defect is most likely in the recognition of the 5′-terminal cap 1 structure that occurs as a required first step in the endonuclease reaction: the cap-dependent binding of a specific capped primer fragment to ts1 viral cores was temperature sensitive under conditions in which binding to wild-type viral cores was not affected by increasing the temperature from 33 to 39.5°C. Thus, our results establish that the viral PB2 protein functions in cap recognition during the endonuclease reaction.
Full text
PDF

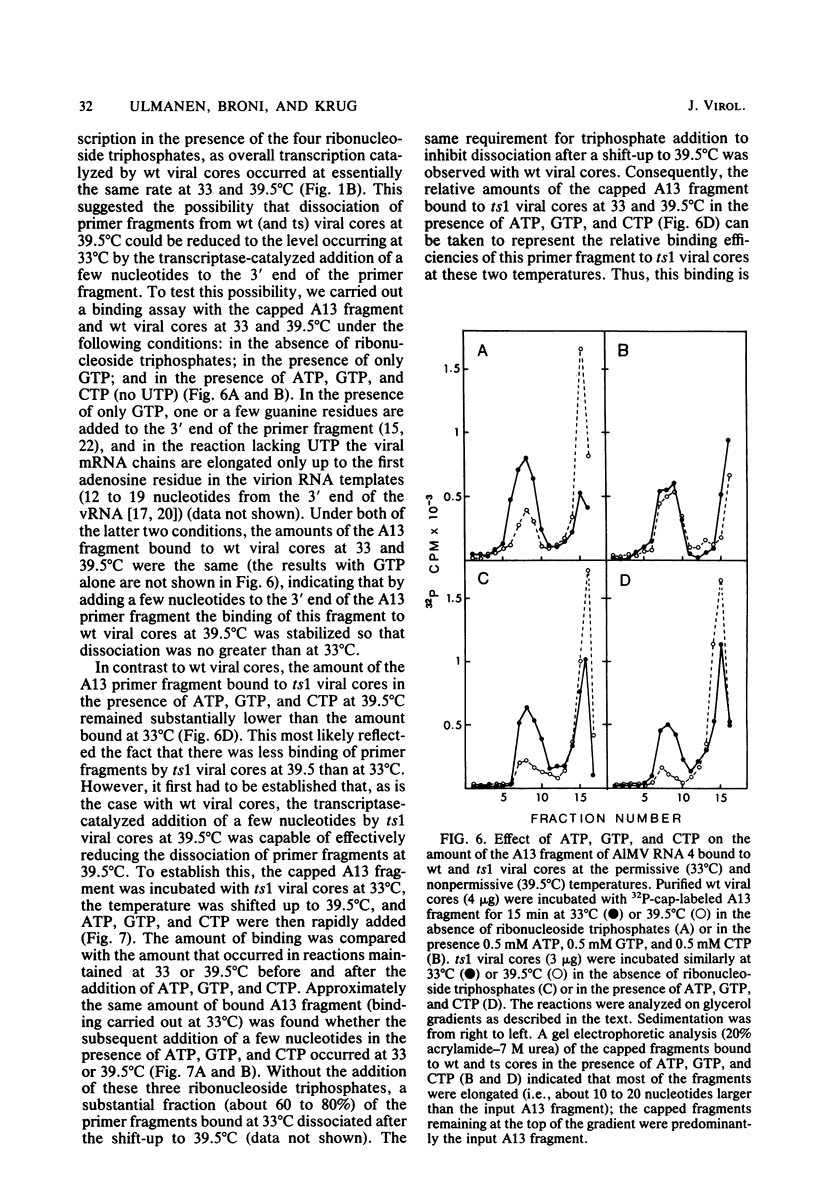
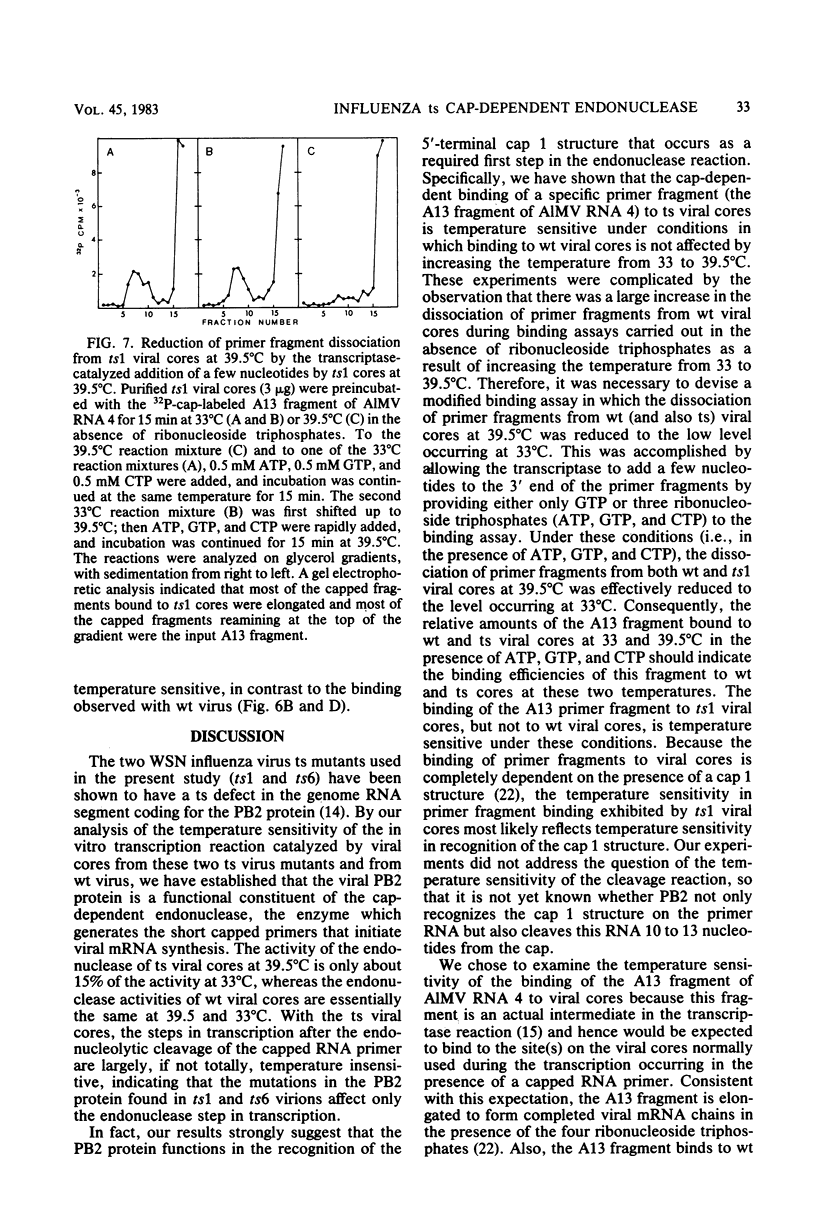


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blaas D., Patzelt E., Kuechler E. Cap-recognizing protein of influenza virus. Virology. 1982 Jan 15;116(1):339–348. doi: 10.1016/0042-6822(82)90425-1. [DOI] [PubMed] [Google Scholar]
- Blaas D., Patzelt E., Kuechler E. Identification of the cap binding protein of influenza virus. Nucleic Acids Res. 1982 Aug 11;10(15):4803–4812. doi: 10.1093/nar/10.15.4803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouloy M., Plotch S. J., Krug R. M. Both the 7-methyl and the 2'-O-methyl groups in the cap of mRNA strongly influence its ability to act as primer for influenza virus RNA transcription. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3952–3956. doi: 10.1073/pnas.77.7.3952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouloy M., Plotch S. J., Krug R. M. Globin mRNAs are primers for the transcription of influenza viral RNA in vitro. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4886–4890. doi: 10.1073/pnas.75.10.4886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compans R. W., Content J., Duesberg P. H. Structure of the ribonucleoprotein of influenza virus. J Virol. 1972 Oct;10(4):795–800. doi: 10.1128/jvi.10.4.795-800.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horisberger M. A. The large P proteins of influenza A viruses are composed of one acidic and two basic polypeptides. Virology. 1980 Nov;107(1):302–305. doi: 10.1016/0042-6822(80)90296-2. [DOI] [PubMed] [Google Scholar]
- Inglis S. C., Carroll A. R., Lamb R. A., Mahy B. W. Polypeptides specified by the influenza virus genome I. Evidence for eight distinct gene products specified by fowl plague virus. Virology. 1976 Oct 15;74(2):489–503. doi: 10.1016/0042-6822(76)90355-x. [DOI] [PubMed] [Google Scholar]
- Kroath H., Shatkin A. J. mRNA 5'-cap binding activity in purified influenza virus detected by simple, rapid assay. J Virol. 1982 Mar;41(3):1105–1108. doi: 10.1128/jvi.41.3.1105-1108.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krug R. M. Influenza viral RNPs newly synthesized during the latent period of viral growth in MDCK cells. Virology. 1971 Apr;44(1):125–136. doi: 10.1016/0042-6822(71)90159-0. [DOI] [PubMed] [Google Scholar]
- Krug R. M., Ueda M., Palese P. Temperature-sensitive mutants of influenza WSN virus defective in virus-specific RNA synthesis. J Virol. 1975 Oct;16(4):790–796. doi: 10.1128/jvi.16.4.790-796.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mowshowitz S. L. P1 is required for initiation of cRNA synthesis in WSN influenza virus. Virology. 1978 Dec;91(2):493–495. doi: 10.1016/0042-6822(78)90398-7. [DOI] [PubMed] [Google Scholar]
- Mowshowitz S. L., Ueda M. Temperature-sensitive virion transcriptase activity in mutants of WSN influenza virus. Arch Virol. 1976;52(1-2):135–141. doi: 10.1007/BF01317872. [DOI] [PubMed] [Google Scholar]
- Nichol S. T., Penn C. R., Mahy B. W. Evidence for the involvement of influenza A (fowl plague Rostock) virus protein P2 in ApG and mRNA primed in vitro RNA synthesis. J Gen Virol. 1981 Dec;57(Pt 2):407–413. doi: 10.1099/0022-1317-57-2-407. [DOI] [PubMed] [Google Scholar]
- Palese P., Ritchey M. B., Schulman J. L. P1 and P3 proteins of influenza virus are required for complementary RNA synthesis. J Virol. 1977 Mar;21(3):1187–1195. doi: 10.1128/jvi.21.3.1187-1195.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plotch S. J., Bouloy M., Ulmanen I., Krug R. M. A unique cap(m7GpppXm)-dependent influenza virion endonuclease cleaves capped RNAs to generate the primers that initiate viral RNA transcription. Cell. 1981 Mar;23(3):847–858. doi: 10.1016/0092-8674(81)90449-9. [DOI] [PubMed] [Google Scholar]
- Plotch S. J., Krug R. M. Segments of influenza virus complementary RNA synthesized in vitro. J Virol. 1978 Feb;25(2):579–586. doi: 10.1128/jvi.25.2.579-586.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson J. S. 5' and 3' terminal nucleotide sequences of the RNA genome segments of influenza virus. Nucleic Acids Res. 1979 Aug 24;6(12):3745–3757. doi: 10.1093/nar/6.12.3745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochovansky O. M. RNA synthesis by ribonucleoprotein-polymerase complexes isolated from influenza virus. Virology. 1976 Sep;73(2):327–338. doi: 10.1016/0042-6822(76)90394-9. [DOI] [PubMed] [Google Scholar]
- Sedmak J. J., Grossberg S. E. A rapid, sensitive, and versatile assay for protein using Coomassie brilliant blue G250. Anal Biochem. 1977 May 1;79(1-2):544–552. doi: 10.1016/0003-2697(77)90428-6. [DOI] [PubMed] [Google Scholar]
- Skehel J. J., Hay A. J. Nucleotide sequences at the 5' termini of influenza virus RNAs and their transcripts. Nucleic Acids Res. 1978 Apr;5(4):1207–1219. doi: 10.1093/nar/5.4.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiura A., Ueda M., Tobita K., Enomoto C. Further isolation and characterization of temperature-sensitive mutants of influenza virus. Virology. 1975 Jun;65(2):363–373. doi: 10.1016/0042-6822(75)90042-2. [DOI] [PubMed] [Google Scholar]
- Ulmanen I., Broni B. A., Krug R. M. Role of two of the influenza virus core P proteins in recognizing cap 1 structures (m7GpppNm) on RNAs and in initiating viral RNA transcription. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7355–7359. doi: 10.1073/pnas.78.12.7355. [DOI] [PMC free article] [PubMed] [Google Scholar]