Abstract
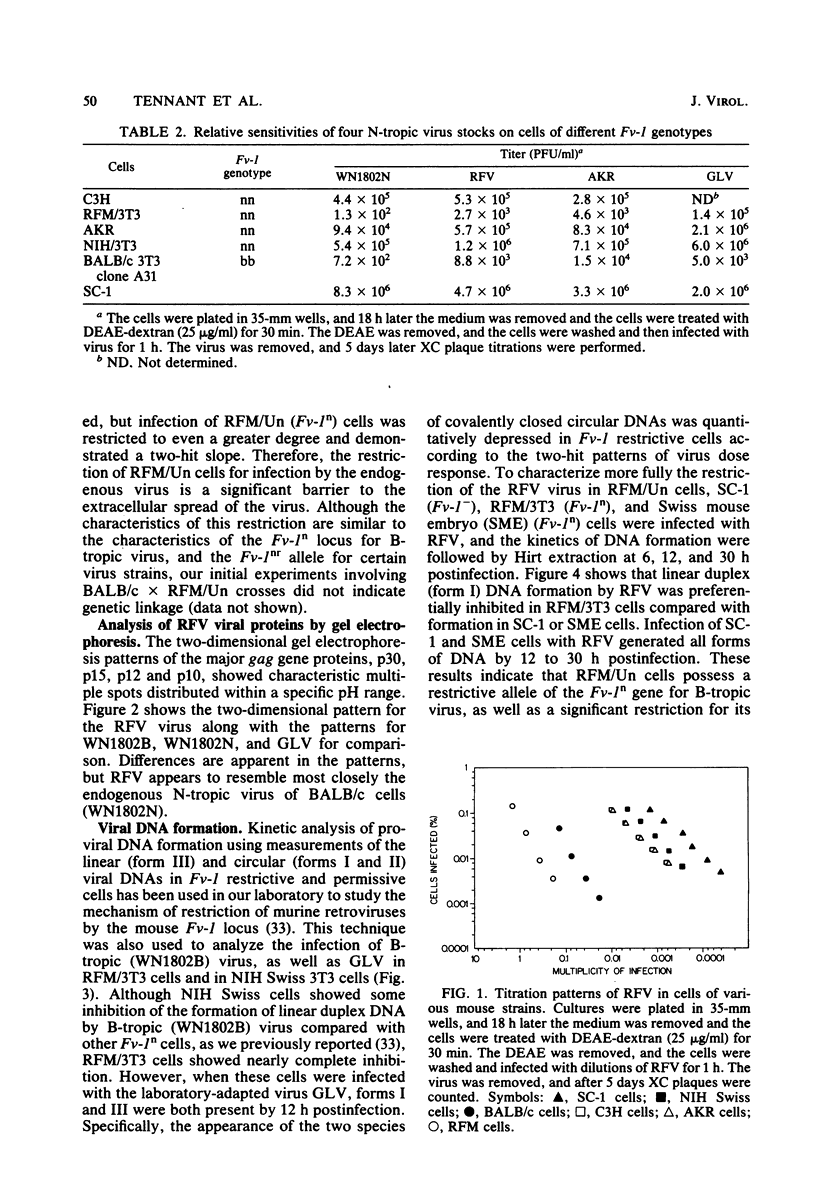
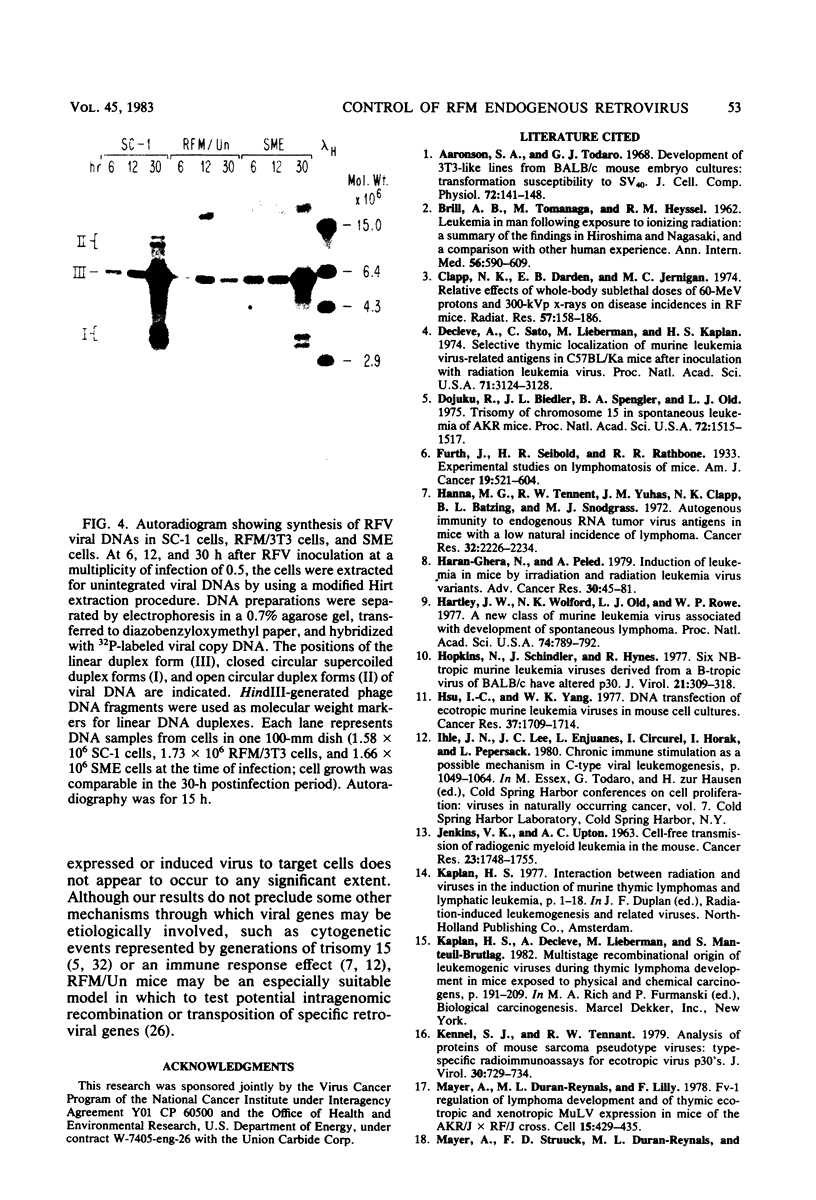
RFM/Un mice express an endogenous type C retrovirus throughout their life span in many tissues; primary or established embryo fibroblast cell cultures do not express a virus but can be induced by exposure to 5-iodo-2'-deoxyuridine. All of our sources yielded a single ecotropic virus (RFV) which appeared to be related more closely to the endogenous N-tropic virus (WN1802N) of BALB/c mice than to Gross leukemia virus on the basis of two-dimensional gel electropherograms of virion proteins. No xenotropic or recombinant viruses were isolated by cocultivation techniques. RFV is N-tropic, and RFM/Un cells possess the Fv-1n allele, as indicated by restriction of B-tropic virus and susceptibility to Gross strain N-tropic virus. However, RFM cells are highly resistant to RFV and other endogenous N-tropic viruses. This resistance is expressed by two-hit titration kinetics and by inhibition of viral linear duplex DNA formation. This is similar to the effects of the Fv-1 locus, but preliminary work has shown no apparent genetic linkage between the two restrictions. The relative strength of the restriction, the presence of a single class of ecotropic virus, and the absence of recombinant viruses suggest that in RFM mice virus is expressed only in cells in which it is induced and not by cell-to-cell transmission.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaronson S. A., Todaro G. J. Development of 3T3-like lines from Balb-c mouse embryo cultures: transformation susceptibility to SV40. J Cell Physiol. 1968 Oct;72(2):141–148. doi: 10.1002/jcp.1040720208. [DOI] [PubMed] [Google Scholar]
- BRILL A. B., TOMONAGA M., HEYSSEL R. M. Leukemia in man following exposure to ionizing radiation. A summary of the findings in Hiroshima and Nagasaki, and a comparison with other human experience. Ann Intern Med. 1962 Apr;56:590–609. doi: 10.7326/0003-4819-56-4-590. [DOI] [PubMed] [Google Scholar]
- Clapp N. K., Darden E. B., Jr, Jernigan M. C. Relative effects of whole-body sublethal doses of 60-MeV protons and 300-kVp X-rays on disease incidences in RF mice. Radiat Res. 1974 Jan;57(1):158–186. [PubMed] [Google Scholar]
- Declève A., Sato C., Lieberman M., Kaplan H. S. Selective thymic localization of murine leukemia virus-related antigens in C57BL-Ka mice after inoculation with radiation virus. Proc Natl Acad Sci U S A. 1974 Aug;71(8):3124–3128. doi: 10.1073/pnas.71.8.3124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dofuku R., Biedler J. L., Spengler B. A., Old L. J. Trisomy of chromosome 15 in spontaneous leukemia of AKR mice. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1515–1517. doi: 10.1073/pnas.72.4.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanna M. G., Jr, Tennant R. W., Yuhas J. M., Clapp N. K., Batzing B. L., Snodgrass M. J. Autogenous immunity to endogenous RNA tumor virus antigens in mice with a low natural incidence of lymphoma. Cancer Res. 1972 Oct;32(10):2226–2234. [PubMed] [Google Scholar]
- Hartley J. W., Wolford N. K., Old L. J., Rowe W. P. A new class of murine leukemia virus associated with development of spontaneous lymphomas. Proc Natl Acad Sci U S A. 1977 Feb;74(2):789–792. doi: 10.1073/pnas.74.2.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins N., Schindler J., Hynes R. Six-NB-tropic murine leukemia viruses derived from a B-tropic virus of BALB/c have altered p30. J Virol. 1977 Jan;21(1):309–318. doi: 10.1128/jvi.21.1.309-318.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu I. C., Yang W. K. DNA transfection of ecotropic murine leukemia viruses in mouse cell cultures. Cancer Res. 1977 Jun;37(6):1709–1714. [PubMed] [Google Scholar]
- JENKINS V. K., UPTON A. C. CELL-FREE TRANSMISSION OF RADIOGENIC MYELOID LEUKEMIA IN THE MOUSE. Cancer Res. 1963 Dec;23:1748–1755. [PubMed] [Google Scholar]
- Kennel S. J., Tennant R. W. Analysis of proteins of mouse sarcoma pseudotype viruses: type-specific radioimmunoassay for ecotropic virus p30's. J Virol. 1979 Jun;30(3):729–734. doi: 10.1128/jvi.30.3.729-734.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer A., Duran-Reynals M. L., Lilly F. Fv-1 regulation of lymphoma development and of thymic ecotropic and xenotropic MuLV expression in mice of the AKR/J x RF/J cross. Cell. 1978 Oct;15(2):429–435. doi: 10.1016/0092-8674(78)90012-0. [DOI] [PubMed] [Google Scholar]
- Mayer A., Struuck F. D., Duran-Reynals M. L., Lilly F. Maternally transmitted resistance to lymphoma development in mice of reciprocal crosses of the RF/J and AKR/J strains. Cell. 1980 Feb;19(2):431–436. doi: 10.1016/0092-8674(80)90517-6. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Otten J. A., Quarles J. M., Tennant R. W. Cell division requirement for activation of murine leukemia virus in cell culture by irradiation. Virology. 1976 Mar;70(1):80–87. doi: 10.1016/0042-6822(76)90237-3. [DOI] [PubMed] [Google Scholar]
- POPP D. M., AMOS D. B. AN H-2 ANALYSIS OF STRAIN RFM/UN MICE. Transplantation. 1965 Jul;3:501–508. doi: 10.1097/00007890-196507000-00005. [DOI] [PubMed] [Google Scholar]
- Rowe W. P., Pugh W. E., Hartley J. W. Plaque assay techniques for murine leukemia viruses. Virology. 1970 Dec;42(4):1136–1139. doi: 10.1016/0042-6822(70)90362-4. [DOI] [PubMed] [Google Scholar]
- Staats J. Standardized nomenclature for inbred strains of mice: seventh listing for the International Committee on Standardized Genetic Nomenclature for Mice. Cancer Res. 1980 Jul;40(7):2083–2128. [PubMed] [Google Scholar]
- Tanaka T., Craig A. W. Cell-free transmission of murine myeloid leukaemia. Eur J Cancer. 1970 Aug;6(4):329–333. doi: 10.1016/0014-2964(70)90098-8. [DOI] [PubMed] [Google Scholar]
- Tennant R. W., Myer F. E., McGrath L. Effect of the Fv-1 gene on leukemia virus in mouse cell heterokaryons. Int J Cancer. 1974 Oct 15;14(4):504–513. doi: 10.1002/ijc.2910140410. [DOI] [PubMed] [Google Scholar]
- Upton A. C., Jenkins V. K., Walburg H. E., Jr, Tyndall R. L., Conklin J. W., Wald N. Observations on viral, chemical, and radiation-induced myeloid and lymphoid leukemias in RF mice. Natl Cancer Inst Monogr. 1966 Sep;22:329–347. [PubMed] [Google Scholar]
- Walburg H. E., Jr, Cosgrove G. E., Upton A. C. Influence of microbial environment on development of myeloid leukemia in x-irradiated RFM mice. Int J Cancer. 1968 Jan 15;3(1):150–154. doi: 10.1002/ijc.2910030118. [DOI] [PubMed] [Google Scholar]
- Wiener F., Ohno S., Spira J., Haran-Ghera N., Klein G. Chromosome changes (trisomies #15 and 17) associated with tumor progression in leukemias induced by radiation leukemia virus. J Natl Cancer Inst. 1978 Jul;61(1):227–237. doi: 10.1093/jnci/61.1.227. [DOI] [PubMed] [Google Scholar]
- Yang W. K., Kiggans J. O., Yang D. M., Ou C. Y., Tennant R. W., Brown A., Bassin R. H. Synthesis and circularization of N- and B-tropic retroviral DNA Fv-1 permissive and restrictive mouse cells. Proc Natl Acad Sci U S A. 1980 May;77(5):2994–2998. doi: 10.1073/pnas.77.5.2994. [DOI] [PMC free article] [PubMed] [Google Scholar]