Abstract
To characterize endogenous molecules and activities of the Golgi complex, proteins in transit were >99% cleared from rat hepatocytes by using cycloheximide (CHX) treatment. The loss of proteins in transit resulted in condensation of the Golgi cisternae and stacks. Isolation of a stacked Golgi fraction is equally efficient with or without proteins in transit [control (CTL SGF1) and cycloheximide (CHX SGF1)]. Electron microscopy and morphometric analysis showed that >90% of the elements could be positively identified as Golgi stacks or cisternae. Biochemical analysis showed that the cis-, medial-, trans-, and TGN Golgi markers were enriched over the postnuclear supernatant 200- to 400-fold with and 400- to 700-fold without proteins in transit. To provide information on a mechanism for import of calcium required at the later stages of the secretory pathway, calcium uptake into CTL SGF1 and CHX SGF1 was examined. All calcium uptake into CTL SGF1 was dependent on a thapsigargin-resistant pump not resident to the Golgi complex and a thapsigargin-sensitive pump resident to the Golgi. Experiments using CHX SGF1 showed that the thapsigargin-resistant activity was a plasma membrane calcium ATPase isoform in transit to the plasma membrane and the thapsigargin-sensitive pump was a sarcoplasmic/endoplasmic reticulum calcium ATPase isoform. In vivo both of these calcium ATPases function to maintain millimolar levels of calcium within the Golgi lumen.
INTRODUCTION
A major focus of cell biology has been to understand the structure–function relationships of the Golgi complex. The characterization of the Golgi ribbon without transiting proteins will allow study of its backbone structure and its functions. Cycloheximide (CHX)1 treatment, which blocks protein synthesis, has been shown to clear albumin from the Golgi complex in rat liver (Taylor et al., 1984) and it was assumed this treatment would clear other proteins in transit through the Golgi complex. We initiated this project in studying the Golgi cleared of proteins in transit with the following long-range objectives: to characterize the basic structure of the Golgi ribbon and to obtain a Golgi fraction where in vivo structure remained relatively intact in order to identify endogenous Golgi proteins and activities. This latter objective led us to revise the Golgi fractionation procedure we had been using for our functional assays of vesicle budding from the trans-Golgi network (TGN; Salamero et al., 1990; Jones et al., 1993). The modified procedure provides highly enriched intact stacked Golgi fractions with and without proteins in transit.
Many different procedures for isolation of Golgi fractions have been described and in general result in two different types of fractions. In one, the cells are homogenized so that the Golgi complex is essentially “microsomalized,” i.e., sheared into small vesicles (Fleischer et al., 1969; Ehrenreich et al., 1973; Bergeron, 1979). The second approach is to keep the Golgi relatively intact, a procedure that requires much milder homogenization conditions (Morré and Mollenhauer, 1964; Leelavathi et al., 1970; Hino et al., 1978; Slusarewicz et al., 1994). Rat liver has been the preferred tissue for Golgi fractionation because of the reduced amount of cytoskeleton in hepatocytes and minimal amount of extracellular matrix (connective tissue) that together allow the release of the Golgi stacks with mild homogenization conditions (Howell et al., 1989).
We had been using the Leelavathi et al. (1970) procedure, which yields an intact stack containing cis-, medial-, and trans-cisternae including the TGN (Salamero et al., 1990; Jones et al., 1993). Major disadvantages of this fraction are that Golgi stacks are never entirely separated from cytosol and that this fraction contains all the proteins in transit through the Golgi complex. Any of the washing procedures used to release soluble luminal or peripheral proteins (i.e., high pH or high salt) result in fragmentation of the stacked structure. This article presents a characterization of the Golgi complex cleared of proteins in transit in vivo and in isolated stacked Golgi fractions. Clearance of proteins in transit in no way alters the ability to isolate an enriched or stacked fraction at high yield, thus providing an optimal preparation for characterization of endogenous proteins and activities. Our studies of endogenous Golgi activities have focused on the identification of Golgi ion pumps as a first step in understanding how the Golgi luminal ionic environment influences the sorting and concentration functions taking place within the Golgi complex.
The ionic composition of the Golgi lumen and its regulation remains poorly defined. Even though the luminal ionic environment of the Golgi complex is hypothesized to play important roles in concentration and processing of proteins in transit through this organelle. Multiple studies have inferred a specific role for calcium in both condensation and processing. Regulated secretory products (e.g., the secretogranins) have been shown in vitro to require millimolar levels of calcium and low pH to achieve the level of concentration present in the granules (Chanat and Huttner, 1991). Consistent with this function, many secretory granules contain calcium chelated with secretory proteins (e.g., in adrenal secretory granules and synaptic vesicles). Proteolytic processing of proinsulin to insulin, which occurs in late Golgi or secretory granules, when studied in vitro also requires millimolar calcium and low pH (Davidson et al., 1988). Other proteolytic processing events require high levels of free calcium, for example, processing of proopiomelanocortin studied in vivo in permeablized AtT-20 cells (Schmidt and Moore, 1995). The presence of millimolar levels of calcium in the Golgi lumen has been reported from studies using ion microscopy (Chandra et al., 1991). These levels are comparable to those estimated to exist within the endoplasmic reticulum (ER) lumen. Where and when calcium enter the lumen of the Golgi is not clear. Is all the calcium pumped into the lumen of the ER and transported to the Golgi with molecules in transit? Or does the Golgi contain its own calcium transport machinery?
The ER lumen is the major store of intracellular calcium in all cell types and the regulation of calcium uptake, storage, and release into the ER has been studied in detail. This process is mediated by calcium-transporting ATPases of the sarcoplasmic/ER calcium ATPase (SERCA) family of p-type pumps (Vegh et al., 1968; Fiehn and Hasselbach, 1970; Knowles and Racker, 1975; Lanini et al., 1992; Lytton et al., 1992). Three genes that are alternatively spliced give rise to five SERCA isoforms (SERCA 1a, 1b, 2a, 2b, and 3; reviewed in Wu et al., 1995). All known isoforms of this family of intracellular calcium pumps are equally sensitive to the pharmacologic inhibitor thapsigargin (Lytton et al., 1991). The mechanism of regulated calcium release from the ER lumen, particularly in the context of signal transduction pathways, has been studied extensively (Spat et al., 1986; Hokin et al., 1987; Gill et al., 1989; Koch, 1990). Stimulation of some signal transduction pathways leads to the formation of inositol 1,4,5-trisphosphate (IP3), which in turn binds an ER transmembrane receptor/channel (the IP3 receptor). Binding of IP3 gates the channel and allows calcium release to the cytosol.
The plasma membrane (PM) also contains a family of p-type calcium-transporting ATPases related to the SERCAs, the PM calcium ATPases (PMCAs). The PMCA family is transcribed from four genes that are alternatively spliced and give rise to 20 transcripts having a variety of tissue distributions (reviewed in Carafoli, 1994). In contrast to the SERCAs, the PMCAs are not sensitive to thapsigargin. All p-type pumps use an aspartyl phosphate enzyme intermediate step in their reaction cycles (Pedersen and Carafoli, 1987). This reaction mechanism can be exploited in two ways: 1) preincubation of the enzyme with sodium vanadate inhibits all p-type ATPases and 2) forming the intermediate in the presence of radiolabeled [γ-32P]ATP on ice traps the aspartyl phosphate intermediate and allows its detection.
A third form of p-type calcium ATPase has been identified in Saccharomyces cerevisiae (Antebi and Fink, 1992). This calcium ATPase is encoded by the PMR1 gene and is about 50% identical to the SERCAs. SERCAs have not been identified in yeast and comparisons of the biochemical properties of the PMR1 gene product and the SERCAs have not been published. The PMR1 gene product has been localized to the yeast Golgi complex and found to have a variety of functions within the cell (Antebi and Fink, 1992; Lapinskas et al., 1995; Verostek and Trimble, 1995; Halachmi and Eilam, 1996; Hartley et al., 1996). In searching for novel SERCAs, a clone (encoding 919 amino acids) was identified from a rat stomach cDNA library that, after sequencing, was found to have identities of 50% to PMR1, 33% to SERCAs, and 23% to the PMCAs (Gunteski-Hamblin et al., 1992). The mammalian homologue is present in liver by Northern blot analysis. It is a calcium ATPase based on sequence analysis, but this has not been confirmed by enzymatic assay. The intracellular localization and functions of the PMR1 homologue have not been defined.
In addition to the functional studies mentioned above, the presence of a calcium ATPase in the Golgi has been implied from demonstration of ATP-dependent calcium uptake into isolated Golgi fractions (Baumrucker et al., 1975; Hodson, 1978; Neville et al., 1981; West, 1981; Virk et al., 1985). These reports used Golgi fractions from lactating mammary glands and rat liver. The consensus from these studies was that the Golgi complex contained a calcium ATPase that was different from the PMCA and SERCA families based on the argument that the fractions were enriched for a Golgi marker, galactosyltransferase activity, from 14-fold (Neville et al., 1981) to 52-fold (Hodson, 1978) and the ATP-dependent calcium uptake could not be accounted for by contaminating ER or PM. These experiments are difficult to evaluate because the authors did not take into account the PMCAs moving through the Golgi complex en route to the PM and the experiments were carried out before the use of thapsigargin to specifically inhibit the SERCA pumps.
Because the ability of the Golgi complex to transport calcium is important to understanding numerous functions and studies with isolated Golgi fractions are dependent on the enrichment and contamination of the fraction, it was essential to characterize the uptake process further. In mitochondria, calcium uptake is oligomycin sensitive. Two groups (West, 1981; Virk et al., 1985) found that 26–40% of the calcium uptake into their isolated Golgi fractions was due to an oligomycin-insensitive proton gradient across the membranes that could be dissipated by using a protonophore. The endosomal/lysosomal and the trans-Golgi/TGN compartments (beside the mitochondria) have a proton gradient across their membranes and could have contributed to this activity but at the time these possibilities were not investigated further.
The availability of well-characterized Golgi fractions, better characterized than those used in the past because of the increased number of markers available, led us to reinvestigate calcium uptake in the Golgi complex. The CHX Golgi fraction facilitated our evaluation of the contribution of PM proteins in transit. The control (CTL) and CHX stacked Golgi fractions (SGF1s) have allowed us to address a long-standing question of the mechanism for import of calcium required at the later stages of the secretory pathway.
MATERIALS AND METHODS
Materials
All chemicals were obtained from Sigma (St. Louis, MO) or Boehringer Mannheim (Indianapolis, IN) unless otherwise indicated.
Fractionation
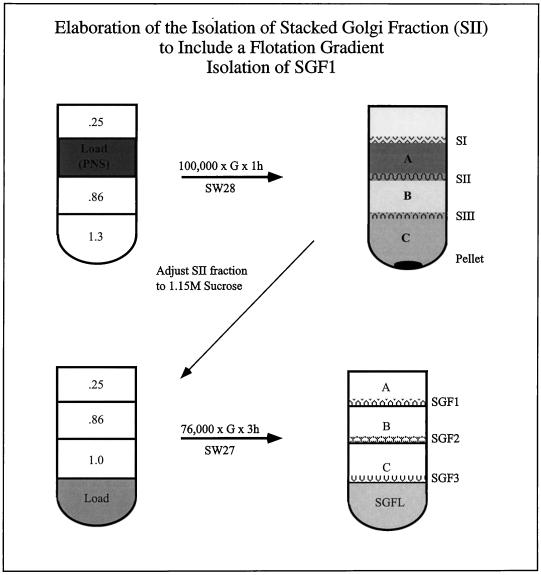
The initial steps in the fractionation procedure were based on the method of Leelavathi et al. (1970). Rats were treated with CHX (50 mg/kg) administered intraperitoneally 4 h before sacrifice. At this dose, animals recover normal function within 24 h. Livers were removed from CTL and CHX-treated animals and placed in precooled glass Petri dishes. All procedures are carried out on ice. Livers were finely minced with scalpels and placed into a preweighed 50-ml conical tube and the wet weight was determined. The minced liver was resuspended at 6 g/10 ml of 0.5 M phosphate-buffered sucrose containing 100 mM KH2PO4/K2HPO4, pH 6.8, 5 mM MgCl2, and 4 μg of the mixture of proteolytic inhibitors (chymostatin, leupeptin, antipain, and pepstatin). All sucrose solutions contained the same buffer and proteolytic inhibitors. Homogenization was in a 50-ml conical tube. The probe of a Polytron PT10/35 (Brinkmann, Westbury, NY), running at setting 3, was placed at the top of the tube and slowly (within 30 s moved to the bottom with a circular motion in only one pass). The homogenate was centrifuged at low speed (1500 × g for 10 min) to pellet unbroken cells, cell debris, and nuclei (nuclear pellet). Because of the mild homogenization procedure the nuclear pellet contained at least 50% of the cell protein. The resulting postnuclear supernatant (PNS, 12 ml) was loaded in the middle of a sucrose step gradient in an SW28 tube: steps of 1.3 M (5 ml) and 0.86 M (12 ml) sucrose were overlaid with the PNS, followed by a 0.25 M layer (5 ml). The gradient was centrifuged at 100,000 × g for 1 h with the brake off (Beckman Instruments, Palo Alto, CA; Figure 1). The following fractions were collected from the top of the gradient by using a wide bore transfer pipet: SI, the 0.25–0.5 M interface; A, the 0.5 M layer; SII, the 0.5–0.86 M interface; B, the 0.86 M layer; SIII, the 0.86–1.3 M interface; C, the 1.3 M layer; and the pellet. After taking an aliquot of the SII fraction, the fraction was adjusted to 1.15 M sucrose with 2 M sucrose. Density was determined by using a refractometer (Bausch and Lomb, Boston, MA). The adjusted SII was loaded into the bottom of a SW28 tube and overlaid with equal volumes (∼10 ml) of 1.0, 0.86, and 0.25 M sucrose and centrifuged at 76,000 × g for 3 h. The following fractions were collected from the top of the gradient: SGFA, the 0.25 M layer; SGF1; the 0.25–0.86 M interface; SGFB, the 0.86 M layer; SGF2, the 0.86–1.0. M interface; SGFC, the 1.0 M layer; SGF3, the 1.0–1.15 M interface; SGFL, the 1.15 M layer (the load zone). All of the fractions from each gradient were collected and protein concentrations were determined by using the DC protein assay (Bio-Rad, Hercules, CA). Small aliquots of these fractions were frozen in liquid nitrogen and stored at −70°C.
Figure 1.
Schematic of preparation of SGF1. The rat liver homogenate was centrifuged (1500 × g for 10 min) to pellet unbroken cells, cell debris, and nuclei. The resulting supernatant (PNS) was loaded in the middle of a step gradient formed in an SW28 tube (upper left) as follows. Two sucrose steps of 0.86 and 1.3 M sucrose were prepared and overlayed with the PNS (∼12 ml) followed by a layer of 0.25 M sucrose. The gradient was centrifuged at 100,000 × g for 1 h. All fractions were collected (upper right), and aliquots were frozen in liquid nitrogen and stored at −70°C. To further enrich the Golgi fraction, the majority of the SII fraction (from the 0.25 M–0.86 M interface) was adjusted to 1.15 M sucrose with 2 M sucrose. The adjusted SII was loaded into the bottom of a second SW28 tube and overlaid with equal volumes of 1.0, 0.86, and 0.25 M sucrose (lower left). The gradient was centrifuged at 76,000 × g for 3 h. All fractions were collected (lower right) and aliquots were frozen in liquid nitrogen and stored at −70°C. The highly enriched stacked Golgi fraction (SGF1) banded at the 0.25–0.86 M interface.
There are two important points in isolating and maintaining an intact Golgi fraction. First is the gentle homogenization procedure; the cells must be broken such that the Golgi “pops” out of the cell intact before ER microsomes are fully formed. Second is the mild handling; the fraction is never pelleted and resuspended or aggressively agitated. All methods of resuspension from a pellet will result in vesiculation of the fraction. Vesiculation also will occur if the fractions are removed from the gradient with a fine bore implement, such as a syringe needle, or are rapidly mixed, e.g., by vortex mixing.
Reporter Molecules
The antibodies used to characterize reporter molecules in the fractions are listed in Table 1 with their respective cellular compartment of predominant localization, reference, and source. We are indebted to many colleagues for generously providing these antibodies.
Table 1.
Antibodies used in these studies
| Marker | Antibody | Localization | Reference | Source |
|---|---|---|---|---|
| p28 | HFD9 | cis-Golgi membrane protein | Subramaniam et al. (1995) | Subramaniam (Univ. of Singapore) |
| MG160 | 10A8 | Medial-Golgi membrane protein | Gonatas et al. (1990) | Gonatas (Univ. of Pennsylvania) |
| TGN38 | 2F7 | trans-Golgi/TGN membrane protein | Horn and Banting (1994) | Banting (Univ. of Bristol) |
| Clathrin | TD.1 | TGN/PM coat protein | Nathke et al. (1992) | ATCC Hybridoma Collectiona |
| p200 | AD7 | Peripheral Golgi protein | Narula et al. (1992) | Burke (Univ. of Calgary) |
| βCOP | M3A5 | Golgi coat protein | Duden et al. (1991) | T. Kreis (Univ. of Geneva) |
| pIgA-R | Membrane secretory protein | Salamero et al. (1990) | Our laboratory | |
| transferrin | Soluble secretory protein | Salamero et al. (1990) | Our laboratory | |
| ApoE | Soluble secretory protein | Scarino and Howell (1987) | Our laboratory | |
| HA4 | HA4 | Plasma membrane protein | Margolis et al. (1988) | Developmental Studies Hybridoma Bank, NICHHD |
| cytochrome P-450p | ER membrane protein | Wrighton et al. (1985) | Quattrochi (Univ. of Colorado) | |
| SERCA | C4 | ER membrane protein | Lytton and MacLennan (1988) | Lytton (Univ. of Calgary) |
| PMCA | 5F10 | Plasma membrane protein | Caride et al. (1996) | Penniston (Mayo Foundation) |
| BiP | Soluble ER protein | Tooze et al. (1989) | Fuller (EMBL) | |
| Ribophorin II | Mab 3D1 | ER membrane protein | Hortsch et al. (1986) | Meyer (Univ. of California Los Angeles) |
ATCC, American Type Culture Collection (Rockville, MD); NICHHD, National Institute of Child Health and Human Development; EMBL, European Molecular Biology Laboratory.
Electron Microscopy
For in situ morphology, rats were anesthetized, perfused with phosphate-buffered saline (PBS) to clear the circulatory system and then perfused for 10 min at 10 ml/min with 2% glutaraldehyde in 100 mM sodium cacodylate, pH 7.3, containing 2% sucrose. When the livers were blanched and firm, they were removed and small pieces were excised and diced into smaller blocks. The tissue was postfixed with 2% OsO4 in 0.8% potassium ferrocyanide buffered with 100 mM sodium cacodylate buffer, pH 7.3, containing 2% sucrose. The tissue blocks were washed with water, en bloc-stained with 2% aqueous uranyl acetate, dehydrated, and embedded in Spur’s resin.
For in vitro morphology, aliquots of fractions were fixed in suspension by addition of an equal volume of 4% glutaraldehyde in 200 mM sodium cacodylate buffer, pH 7.3. After 2 h at 4°C, the fraction was pelleted at 50,000 × g for 30 min in a TLA100 rotor (Beckman). Pellets were washed twice in 100 mM sodium cacodylate buffer, pH 7.3, and postfixed with 2% OsO4 in 0.8% potassium ferrocyanide before embedding in Spur’s resin. Sections were poststained with 5% uranyl acetate in methanol and Reynolds’ lead citrate (Reynolds, 1963).
Morphometric Analysis
Systematic random sampling methods were used to examine preparations of hepatocytes and SGF1 from three CTL animals and three CHX-treated animals (Weibel, 1969; Lucocq, 1993). For analysis of the SGF1s, the entire pellets were sampled by photographing adjacent consecutive fields from top to bottom. Each field was photographed at 15,000× and printed with a 2.8-fold enlargement. Twelve micrographs were used to sample each SGF1 preparation. To determine the relative number of cellular components, the micrograph was overlaid with a 2 cm × 2 cm grid and those structures lying at the intersections of the grid were identified and counted. The micrographs were scored by three individuals without knowledge of the treatment groups, and subsequently, the scores were tallied. The total numbers of scored structures for CTLs were 2692 and for CHX-treated were 3722.
Gel Electrophoresis and Immunoblots
One-dimensional SDS-PAGE was carried out using a 5–15% polyacrylamide gradient and the buffer system of Maizel (1971). Molecular weight standards were from Bio-Rad. For immunoblots, samples were transferred to Immobilon-P (Millipore, Bedford, MA) and were blocked for 1 h in 5% defatted milk/PBS/0.02% sodium azide. The filters were incubated overnight with primary antibody and washed. When using a mouse primary antibody, the filters were incubated with rabbit anti-mouse IgG for 2 h. Bound antibody was detected using 125I-labeled protein A (Dupont, New England Nuclear, Boston, MA). When imunoblotting using antibodies against PMCA (5F10) the procedure was varied, in that binding was carried out in PBS/0.1% bovine serum albumin/0.02% sodium azide. The positive control used for anti-PMCA immunoblots was COS cell microsomes from cells over expressing the human PMCA4b isoform (which were kindly provided by Dr. J. Penniston, Mayo Foundation, Rochester, MN). Immunoblots and autophosphorylation reactions were both quantitated by using a PhosphorImager (PI; Molecular Dynamics, Sunnyvale, CA) and exposed to film for autoradiography. All figures are from autoradiographs.
Enzymatic Assays
Enzymatic assays for β-N-acetylglucosaminidase, lactate dehydrogenase, NADPH-cytochrome C reductase, succinate dehydrogenase, and catalase were carried out as described by Beaufay et al., (1974). Galactosyltransferase was assayed according to Bretz and Stäubli (1977).
Method for Calculating Enrichments
For immunoblots, the protein load of each fraction was adjusted to observe a signal that could be reliably quantitated. This required very large protein loads for the PNS, intermediate loads for the fractions from the first gradient, and much lower loads for the fractions of the second gradient. The protein concentration loaded on the gels is noted on the respective lane in all figures. Enrichment was calculated relative to the PNS as (sample PI units/μg of protein)/(PNS PI units/μg of protein). No detectable antigen (NDA) is defined as an enrichment value of less than 0.5. Enrichment values are noted at the bottom of each lane in all panels. Yield was calculated as the (sample PI units/mg of protein)(total sample volume)(sample protein concentration in mg/ml)/(PNS PI units/mg of protein)(total PNS volume)(PNS protein concentration in mg/ml). Traditionally, enrichment is measured from the starting homogenate, but in the case of this fractionation procedure, the first homogenate contains large amounts of unbroken cells, cell debris, and nuclei making this type of analysis impractical. The amount of this fraction that one would need to load onto an SDS gel to obtain a reliable signal is beyond the resolving capacity of the gel system; therefore, we have calculated yields and enrichment from PNS.
Calcium Uptake Assays
Uptake assays were carried out at 37°C for 10 min. SGF1 (25 μg) or ER (220 μg) was suspended in 100 mM KCl, 30 mM choline chloride, 1 mM MgCl2, 1 mM sodium azide, 1 mM dithiothreitol, 0.1 mM ethylene glycol-bis(β-aminoethyl ether)-N,N,N′,N′-tetraacetic acid (EGTA), 25.5 μM CaCl2 (free Ca2+ = 75 nM), and 20 mM HEPES, pH 7.0. Two percent of the calcium in each assay was 45Ca2+ (30 mCi/mg; Dupont, New England Nuclear). Uptake was defined as pmoles of Ca2+ per min per μg of protein that pelleted during centrifugation at 16,000 × g at 4°C for 22 min in a Brinkmann microcentrifuge. The entire pellet was solubilized in 1% Triton X-100 for 45 min at 37°C followed by addition of counting cocktail (Bio-Safe II, Research Products International, Mount Prospect, IL) and measuring radioactivity in a Beckman LS 1801 scintillation counter. Identical assays that had no ATP added were used to define the background amount of Ca2+ associated with the pelleted fraction, and that value was subtracted from the total uptake value. Pharmacologic agents (A23187, thapsigargin, and oligomycin; Calbiochem, San Diego, CA) were first dissolved in dimethyl sulfoxide (thapsigargin) or in EtOH (A23187 and oligomycin) as stock solutions before addition to uptake assays. Control assays contained equivalent amounts of dimethyl sulfoxide/EtOH as were added to assays given the highest amount of a particular agent.
Time course experiments were performed by using a variant of the standard uptake reaction. Reactions were initiated by the addition of ATP (reactions were reduced by 50% for these assays) to buffer containing SGF1 or ER prewarmed to 25°C and uptake was allowed to occur for various lengths of time before being diluted into 1 ml of ice-cold reaction buffer, which did not contain radioactive calcium, and pelleted for 22 min at 4°C. These reactions were solubilized and radioactivity was measured as above.
Additional control experiments were performed to further define the assay system. Uptake reactions were performed for 10 min at 37°C and then kept on ice for 0, 10, or 20 min before pelleting for 22 min and measuring radioactivity. No loss of counts taken up was seen between the 0 and 20 min time points. In addition, uptake reactions were performed for 10 min at 37°C, the membranes were pelleted, and the supernatant was reused for another round of uptake for 10 min at 37°C. In these reactions fresh CTL-SGF1 also took up calcium, providing evidence that substrate was still available for further calcium uptake at the end of the standard 10-min incubations.
Autophosphorylation Reactions
Autophosphorylation reactions were performed according to Lytton et al. (1992). SGF1 or ER were incubated on ice for 15 s in the presence of either zero free Ca2+ (1 mM EGTA) or 100 μM Ca2+. Reactions were stopped by diluting with a 20× volume of ice-cold 10% trichloroacetic acid and 1 mM H3PO4. Precipitated proteins were solubilized into sample buffer and separated on SDS-PAGE (pH 6.3) by the method of Weber and Osborn (1969).
RESULTS
CHX Treatment Clears Proteins in Transit from the Golgi Complex
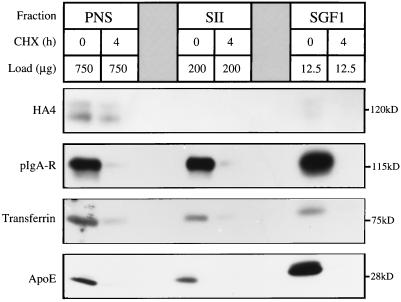
Taylor et al. (1984) showed that 90 min after a block of protein synthesis with CHX, 90% of albumin, the major secretory protein of hepatocytes, had cleared the Golgi complex. Because secretory and transmembrane proteins in transit make up a large proportion of the total protein component of Golgi fractions, even after reduction of these molecules by 90%, they may still be dominant proteins of the fraction and obscure endogenous proteins (Howell and Palade, 1982). To obtain clearance values approaching 100%, we increased the dose of CHX and the time between administration and sacrifice of the animal. Our initial experiments showed the rate of transport of various reporter molecules from synthesis to secretion or insertion into the PM was significantly increased in the presence of CHX. After ∼4 h, secretory and transmembrane proteins in transit were no longer detected in the CHX SGF1 (Figure 2).
Figure 2.
CHX treatment clears proteins in transit from SGF1. The efficiency of CHX treatment in clearing nonresident proteins from the Golgi complex was determined by quantitative immunoblotting of PNS, SII, and SGF1 from CTL and CHX-treated animals. To optimize detection and quantitation, the gel was loaded with 750 μg of PNS, 200 μg of SII, and 12.5 μg of SGF1. The four proteins analyzed were HA4, pIgA-R, transferrin, and ApoE shown from top to bottom. The molecular size of each reporter molecule is noted at the right of each panel.
Four “marker” proteins were followed by immunoblot analysis of PNS, SII, and SGF1 isolated from CTL and CHX-treated animals. The first was the hepatocyte PM ectoATPase recognized by monoclonal antibody HA4. The second was the polymeric IgA receptor (pIgA-R), a transmembrane protein that follows the same route as ectoATPase but is cleaved upon delivery to the apical PM. Its ectoplasmic portion bound to pIgA (secretory IgA) is secreted into bile. Studies of the kinetics of transport from synthesis to secretion in bile for the pIgA-R have shown that in untreated rats the receptor was totally cleared from the hepatocyte by 90 min (Sztul et al., 1983, 1985). Thus, the CHX treatment slows the rate of transport of the pIgA-R and presumably other molecules moving to the PM. The other reporter molecules were two soluble (luminal) secretory proteins, transferrin and apolipoprotein E (apoE). Transferrin was selected because it was shown to have slower clearance from the ER than other secretory proteins (Lodish et al., 1983). ApoE was selected to provide a biochemical correlate with the morphological evaluation of the fractions (the appearance of lipoprotein particles). ApoE is associated with very low density lipoprotein and high density lipoprotein in transit through the secretory pathway of rat hepatocytes. As seen in Figure 2, a 4-h CHX treatment efficiently cleared the Golgi of both secretory and transmembrane proteins in transit. This time point was used for all subsequent studies.
Morphological Characterization
Structural Changes in the Golgi Ribbon In Situ after Proteins in Transit Are Cleared.
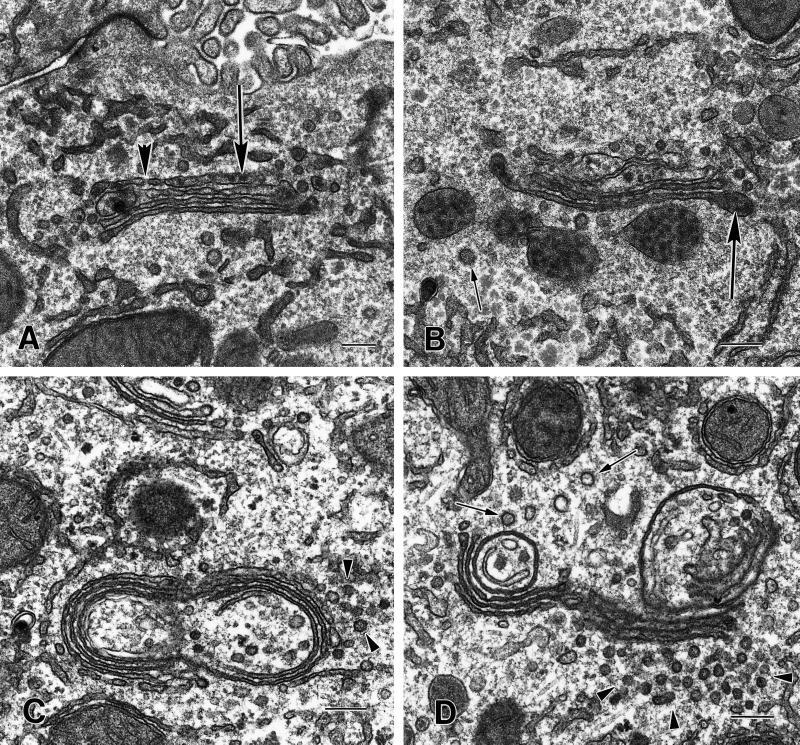
The loss of proteins in transit resulted in extensive structural changes of the Golgi complex that were evaluated by ultrastructure analysis of CTL and CHX-treated hepatocytes (Figure 3). In regions of Golgi ribbons adjacent to bile canaliculi from nonfasted CTL animals, the cisternae and dilated rims and/or vesicles are distended with secretory product, including lipoprotein particles (Figure 3, A and B). In contrast, the compact regions of the Golgi ribbon from CHX-treated animals are noticeably compressed and there is no evidence of dilated rims and/or vesicles distended with secretory product (Figure 3, C and D). Small vesicles (50–70 nm in diameter) and/or tubules are abundant adjacent to the Golgi. The cisternae in the compact regions are not as linear as in the control and frequently appear circular, as if they were no longer connected with the tubules of the noncompact region. Unlike mitotic Golgi fragments that distribute throughout the cytosol, the Golgi depleted of proteins in transit by CHX treatment retains its central localization and stacked structure (Novikoff et al., 1971; Lucocq and Warren, 1987; Lucocq et al., 1989). The morphology of the hepatocytes from the CHX-treated animals appears otherwise normal (our unpublished data).
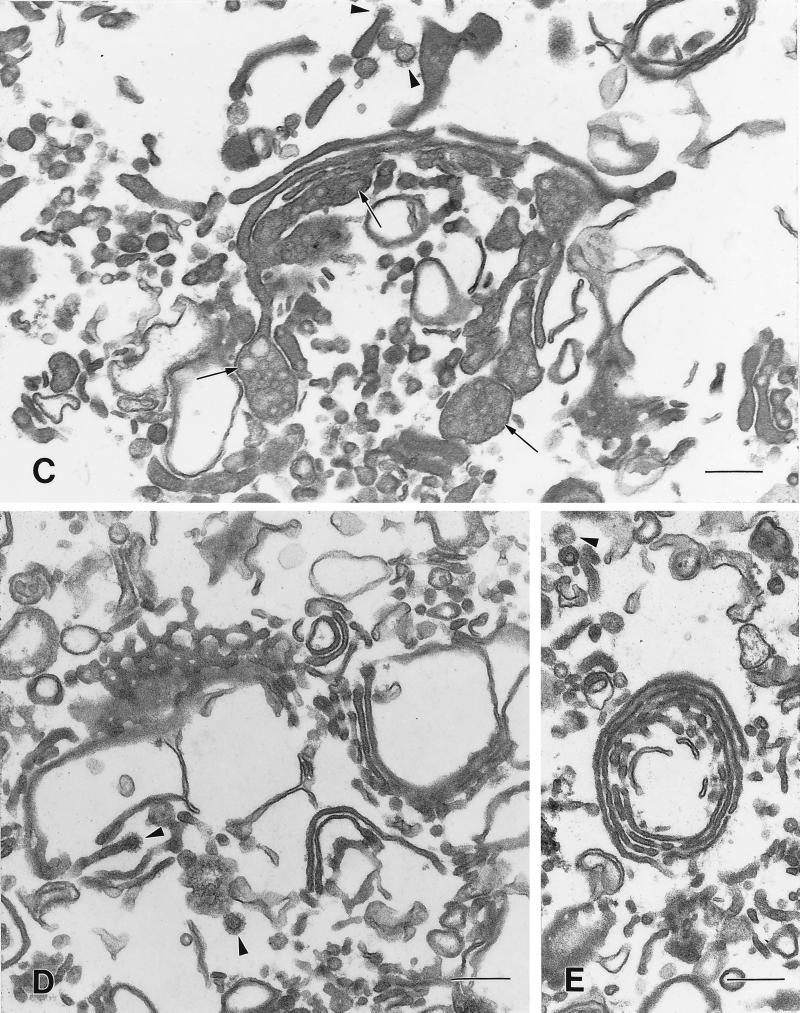
Figure 3.
Morphology of the compact regions of the Golgi ribbon in hepatocytes from CTL and CHX-treated animals. (A and B) The cisternae of the compact region of the Golgi ribbon from CTL animals are relatively straight. The cis cisternae (cis) were identified by their fenestrations (A, large arrowhead). Lipoprotein particles (large arrows) are present in the cisternae, particularly in the distended rims and/or vesicles in the trans region. Clathrin-coated vesicles (small arrow) are present in the trans region (B). (C and D) In the compact region of the Golgi stack the cisternae from CHX-treated animals are more tightly packed together and the width of the cisternal lumen is reduced when compared with CTLs. Lipoprotein particles are absent from the cisternae and vesicles. There are a large number of what appear to be vesicles (arrowheads), 50–70 nm in diameter, associated with the Golgi. Often the Golgi cisternae appear to be circular; this could result from a disruption at the noncompact region of the Golgi ribbon and some cisternae of the compact region folding back on themselves. The circularized Golgi cisternae are surrounded (both inside and out with many vesicles or tubules, some with clathrin-coats (small arrows, D). Bars, 200 nm.
Structural Changes Observed In Situ Are Preserved in the Isolated Golgi Fractions.
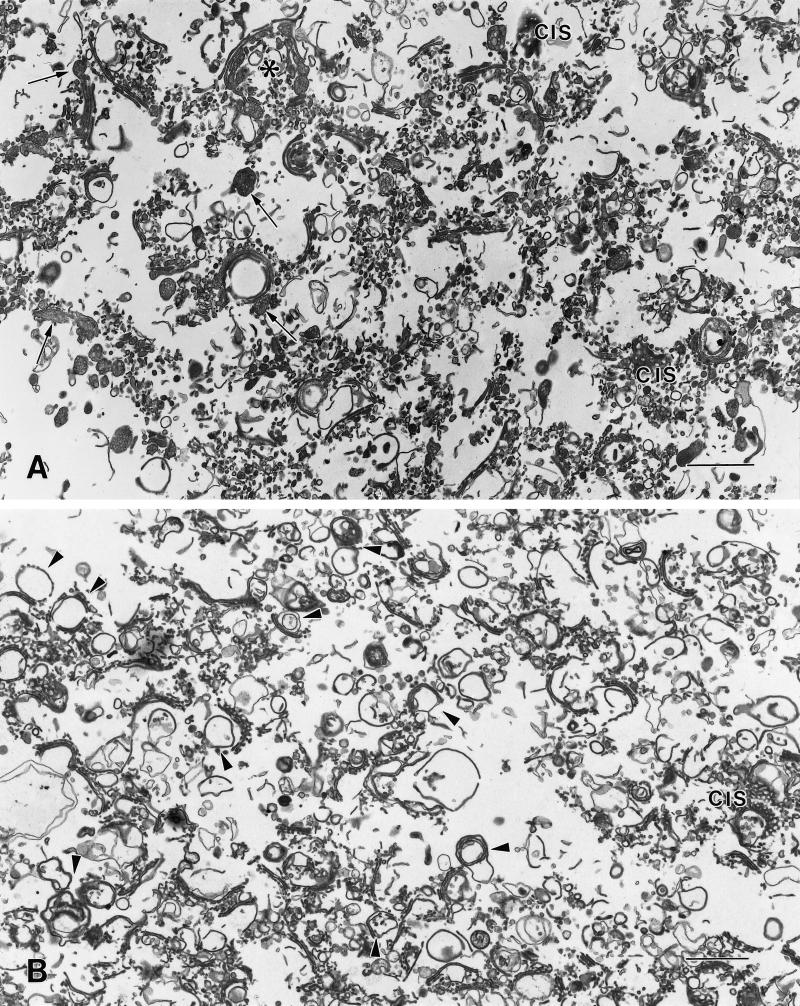
The major components of both CTL and CHX SGF1s are stacked and single cisternae (Figure 4). Some of the stacks retain adjacent tubular regions similar to the noncompact zones of the Golgi ribbon. In the CHX SGF1, the cisternae are more tightly packed within the stack (Figure 4, B, D, and E). The fenestrations of the cis-cisternae are readily apparent in the isolated fractions, particularly in the CHX SGF1 (Figure 4D). In the CTL SGF1, the cisternae have dilated rims and/or associated vesicles that are filled with lipoprotein particles (Figure 4, A and C). In CHX SGF1, there is no evidence of distention of the cisternae due to secretory products. Many of the compact regions of the CHX SGF1 appear to be circular (Figure 4B, arrowheads), similar to the morphologic changes observed in situ. Although vesicles are less abundant in the CHX SGF1, many clathrin-coated buds and vesicles are still present.
Figure 4.
Overview of SGF1s isolated from livers of CTL and CHX-treated animals. Low-magnification overview of CTL (A) and CHX (B) SGF1s. The majority of the components of the fractions are Golgi stacks, single cisternae, and associated vesicles. (A) In CTL SGF1, the cisternae are distended and contain secretory products (arrows). Most of the stacks are in a linear ribbon, although a few stacks appear circular. The asterisk indicates a Golgi region shown at higher magnification in C. (B) In CHX SGF1, the isolated stacks are condensed and do not contain obvious luminal secretory products. Compared with CTL SGF1, a higher proportion of the cisternae appear to be circular (arrowheads), similar to that observed in situ. The anastomosing tubular pattern of cis cisternae (cis) is particularly evident in the CHX SGF1. Higher magnifications of Golgi compact zones in CTL SGF1 (C) and CHX SGF1 (D and E). The stacked regions in the isolated CTL and CHX SGF1 have three or four cisternae. Arrowheads denote clathrin-coated structures. (C) In CTL SGF1, lipoprotein particles (arrows) are evident in the stacked cisternae, the dilated rims of the cisternae, and what appear to be adhering vesicles in the trans region. (D and E) In CHX SGF1, the cisternae of the Golgi stacks are more tightly packed and have reduced luminal widths when compared with the CTL. Lipoprotein particles are absent from the cisternae and vesicles. Circular profiles and cis regions are prominent. Bars: A and B, 1 μm; C–E, 200 nm.
Morphometric Analysis of CTL and CHX SGF1s.
The components, integrity, and level of contamination of the CTL and CHX SGF1s were assessed by morphometric analysis (Table 2). The composition of CTL and CHX SGF1s was >90% identifiable Golgi stacks, intact cisternae, associated vesicles, or “blown-up” cisternae. The CHX SGF1 shows greater integrity of the compact region; ∼44% of the Golgi elements of CHX SGF1 were stacked but only 32% of the elements were stacked in the CTL SGF1. In CTL SGF1, the cisternal width varied reflecting the presence of the secretory proteins, especially the lipoproteins, whereas the widths of the cisternae in CHX SGF1 were approximately equal. Further, the amount of vesicles in the CHX SGF1 was reduced compared with CTL SGF1; however, the proportion of those vesicles present that are clathrin-coated remained the same (∼16%). The non-Golgi components scored in both fractions were lipid droplets, lysosomes, ER, and non-Golgi membranes and make up ∼7% of both CTL and CHX SGF1s. In vivo, Golgi stacks had four or five cisternae, and CHX treatment did not result in a change of cisternal number but did result in a decrease in luminal width of all cisternae. The CHX treatment resulted in a decrease in luminal width of the cisternae by about 30% in the hepatocyte and 20% in the SGF1s (our unpublished results).
Table 2.
Morphometric analysis of CTL and CHX SGF1s
| Percent
|
||
|---|---|---|
| CTL | CHX | |
| Golgi components (total) | (91 ± 2) | (90 ± 2) |
| Stacks | 32 ± 21 | 44 ± 8 |
| Single cisternae | 39 ± 21 | 35 ± 6 |
| Vesicles | 13 ± 4 (16 ± 7a) | 7 ± 6 (16 ± 6a) |
| Other membranes | 7 ± 4 | 4 ± 2 |
| Lysosomes | 3 ± 3 | 3 ± 1 |
| ER | 1 ± 1 | 1 ± 2 |
| Lipid droplets | 1 ± 1 | 1 ± 1 |
| Other non-Golgi membranes | 4 ± 3 | 5 ± 2 |
Percent of total profiles scored.
Percent clathin-coated vesicles.
Biochemical Characterization
Distribution of Golgi Markers in CTL and CHX SGF1s.
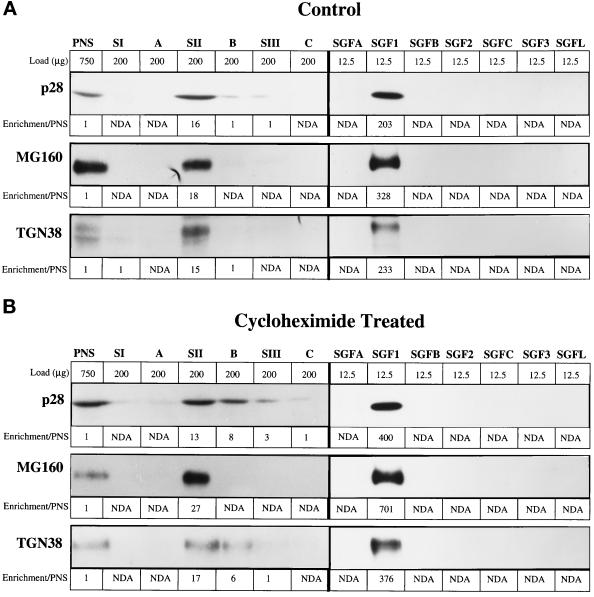
The biochemical characterization provides parallel and complementary data to the morphology on Golgi proteins and contaminants present in the CTL and CHX SGF1s. The distribution of four Golgi markers was determined in the PNS and fractions from both gradients in the SGF isolation protocol by quantitative immunoblot and enzymatic assay (Figure 5 and Tables 3 and 4). Transmembrane proteins predominately localized to cis-, medial-, trans-, and TGN were selected to determine whether all cisternal subcompartments of the Golgi complex were equally isolated. The markers used were p28 for cis- (Subramaniam et al., 1995), MG160 for medial- (Gonatas et al., 1989), and galactosyltransferase (Berger et al., 1981) and TGN38 (Luzio et al., 1990) for trans- and TGN. Enrichment of each marker over its concentration in the PNS was calculated for each fraction and that value is noted below each lane (Figure 5 and Table 3). The Golgi markers were enriched ∼15- to 20-fold over PNS in the SII fraction with no significant differences apparent between CTL and CHX SGF1s.
Figure 5.
Enrichment of Golgi markers in the stacked Golgi fraction. Golgi markers were analyzed in the fractions of the SGF1 isolation protocol by quantitative immunoblot of fractions from CTL (A) and CHX-treated (B) animals. The fractions are noted at the top and directly below is the load (micrograms of protein) of that fraction. It was necessary to increase the protein load of fraction in which the markers were present in low concentrations to obtain a reliable signal. For each marker, the values for enrichment over PNS are placed below the gel bands: cis-, p28; medial-, MG160; trans- and TGN, TGN38.
Table 3.
Enrichment and yield of markers in CTL and CHX SGF1s
| Marker | Localization | Enrichment (fold/PNS)
|
% Yield/PNS
|
||
|---|---|---|---|---|---|
| CTL | CHX | CTL | CHX | ||
| p28 | cis-Golgi | 204 | 400 | 51 | 46 |
| MG160 | medial-Golgi | 328 | 701 | 68 | 61 |
| TGN38 | trans-Golgi/TGN | 233 | 376 | 42 | 37 |
| Clathrin | TGN/PM coat protein | 23 | 12 | ||
| p200a | Peripheral Golgi protein | 68 | 30 | ||
| βCOP | Golgi coat protein | 35 | 20 | ||
| pIgA-R | Membrane secretory protein | 110 | NDA | ||
| Transferrin | Soluble secretory protein | 47 | NDA | ||
| ApoE | Soluble secretory protein | 50 | NDA | ||
| HA4 | Plasma membrane protein | 16 | NDA | ||
| Ribophorin II | ER | 0.15 | 1.00 | ||
| Cytochrome p450 | ER | 0.76 | 2.51 | ||
| BiP | ER | 0.9 | 1.6 | ||
| SERCA | ER | 1.3 | 3.2 | ||
p200 is a nonmuscle myosin IIA.
Table 4.
Enzymatic marker analysis of the stacked Golgi fraction
| Enzyme | Localizaton | SII
|
SGF1
|
||
|---|---|---|---|---|---|
| Enrichment (fold/PNS)
|
Enrichment (fold/PNS)
|
||||
| CTL | CHX | CTL | CHX | ||
| Galactosyltransferase | trans/TGN membrane | 13 | 14 | 327 | 748 |
| β-N-Acetylglucosaminidase | Lysosome content | 2.79 | 4.25 | 0.79 | 1.00 |
| Lactate dehydrogenase | Cytosol | 2.71 | 3.17 | 0.001 | 0.002 |
| NADPH-cytochrome c reductase | ER membrane | 4.7 | 5.9 | 0.81 | 1.15 |
| Succinate dehydrogenase | Mitochondria membrane | 0.296 | 0.186 | 0.057 | 0.047 |
| Catalase | Peroxisome content | 0.081 | 0.127 | 0.018 | 0.020 |
The primary rationale for the second gradient was to float the stacked Golgi membranes away from the large pool of soluble cytosolic proteins present in the SII fraction with a secondary benefit being the loss of more dense components that had not completely cleared the load zone in the first gradient. The second gradient was effective in removing cytosol, resulting in enrichments for lactic dehydrogenase of less than 0.01 in the CTL and CHX SGFIs (Table 4).
A considerable increase in enrichment of the Golgi markers was attained by using the floatation gradient. The enrichment data, combined with the clearance data (Figure 2), are summarized in Table 3. The CTL SGF1 is enriched 204-fold for p28, 328-fold for MG160, 327-fold for galactosyltransferase, and 233-fold for TGN38. The enrichment in CHX SGF1 was about two times greater, 400-fold for p28, 701-fold for MG160, 748-fold for galactosyltransferase, and 376-fold for TGN38, suggesting that ∼50% of the protein in CTL SGF1 is accounted for by proteins in transit. Importantly, the proteins in transit through the secretory pathway were enriched 20- to 100-fold over PNS in CTL SGF1 but were below the limit of detection in CHX SGF1.
Minimal Enrichment of ER Markers in CTL and CHX SGF1.
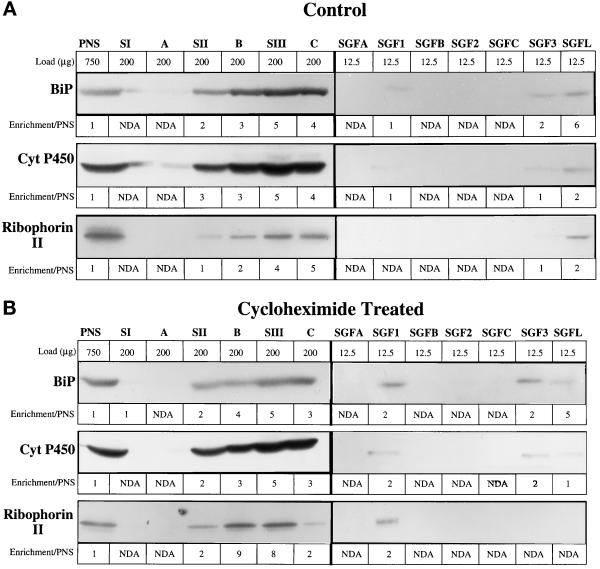
As our goal is to examine the calcium uptake activity associated with the Golgi complex, it is critical to evaluate the amount of ER markers in the SGF1s. The ER contains a family of well-characterized calcium pumps, the SERCAs, that transport calcium into the lumen of the ER. We selected markers that are significantly enriched in the smooth endoplasmic reticulum (SER), cytochrome P450 and NADPH-cytochrome c reductase activity (Wrighton et al., 1985); equally distributed in rough endoplasmic reticulum (RER) and SER, SERCA and BiP, a soluble ER protein (Lytton and MacLennan, 1988; Tooze et al., 1989); and an RER-specific transmembrane protein, ribophorin II (Hortsch et al., 1986). The major distribution of the ER markers was in the heavier SIII and C fractions on the first gradient (Figure 6). However, ∼5% of each ER marker isolated in the SII fraction but remained in the load zone of the second gradient. Nonetheless, there was always a detectable amount of ER markers in the SGF1 fractions (Figure 6 and Tables 3 and 4). In CTL SGF1, the markers cytochrome P450, SERCA, BiP, NADPH cytochrome c reductase, and ribophorin II showed minimal enrichment and were at the same concentration as in the PNS. In the CHX SGF1, the enrichment of the ER markers increased twofold. The enrichment of ER markers in the SGF1s is several hundred fold smaller than the enrichment for the Golgi markers, indicating that ER contamination is minimal.
Figure 6.
Enrichment of ER markers in the stacked Golgi fraction. ER markers were analyzed in the fractions of the SGF1 isolation protocol by quantitative immunoblot of fractions from CTL (A) and CHX-treated (B) animals. The fractions are noted at the top of each panel and directly below is the load (μg of protein) of that fraction. It was necessary to increase the protein load of fractions in which the markers were present in low concentrations to obtain a reliable signal. For the following markers, the values for enrichment over PNS are indicated: BiP, soluble luminal protein; cytochrome P450, a transmembrane protein enriched in SER; Ribophorin II, a membrane protein predominately localized to RER. NDA, no detectable antigen or activity.
Evaluation of Enrichment for Other Markers in CTL and CHX SGF1s.
Because lysosomes were detected in the morphological evaluation, the lysosomal enzyme, β-N-acetylglucosaminidase was assayed and found to be present in the SGF1s at the same concentration as the PNS (about onefold enrichment; Table 4). Markers of mitochondrial membrane (succinate dehydrogenase) and peroxisomal content (catalase) showed minimal enrichment in both CTL and CHX SGF1s (Table 4). The following peripheral and coat proteins associated with the Golgi complex were evaluated: p200, clathrin, and β-COP (Table 3). Each of these molecules is enriched in CTL SGF1 (p200, 68-fold; clathrin, 23-fold; β-COP, 35-fold), and these enrichments are reduced by half in CHX SGF1.
Yield of CTL and CHX SGF1s.
The yields of the Golgi markers shown in Table 3 nicely parallel the enrichment data and in CTL and CHX SGF1 are, respectively: cis- p28, 51% and 46%; medial- MG160, 68% and 61%; trans- and TGN galactosyltransferase, 41% and 36%; and TGN38, 42% and 37%. The parallel protein yields per gram wet weight liver are 1.0 mg and 0.75 mg. Thus, the protocol provides isolation of fractions with intact structure and sufficient material to undertake most studies of Golgi function.
SGF1 Takes Up Calcium into a Membrane-Bound Compartment in an ATP-dependent Manner and Is Blocked by Known Inhibitors of SERCA and All p-Type ATPases.
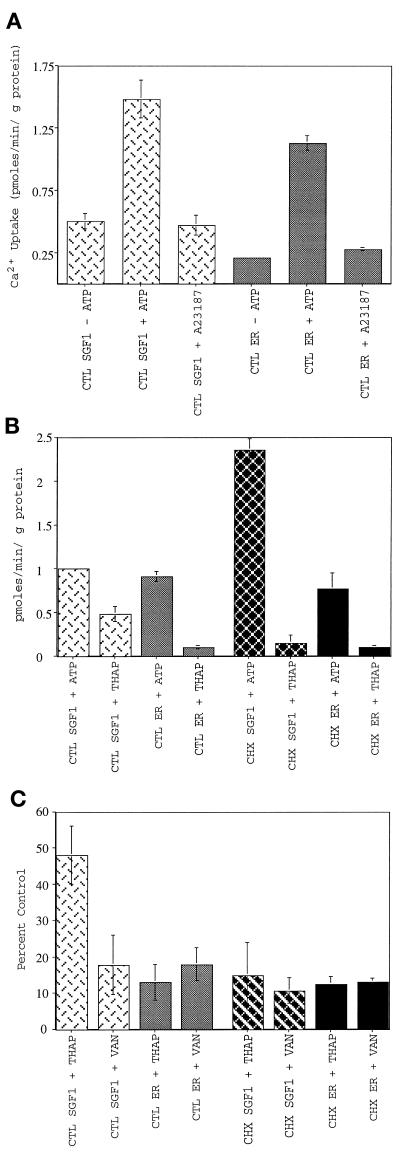
Calcium uptake was first analyzed with the CTL SGF1 and the CTL ER fraction. When incubated with ATP, both fractions accumulated Ca2+ at 1 pmol per min per μg of protein (Figure 7A). Uptake into a membrane-bound compartment was confirmed by the addition of the calcium ionophore A23187, which reduced the specific calcium uptake to the background levels observed in the absence of ATP. The requirement for ATP hydrolysis was established by performing the reaction on ice or in the presence of 1 mM adenosine 5′-[β,γ-imido]triphosphate (AMP-PNP), a nonhydrolyzable analogue of ATP, conditions in which background levels of calcium uptake were observed (our unpublished results).
Figure 7.
Characterization of calcium uptake into SGF1s. SGF1 takes up calcium into a membrane-bound compartment in an ATP-dependent manner and is blocked by known inhibitors of SERCA and all p-type ATPases. CTL SGF1 (light cross-hatched bars), CTL ER (light solid bars), CHX SGF1 (dark cross-hatched bars), and CHX ER (dark solid bars) are indicated. (A) ATP-dependent calcium uptake into CTL SGF1 and CTL ER fraction. Calcium uptake in pmoles per minute per microgram of protein is plotted. Uptake reactions were performed in the presence of 75 nM free calcium by using 25 μg of SGF1 or 100 μg of ER for 10 min at 37°C in the absence of ATP, in the presence of 1 mM ATP, or in the presence of 1 mM ATP and 1 μM A23187. The 10-min time point was within the linear range of calcium uptake (see Figure 11). Note that background (uptake in the absence of ATP) is not subtracted from any of these experiments. (B) Thapsigargin sensitivity of calcium uptake into CTL and CHX SGF1s and CTL and CHX ER fractions. ATP-dependent calcium uptake in pmoles per minute per microgram of protein (after background subtraction from a minus ATP reaction) is plotted for all four fractions. Thapsigargin (2 μM) was added to the reaction mixture containing 1 mM ATP at 4°C for 5 min before initation of the assay by rapid warming to 37°C. (C) Inhibition of calcium uptake by thapsigargin and sodium vanadate. Calcium uptake is expressed as percent of CTL (no inhibitor) for all four fractions. Thapsigargin (2 μM) was added to the reaction mixture at 4°C for 5 min before initation of the assay by rapid warming to 37°C. The fractions were pretreated with 1 mM sodium vanadate for 10 min at room temperature in the absence of ATP before warming to 37°C and addition of ATP. Error bars represent SD, and n = 25 for A, and n = 6 for B and C.
In an initial experiment to characterize the type of calcium pump in SGF1, uptake reactions were carried out on both fractions in the presence of pharmacologic agents that specifically inhibit intracellular SERCA pumps and have no effect on PMCA pumps (Thastrup et al., 1990). SERCA pumps are thought to reside solely in the membrane of the RER and SER (Grover and Khan, 1992). In the presence of 2 μM thapsigargin (Figure 7B), uptake into CTL ER was completely blocked. In contrast, uptake into CTL SGF1 was only inhibited 50% by thapsigargin. In parallel experiments, CHX ER fractions take up the same amount of calcium as the CTL ER fraction and this activity was completely inhibited by thapsigargin. Interestingly, the CHX SGF1 took up significantly more calcium than the CTL SGF1 (2.3 vs. 1.0 pmol per min per μg of protein) and was now totally sensitive to thapsigargin. These data suggest that two populations of calcium pump are present in CTL SGF1.
As discussed in the introduction, others (West, 1981; Virk et al., 1985) have reported that 26–40% of lactating mammary Golgi fraction calcium uptake was through a proton-gradient–dependent mechanism that could be inhibited by protonophores. V-type ATPases (e.g., the vacuolar-ATPase) transport protons and establish proton gradients across membranes (Carafoli, 1994). P-type ATPases can be distinguished from v-type ATPases by their vanadate sensitivity. To examine whether the thapsigargin-resistant pool of calcium uptake in CTL SGF1 was due to a p-type calcium ATPase, vanadate sensitivity was tested. The samples were mixed with 1 mM sodium vanadate for 10 min at room temperature in the absence of ATP before initiating calcium uptake by addition of ATP and warming to 37°C. Uptake in all fractions was blocked to background levels (12–15%) in the presence of vanadate (Figure 7C). Additionally, oligomycin, an inhibitor of mitochondrial calcium uptake, had no effect on calcium uptake in the ER or SGF1 fractions (our unpublished results). These results demonstrate that, in rat liver ER and Golgi fractions, all calcium transport is due to members of the p-type family of ATPases.
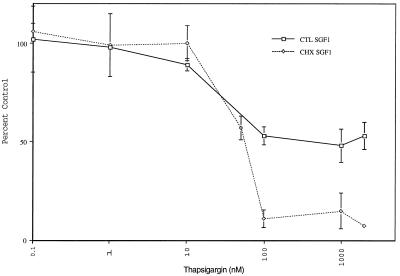
To more fully characterize the thapsigargin-sensitive activity, a thapsigargin dose–response curve was carried out by using CTL SGF1 and CHX SGF1 (Figure 8). Half-maximal inhibition of both SGF1 fractions was obtained at 50 nM, and maximal inhibition was obtained at 100 nM thapsigargin. The inhibition values for the ER fractions were the same as those for the SGF1s (our unpublished results). The thapsigargin-resistant portion of CTL SGF1 was not the result of insufficient thapsigargin present to fully inhibit all calcium pumps in the fraction, but rather maximal inhibition had been achieved. The activity that remained was an indication of a thapsigargin-resistant pool of calcium uptake in CTL Golgi membranes.
Figure 8.
Thapsigargin dose–response curves for CTL SGF1 and CHX SGF1. Calcium uptake is measured in the presence of increasing amounts of thapsigargin (0–2000 nM) for CTL SGF1 (□) and CHX SGF1 (♦). Calcium uptake is expressed as percent of control (no thapsigargin). Error bars represent SD, and n = 6.
Higher concentrations of free calcium might activate a calcium ATPase not seen in the above experiments using 75 nM calcium. To test this possibility, we carried out a thapsigargin dose–response experiment, as in Figure 3, in the presence of 25 μM free calcium (our unpublished results). The thapsigargin sensitivity for the calcium uptake activity of both SGF1s remain the same in the presence of 75 nM or 25 μM free calcium, indicating that other calcium ATPases were not present in the SGF1s and presumably the Golgi complex.
Immunoblot Analysis Reveals the Presence of a PMCA in CTL SGF1 but Not in CHX SGF1 and Confirms a Comparable Enrichment of SERCA Protein in SGF1 and ER.
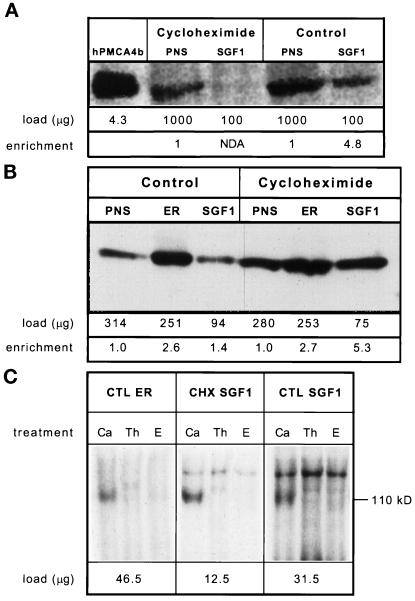
The loss of the thapsigargin-resistant uptake activity from CHX SGF1 led us to hypothesize this activity was due to a PMCA isoform en route to the PM. To test this directly, we obtained monoclonal antibodies that recognize all PMCA isoforms (5F10) and used them to probe our SGF1 and CTL fractions. The 5F10 antibody recognized an ∼140-kDa protein in the PNS from livers of CTL and CHX-treated rats, as well as a similar-sized protein in CTL SGF1 (Figure 9A). Importantly, no signal is detected in the CHX SGF1 fraction.
Figure 9.
Immunoblot and autophosphorylation analysis of CTL and CHX fractions. Samples of each fraction were solubilized in SDS-PAGE sample buffer at room temperature for 1 h before running on a 5–15% polyacrylamide gel and transfer to Immobilon-P. The sample size was optimized for each fraction and the protein loaded per lane is given directly below each lane. Signal was detected with 125I-labeled protein A and quantitated by using a PhosphorImager and reported in PI units. Enrichment was calculated as (sample PI units/μg of protein)/(PNS PI units/μg of protein) and is given at the bottom of the figure. Shown is an autoradiograph of the same immunoblot. (A) PMCA analysis by immunoblotting: lane 1, COS microsomes overexpressing hPMCA4b; lane 2, CHX PNS; lane 3, CHX SGF1; lane 4, CTL PNS; lane 5, CTL SGF1. (B) SERCA analysis: lane 1, CTL PNS; lane 2, CTL ER; lane 3, CTL SGF1; lane 4, CHX PNS; lane 5, CHX ER; lane 6, CHX SGF1. NDA is defined as an enrichment of less than 0.5. (C) Calcium-dependent autophosphorylation of fractions. CTL ER (left), CHX SGF1 (middle), and CTL SGF1 (right) were incubated with [γ-32P]ATP. The sample size was optimized for each fraction and the protein per reaction is given below the autoradiograph. Reactions were carried out in the presence of 100 μM calcium (Ca), 2 μM thapsigargin (Th), or 1 mM EGTA (E) for 15 s on ice and processed as described in MATERIALS AND METHODS. The molecular mass of SERCA (110 kDa) is shown on the right. This was the only band phosphorylated in a calcium-stimulated and an EGTA-inhibited manner.
The finding that the SGF1s had the same or greater calcium uptake activity as the ER fractions led us to consider that a resident calcium-transporting ATPase was present in the rat liver Golgi and that this pump was related to the SERCA family. To test for the presence of a SERCA family protein in the SGF1s, blots of these fractions were probed with a polyclonal antibody (C4) that recognizes all known isoforms of the SERCA family (Lytton et al., 1991). SERCA protein was enriched only 1.4-fold in CTL SGF1 and 5.3-fold in CHX SGF1 (Figure 9B). This enrichment is consistent with the previous observation on rate of uptake of calcium into ER and SGF1 fractions. CHX causes a fourfold increase in the specific activity in SGF1 for both calcium uptake and for SERCA signal.
To examine whether a thapsigargin-sensitive calcium pump is present in the SGF1s that is not antigenically related to the known SERCA isoforms, autophosphorylation assays were performed on the CTL ER, CTL SGF1, and CHX SGF1. Autophosphorylation assays have been used to reveal p-type calcium ATPases in subcellular fractions resolved in acidic SDS gels (Lytton et al., 1992). If the low levels of SERCA were not responsible for the majority of calcium uptake activity, a second band at a different molecular weight from SERCA (110 kDa) or a disproportionately intense signal at 110 kDa between the immunoblot and the autophosphorylation gel would be seen. The autophosphorylation gel showed a 110-kDa band corresponding to SERCA protein in the CTL ER, CHX SGF1, and CTL SGF1 when the reaction was carried out in the presence of calcium (Figure 9C). Both EGTA and thapsigargin inhibited the phosphorylation of the 110-kDa bands. Other EGTA- or thapsigargin-sensitive bands were not seen in the SGF1s. The relative signal between ER and Golgi fractions in immunoblot and autophosphorylation assays is approximately the same, suggesting that SGF1s do not contain additional abundant autophorphorylatable species of the same or different molecular weights. We did not detect a thapsigargin-resistant phosphorylated band corresponding to the 140-kDa PMCAs. Perhaps the concentration of this protein was too low to be observed. Both the immunoblot and autophosphorylation studies demonstrate that other Golgi isoforms, if they exist, must have the same apparent molecular weight as previously characterized SERCAs. Thus, these data lead us to conclude that all thapsigargin-sensitive calcium uptake activity in the SGF1s is due to the activity of SERCA pumps.
Properties of Calcium Uptake Are Different in SGF1 and ER.
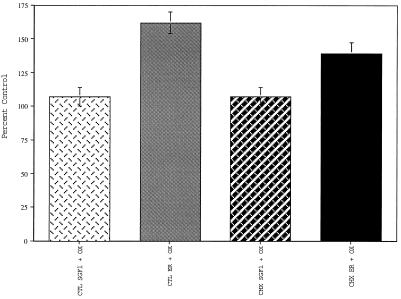
Addition of oxalate anions to uptake assays enhances calcium uptake into the ER by precipitating calcium in the form of calcium oxalate, thereby decreasing leak out of the ER lumen (Moore et al., 1975). When uptake assays were carried out in the presence of 2.5 mM potassium oxalate, the calcium uptake remained the same in CTL and CHX SGF1s, but it increased 60% in the CTL and 40% in the CHX ER fractions (Figure 10). These data indicate that the membrane-bound compartment taking up calcium in the SGF1s is fundamentally different from the membrane-bound compartment in the ER fraction. This is not what would have happened if all the calcium uptake in SGF1s was due to ER membrane contamination of the Golgi fractions and correlates with the conclusions drawn from the morphological and biochemical data demonstrating minimal contamination of the Golgi fractions with ER.
Figure 10.
Effect of oxalate on calcium uptake. Calcium uptake in the presence and absence of oxalate is expressed as percent of CTL (no oxalate) for all four fractions: CTL SGF1 (light cross-hatched bars), CTL ER (light solid bars), CHX SGF1 (dark cross-hatched bars), and CHX ER (dark solid bars). Plus oxalate samples contained 2.5 mM potassium oxalate. Error bars represent SD and n = 3.
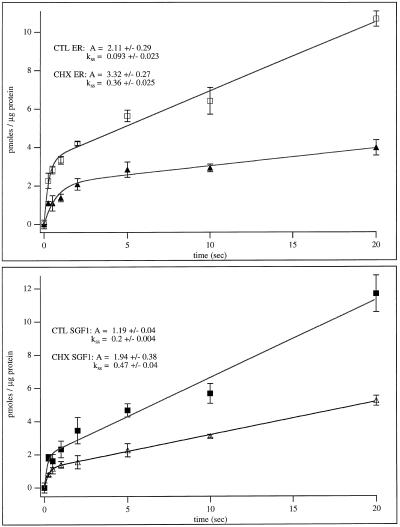
Time course of uptake experiments have the potential to reveal differences between the pumps in the various fractions. Parameters that could effect initial rates include: differential regulation (e.g., calmodulin has a regulatory effect on the PM pump but not the ER pump) or differences in pump densities. The time course data for all four fractions were analyzed by using waveform analysis software (Igor, Lake Oswego, OR) and are shown in Figure 11. All fractions displayed an initial rapid burst followed by a linear rate of steady-state uptake. The steady-state rates of calcium uptake for CTL SGF1, 0.2 ± 0.004 pmol per min per μg protein, and CTL ER, 0.093 ± 0.023, were less than the rates of either the CHX SGF1, 0.47 ± 0.04, and CHX ER, 0.36 ± 0.025. This corresponds to an approximately two- to threefold higher rate of uptake in the CHX fractions than in the CTL fractions. Further, when the CTL SGF1 and CHX SGF1 fractions are compared (or CTL ER and CHX ER), a higher burst height is seen in the CHX SGF1 fraction (or CHX ER). The burst rate could not be reliably determined due to the rapid nature of the burst and the limits of our technology. The simplest interpretation of these data is that CHX treatment results in an increase in the number of calcium pumps within the membrane of the ER and Golgi per microgram of protein.
Figure 11.
Time course of calcium uptake into CTL and CHX SGF1 and ER fractions. (Upper panel) Calcium uptake versus time is plotted for CTL ER (▴) and CHX ER (□). (Lower panel) Calcium uptake (pmoles/μg of protein) versus time (seconds) is plotted for CTL SGF1 (▵) and CHX SGF1 (▪). Curves were fitted and rates were calculated by using Igor software and the formula: f(x) = A × [1 − exp(−kb × t)] + kss × t, where A is the burst amplitude, kb is the burst rate, and kss is the steady-state rate. The burst height (A) and steady-state rate of uptake (kss) for each fraction are given.
DISCUSSION
Reduction of the Golgi complex to it most basic components is a practical way to gain new insight into its functions. Herein we have established conditions for CHX treatment and demonstrate that these conditions effectively clear transmembrane and secretory proteins in transit from the Golgi complex. This reductionist approach has revealed basic properties inherent to the Golgi ribbon. First, the compact region of the ribbon is stable and lack of proteins in transit results in a reduction in diameter of all cisternae without changing their number. The noncompact regions appear less stable and seem to dissociate from the compact regions. Second, the compliment of cisternae remain the same and can be isolated with higher efficiency than the control. Third, proteins in transit account for approximately 50% of the protein in rat liver Golgi at steady state. Fourth, calcium transport into the lumen of the Golgi complex is mediated by two p-type calcium pumps. Finally, dissection of the proteins of the CHX-CHX SGF1 will lead to identification and understanding of the building blocks of this organelle.
The finding, that cis-, medial-, trans-, and TGN components of the Golgi complex remain in approximately the same proportions after CHX treatment has blocked entry of “new” proteins from the ER suggests that the Golgi is a very stable structure and that endogenous molecules are not degraded or mislocalized. Proteins in transit account for about 50% of the total protein of the Golgi fraction, based on the increase in enrichment of the CHX SGF1 over the CTL SGF1. This is in reasonable agreement with the earlier estimate of ∼40% obtained by protein assay after high pH washing a Golgi fraction (Howell and Palade, 1982). The difference in the two estimates can be partially accounted for by transmembrane proteins in transit, as high pH washing cannot remove this category of proteins from Golgi membranes. High pH washing has the following three disadvantages in preparing a Golgi fraction for identification of endogenous molecules: 1) transmembrane proteins in transit are not removed, 2) peripherally associated proteins (both luminal and cytoplasmic) that have unique Golgi functions are removed, and 3) enzymes may become inactivated. CHX treatment avoids all of these problems and is, therefore, an ideal method to use in the identification of the endogenous proteins of the Golgi complex. We have resolved the SGF1s by high resolution two-dimensional gel electrophoresis and the most abundant 173 Golgi specific proteins have been placed into three categories: cargo, cytosolic Golgi-associated, and resident Golgi proteins (Taylor et al., 1997). We are working toward identifying already characterized proteins by using immunoblot analysis and identifying unknown species.
The fractionation scheme reported herein is a significant improvement over the 1970 protocol of Leelavathi et al. (1970); the stacked Golgi cisternae are separated from cytosol and other contaminating membranes increasing the enrichment 10-fold, resulting in a final enrichment of ∼400-fold over PNS. In 1970 very few Golgi markers had been identified, so our evaluation of enrichment and yields of markers from the different subcompartments of the Golgi complex are clearly more complete. The enrichment of biochemical markers for cis-, medial-, and trans-Golgi in SGF1 isolated from CTL and CHX-treated animals is 300- to 700-fold. This is paralleled by an enrichment of morphologically defined Golgi stacks, cisternae, and vesicles of >90%. Strikingly, the morphometric analysis shows that the percent of vesicles observed immediately adjacent to Golgi stacks is reduced by half in the CHX fraction (7%) compared with CTL (13%). These data are consistent with the reduction in the enrichment of the coat proteins clathrin and β-COP from 23- and 35-fold in CTL SGF1 to 12- and 20-fold in CHX SGF1. Secretory proteins in transit, transferrin and apoE, are enriched 50-fold in the CTL SGF1 and could not be detected in CHX SGF1. These data demonstrate that secretory proteins have cleared the Golgi. This is dramatically corroborated by morphology. The cisternae of CTL SGF1 are wider and filled with lipoprotein particles. This is especially evident at the cisternal rims and what appear to be vesicles in the trans region of the Golgi stack. After treatment with CHX, the cisternae are condensed and have reduced cisternal width, and lipoprotein particles are not evident within cisternae and vesicles surrounding the isolated stacks. Although there is no morphological correlate of transmembrane proteins in transit, the biochemical enrichment of HA4 and the pIgA-R shows these PM proteins enriched 16- and 110-fold, respectively, in the CTL SGF1, and both are effectively cleared from the CHX SGF1. A low level of contamination of the SGF1s determined by morphometric evaluation is borne out by the barely detectable levels of lysosomal and ER markers and their minimal enrichments in SGF1.
One noticeable difference in the Golgi marker enrichment data for both CTL and CHX fractions is that MG160, the medial marker, was found to enrich 150% more than p28 (cis) and TGN38 (trans). We attribute this difference to a greater stability and hence better recovery of the medial cisternae during the fractionation. The cis and trans cisternae are more susceptible to shear in the homogenization and fractionation procedures. However, both the cis and trans markers are recovered at similar levels. This level of enrichment and yields for p28 (204- and 400-fold enrichment and 51% and 46% yields for CTL and CHX SGF1s, respectively) and TGN38 (233- and 376-fold enrichment and 42% and 37% yields for CTL and CHX SGF1s, respectively) are certainly sufficient for studies of Golgi function.
One might wonder why we have spent our time and resources to develop a fractionation procedure using rat liver rather than using cultured cells. To address this concern, we have modified this fractionation protocol to obtain a stacked Golgi fraction from normal rat kidney (NRK) cells. The fractions obtained contained fewer stacked Golgi cisternae, greater contamination and the yield was extremely low, 50 μg of protein/3 × 107 cells (our unpublished results). Furthermore, studies of function would not be time and cost effective nor would it be practical to use such fractions to define endogenous proteins.
The clearing of proteins in transit by CHX treatment has revealed many properties of the organelle and allowed isolation of a stacked Golgi fraction enriched in endogenous proteins. Our characterization of calcium uptake indicates that these fractions are suitable for the study of endogenous Golgi proteins and functions. Two different calcium uptake activities are characterized in the CTL SGF1. The first activity, accounting for ∼50% of the total, is consistent with a PMCA isoform(s). It is thapsigargin resistant, vanadate sensitive, oligomycin resistant and is cleared from the Golgi fraction after CHX treatment of the animals. A signal for PMCA was detected in CTL SGF1 but was not detected in the CHX SGF1 by immunoblot analysis. These data provide convincing evidence that the thapsigargin-resistant activity is not a resident Golgi activity and is most likely due to the PMCA isoform(s) in transit. Thus molecules in transit can function en route and contribute significantly to total calcium transport within the Golgi complex.
The other calcium uptake activity in the SGF1s corresponds to a SERCA class of intracellular calcium pumps. This activity is thapsigargin and vanadate sensitive, oligomycin resistant, and could not be distinguished from the ER calcium pump activity, in all experiments performed. The thapsigargin dose–response curve and IC50 values were the same for SGF1 and ER fractions in the presence of low and high free calcium levels. Immunoblots of the fractions, with an antibody that recognizes all isoforms of SERCA, revealed the same 110-kDa band in both the ER and SGF1s with enrichment levels of one- to fivefold. Upon further examination using autophosphorylation experiments, the only autophosphorylated band in the SGF1s that was EGTA sensitive and calcium stimulated was of the same molecular weight as the SERCA pump of the ER (110 kDa). The autophosphorylation was also thapsigargin sensitive. Wu et al. (1995) suggest that SERCA2b is the only isoform expressed in liver and it is reasonable to expect this autophosphorylated band to correspond to a SERCA2b isoform.
These data argue that there is no biochemically unique resident calcium ATPase in the rat liver Golgi fractions. On the other hand, there is both SERCA protein and activity associated with the Golgi complex. Any Golgi-specific activity would be expected to enrich to the same level as the well characterized resident Golgi transmembrane proteins (200- to 400-fold). The much lower enrichment for SERCA results from it being distributed in two compartments, ER and Golgi. Because in rat liver hepatocytes, the ER is a large compartment containing approximately 50% of total cellular membranes, and the Golgi is a much smaller compartment, containing approximately 7% of total cellular membrane, a much lower level of enrichment for a protein equally distributed in both membranes is expected (Weibel et al., 1969).
Data to suggest that the SERCA activity is actually in the Golgi and not a result of ER contamination come from two different experimental approaches. First is the morphometic and biochemical characterization of the fractions presented here. The ER contamination was at the level of 1%. Because both fractions had approximately the same uptake activity (pmoles of calcium per minute per microgram of protein), this would translate into the contaminating ER in the SGF1 transporting calcium at 100 times the rate it transports it in the ER fraction. Second is the data that in the presence of oxalate, calcium uptake was enhanced 40% in CTL and 60% in CHX ER. However, SGF1 calcium uptake showed no oxalate enhancement. This means that the membranes in the SGF1s containing the calcium uptake activity do not transport oxalate into the lumen of the Golgi fractions but the ER fractions have oxalate transporting activities.
What about the mammalian homologue of the yeast PMR1? Because PMR1 is localized to the Golgi, perhaps the mammalian homologue is as well. Too little is known at the present to draw reasonable conclusions and appropriate reagents are not avaliable to test its presence in rat liver fractions. If the PMR1 homologue is present in the Golgi fractions, it must be biochemically indistinguishable from the SERCAs.
We conclude that the Golgi complex does not contain a unique resident calcium transporting ATPase and that all calcium uptake into SGF1 can be attributed to two calcium uptake mechanisms: first, via a thapsigargin-resistant p-type pump that is not resident to the Golgi complex and corresponds to PMCA isoform(s) in transit to the PM, and second, SERCA pumps that are not restricted to the ER membrane. These two calcium pumps, along with any free calcium and/or calcium bound to soluble luminal proteins moving from the ER to and through the Golgi, supply the calcium required for the many functions of the Golgi complex.
ACKNOWLEDGMENTS
We thank our many colleagues listed in Table 1 for their generous gift of antibodies. We are indebted to Kathryn Bowenkamp, D.V.M., for providing support in all aspects of the animal protocols. We are grateful to Dr. Jonathan Lytton for sharing his knowledge on the calcium ATPases. We especially thank Dr. Carlos Catalano (University of Colorado School of Pharmacy) for expert advice on the kinetic experiments and analysis. We thank John Caldwell, Jiaming Wang, and Jonathan Sherman for critical reading of this manuscript. This work was supported by National Institute of Health grant GM 42629 to K.E.H. and additional support from Cell Biology Cores of the Hepatobilary Center, grant P30-DK34914, and the Monoclonal Core of the Cancer Center, grant P30-CA46934.
Footnotes
Abbreviations used: CHX, cycloheximide; CTL, control; IP3, inositol 1,4,5-trisphosphate; PM, plasma membrane; PMCA, plasma membrane calcium ATPase; PNS, postnuclear supernatant; SERCA, sarcoplasmic/endoplasmic reticulum calcium ATPase; SGF1, stacked Golgi fraction; TGN, trans-Golgi network.
REFERENCES
- Antebi A, Fink GR. The yeast Ca(2+)-ATPase homologue, PMR1, is required for normal Golgi function and localizes in a novel Golgi-like distribution. Mol Biol Cell. 1992;3:633–654. doi: 10.1091/mbc.3.6.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumrucker CR, Keenan TW. Membranes of mammary gland. X. Adenosine triphosphate dependent calcium accumulation by Golgi apparatus rich fractions from bovine mammary gland. Exp Cell Res. 1975;90:253–260. doi: 10.1016/0014-4827(75)90314-6. [DOI] [PubMed] [Google Scholar]
- Beaufay H, Amar-Costesec A, Feytmans E, Thines-Sempoux D, Wibo M, Robbi M, Berthet J. Analytical study of microsomes and isolated subcellular membranes from rat liver. 3. Subfractionation of the microsomal fraction by isopycnic and differential centrifugation in density gradients. J Cell Biol. 1974;61:188–200. doi: 10.1083/jcb.61.1.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger EG, Mandel TH, Schilt U. Immunohistochemical localization of galactosyltransferase in human fibroblasts and HeLa cells. J Histochem Cytochem. 1981;29:364–371. doi: 10.1177/29.3.6787115. [DOI] [PubMed] [Google Scholar]
- Bergeron JJM. Golgi fractions from livers of control and ethanol-intoxicated rats. Enzymic and morphologic properties following rapid isolation. Biochim Biophys Acta. 1979;555:493–503. doi: 10.1016/0005-2736(79)90402-4. [DOI] [PubMed] [Google Scholar]
- Bretz R, Stäubli W. Detergent influence on rat-liver galactosyltransferase activities toward different receptors. Eur J Biochem. 1977;77:181–192. doi: 10.1111/j.1432-1033.1977.tb11656.x. [DOI] [PubMed] [Google Scholar]
- Carafoli E. Biogenesis: plasma membrane calcium ATPase: 15 years of work on the purified enzyme. FASEB J. 1994;8:993–1002. [PubMed] [Google Scholar]
- Caride AJ, Filoteo AG, Enyedi A, Verma AK, Penniston JT. Detection of isoform 4 of the plasma membrane calcium pump in human tissues by using isoform-specific monoclonal antibodies. Biochem J. 1996;316:353–359. doi: 10.1042/bj3160353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanat E, Huttner WB. Milieu-induced, selective aggregation of regulated secretory proteins in the trans-Golgi network. J Cell Biol. 1991;115:1505–1519. doi: 10.1083/jcb.115.6.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandra S, Kable EP, Morrison GH, Webb WW. Calcium sequestration in the Golgi apparatus of cultured mammalian cells revealed by laser scanning confocal microscopy and ion microscopy. J Cell Sci. 1991;100:747–752. doi: 10.1242/jcs.100.4.747. [DOI] [PubMed] [Google Scholar]
- Davidson HW, Rhodes CJ, Hutton JC. Intraorganellar calcium and pH control proinsulin cleavage in the pancreatic b cell via two distinct site-specific endopeptidases. Nature. 1988;333:93–96. doi: 10.1038/333093a0. [DOI] [PubMed] [Google Scholar]
- Duden R, Griffiths G, Frank R, Argos P, Kreis TE. Beta-COP, a 110 kd protein associated with non-clathrin-coated vesicles and the Golgi complex, shows homology to beta-adaptin. Cell. 1991;64:649–665. doi: 10.1016/0092-8674(91)90248-w. [DOI] [PubMed] [Google Scholar]
- Ehrenreich JH, Bergeron JJM, Siekevitz P, Palade GE. Golgi fractions prepared from rat liver homogenates. I. Isolation procedure and morphological characterization. J Cell Biol. 1973;59:45–72. doi: 10.1083/jcb.59.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiehn W, Hasselbach W. The effect of phospholipase A on the calcium transport and the role of unsaturated fatty acids in ATPase activity of sarcoplasmic vesicles. Eur J Biochem. 1970;13:510–518. doi: 10.1111/j.1432-1033.1970.tb00954.x. [DOI] [PubMed] [Google Scholar]
- Fleischer B, Fleischer S, Ozawa H. Isolation and characterization of Golgi membranes from bovine liver. J Cell Biol. 1969;43:59–79. doi: 10.1083/jcb.43.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill DL, Ghosh TK, Mullaney JM. Calcium signaling mechanisms in endoplasmic reticulum activated by inositol 1,4,5-trisphosphate and GTP. Cell Calcium. 1989;10:363–374. doi: 10.1016/0143-4160(89)90062-6. [DOI] [PubMed] [Google Scholar]
- Gonatas JO, Mezitis SG, Stieber A, Fleischer B, Gonatas NK. MG-160. A novel sialoglycoprotein of the medial cisternae of the Golgi apparatus. J Biol Chem. 1989;264:646–653. [PubMed] [Google Scholar]
- Grover AK, Khan I. Calcium pump isoforms: diversity, selectivity and plasticity. Cell Calcium. 1992;13:9–17. doi: 10.1016/0143-4160(92)90025-n. [DOI] [PubMed] [Google Scholar]
- Gunteski-Hamblin AM, Clarke DM, Shull GE. Molecular cloning and tissue distribution of alternatively spliced mRNAs encoding possible mammalian homologues of the yeast secretory pathway calcium pump. Biochemistry. 1992;31:7600–7608. doi: 10.1021/bi00148a023. [DOI] [PubMed] [Google Scholar]
- Halachmi D, Eilam Y. Elevated cytosolic free Ca2+ concentrations and massive Ca2+ accumulation within vacuoles, in yeast mutant lacking PMR1, a homolog of Ca2+-ATPase. FEBS Lett. 1996;392:194–200. doi: 10.1016/0014-5793(96)00799-5. [DOI] [PubMed] [Google Scholar]
- Hartley AD, Bogaerts S, Garrett S. cAMP inhibits bud growth in a yeast strain compromised for Ca2+ influx into the Golgi. Mol Gen Genet. 1996;251:556–564. doi: 10.1007/BF02173645. [DOI] [PubMed] [Google Scholar]
- Hino Y, Asano A, Sato R, Shimizu S. Biochemical studies of rat liver Golgi apparatus. I. Isolation and preliminary characterization. J Biochem. 1978;83:909–923. doi: 10.1093/oxfordjournals.jbchem.a132018. [DOI] [PubMed] [Google Scholar]
- Hodson S. The ATP-dependent concentration of calcium by a Golgi apparatus-rich fraction isolated from rat liver. J Cell Sci. 1978;30:117–128. doi: 10.1242/jcs.30.1.117. [DOI] [PubMed] [Google Scholar]
- Hokin LE, Dixon JF, Reichman M, Sekar MC. Biochemical aspects of the phosphoinositide signaling system with special reference to the formation of inositol cyclic phosphates and arachidonic acid and metabolites on agonist stimulation. Adv Enzyme Regul. 1987;26:117–132. doi: 10.1016/0065-2571(87)90009-4. [DOI] [PubMed] [Google Scholar]
- Horn M, Banting G. Okadaic acid treatment leads to a fragmentation of the trans-Golgi network and an increase in expression of TGN38 at the cell surface. Biochem J. 1994;301:68–73. doi: 10.1042/bj3010069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hortsch M, Avossa D, Meyer DI. Characterization of secretory protein translocation: ribosome-membrane interaction in endoplasmic reticulum. J Cell Biol. 1986;103:241–253. doi: 10.1083/jcb.103.1.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell KE, Devaney E, Gruenberg J. Subcellular fractionation of tissue culture cells. Trends Biochem Sci. 1989;2:44–47. doi: 10.1016/0968-0004(89)90040-6. [DOI] [PubMed] [Google Scholar]
- Howell KE, Palade GE. Hepatic Golgi fractions resolved into membrane and content subfractions. J Cell Biol. 1982;92:822–832. doi: 10.1083/jcb.92.3.822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones SM, Crosby JR, Salamero J, Howell KE. A cytoplasmic complex of p62 and rab6 associates with TGN38/41 and is involved in budding of exocytic vesicles from the trans-Golgi network. J Cell Biol. 1993;122:775–788. doi: 10.1083/jcb.122.4.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles AF, Racker E. Properties of a reconstituted calcium pump. J Biol Chem. 1975;250:3538–3544. [PubMed] [Google Scholar]
- Koch GL. The endoplasmic reticulum and calcium storage. Bioessays. 1990;12:527–531. doi: 10.1002/bies.950121105. [DOI] [PubMed] [Google Scholar]
- Lanini L, Bachs O, Carafoli E. The calcium pump of the Liver Nuclear membrane is Identical to that of endoplasmic reticulum. J Biol Chem. 1992;267:11548–11552. [PubMed] [Google Scholar]
- Lapinskas PJ, Cunningham KW, Liu XF, Fink GR, Culotta VC. Mutations in PMR1 suppress oxidative damage in yeast cells lacking superoxide dismutase. Mol Cell Biol. 1995;15:1382–1388. doi: 10.1128/mcb.15.3.1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leelavathi DE, Estes LW, Feingold DS, Lombardi B. Isolation of a Golgi-rich fraction from rat liver. Biochim Biophys Acta. 1970;211:124–138. [Google Scholar]
- Lodish HF, Kong N, Snider M, Strous GJ. Hepatoma secretory proteins migrate from rough endoplasmic reticulum to Golgi at characteristic rates. Nature. 1983;304:80–83. doi: 10.1038/304080a0. [DOI] [PubMed] [Google Scholar]
- Lucocq J. Unbiased 3-D quantitation of ultrastructure in cell biology. Trends Cell Biol. 1993;3:354–358. doi: 10.1016/0962-8924(93)90106-b. [DOI] [PubMed] [Google Scholar]
- Lucocq J, Berger EG, Warren G. Mitotic Golgi fragments in HeLa cells and their role in the reassembly pathway. J Cell Biol. 1989;109:463–474. doi: 10.1083/jcb.109.2.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucocq J, Warren G. Fragmentation and partitioning of the Golgi during mitosis in HeLa cells. EMBO J. 1987;6:3239–3246. doi: 10.1002/j.1460-2075.1987.tb02641.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luzio PJ, Brake B, Banting G, Howell KE, Braghetta P, Stanley KK. Identification, sequencing and expression of an integral membrane protein of the trans-Golgi network (TGN38) Biochem J. 1990;270:97–102. doi: 10.1042/bj2700097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lytton J, MacLennan DH. Molecular cloning of cDNAs from human kidney coding for two alternatively spliced products of the cardiac Ca2+-ATPase gene. J Biol Chem. 1988;263:15024–15031. [PubMed] [Google Scholar]
- Lytton J, Westlin M, Burk SE, Shull GE, MacLennan DH. Functional comparisons between isoforms of the sarcoplasmic or endoplasmic reticulum family of calcium pumps. J Biol Chem. 1992;267:14483–14489. [PubMed] [Google Scholar]
- Lytton J, Westlin M, Hanley MR. Thapsigargin inhibits the sarcoplasmic or endoplasmic reticulum Ca-ATPase family of calcium pumps. J Biol Chem. 1991;266:17067–17071. [PubMed] [Google Scholar]
- Maizel JV. Polyacrylamide electrophoresis of viral proteins. Methods Virol. 1971;5:179–246. [Google Scholar]
- Margolis R, Taylor SI, Seminara D, Hubbard AL. Identification of Pp 120, an endogenous substrate for the hepatocyte insulin receptor tyrosine kinase, as an integral membrane glycoprotein of the bile canalicular domain. Proc Natl Acad Sci USA. 1988;85:7256–7259. doi: 10.1073/pnas.85.19.7256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore L, Chen T, Knapp HR, Jr, Landon EJ. Energy-dependent calcium sequestration activity in rat liver microsomes. J Biol Chem. 1975;250:4562–4568. [PubMed] [Google Scholar]
- Morré DJ, Mollenhauer HM. Isolation of the Golgi apparatus from plant cells. J Cell Biol. 1964;23:295–305. doi: 10.1083/jcb.23.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narula N, McMorrow I, Plopper G, Doherty J, Matlin KS, Burke B, Stow JL. Identification of a 200-kD, brefeldin-sensitive protein on Golgi membranes. J Cell Biol. 1992;117:27–38. doi: 10.1083/jcb.117.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathke I, Heuser J, Lupas A, Stock J, Turck CW, Brodsky FM. Folding and trimerization of clathrin subunits at the triskelion hub. Cell. 1992;68:899–910. doi: 10.1016/0092-8674(92)90033-9. [DOI] [PubMed] [Google Scholar]
- Neville MC, Selker F, Semple K, Watters C. ATP-dependent calcium transport by a Golgi-enriched membrane fraction from mouse mammary gland. J Membr Biol. 1981;61:97–105. doi: 10.1007/BF02007636. [DOI] [PubMed] [Google Scholar]
- Novikoff PM, Novikoff AB, Quintana H, Hauw J-J. Golgi apparatus, GERL and lysosomes of neurons in rat dorsal root ganglia, studied by thick section and thin section cytochemistry. J Cell Biol. 1971;50:859–886. doi: 10.1083/jcb.50.3.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen PL, Carafoli E. Ion motive ATPases. I. Ubiquity, properties, and significance to cell function. Trends Biochem Sci. 1987;12:146–189. [Google Scholar]
- Reynolds ES. The use of lead citrate at high pH as an electron opaque stain in electron microscopy. J Cell Biol. 1963;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salamero J, Sztul ES, Howell KE. Exocytic transport vesicles generated in vitro from the trans-Golgi network carry secretory and plasma membrane proteins. Proc Natl Acad Sci USA. 1990;87:7717–7721. doi: 10.1073/pnas.87.19.7717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarino ML, Howell KE. The Fao cell. A tissue culture model for lipoprotein synthesis and secretion. I. Characterization of the system. Exp Cell Res. 1987;170:1–14. doi: 10.1016/0014-4827(87)90112-1. [DOI] [PubMed] [Google Scholar]
- Schmidt WK, Moore HP. Ionic milieu controls the compartment-specific activation of pro-opiomelanocortin processing in AtT-20 cells. Mol Biol Cell. 1995;6:1271–1285. doi: 10.1091/mbc.6.10.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slusarewicz P, Hui N, Warren G. Purification of rat liver Golgi stacks. In: Celis J, editor. Cell Biology: A Laboratory Handbook. London: Academic Press; 1994. [Google Scholar]
- Spat A, Bradford PG, McKinney JS, Rubin RP, Putney JW., Jr A saturable receptor for 32P-inositol-1,4,5-triphosphate in hepatocytes and neutrophils. Nature. 1986;319:514–516. doi: 10.1038/319514a0. [DOI] [PubMed] [Google Scholar]
- Subramaniam VN, Krijnse-Locker J, Tang BL, Ericsson M, Yusoff AR, Griffiths G, Hong W. Monoclonal antibody HFD9 identifies a novel 28 kDa integral membrane protein on the cis-Golgi. J Cell Sci. 1995;108:2405–2414. doi: 10.1242/jcs.108.6.2405. [DOI] [PubMed] [Google Scholar]
- Sztul ES, Howell KE, Palade GE. Intracellular and transcellular transport of secretory component and albumin in rat hepatocytes J. Cell Biol. 1983;97:1582–1591. doi: 10.1083/jcb.97.5.1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sztul ES, Howell KE, Palade GE. Biogenesis of the polymeric IgA-R in rat hepatocytes. I. Kinetic studies of its intracellular forms. J Cell Biol. 1985;100:1248–1254. doi: 10.1083/jcb.100.4.1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JA, Limbrick AR, Allan D, Judah JD. Isolation of highly purified Golgi membranes from rat liver use of cycloheximide in vivo to remove Golgi contents. Biochim Biophys Acta. 1984;769:171–178. doi: 10.1016/0005-2736(84)90020-8. [DOI] [PubMed] [Google Scholar]
- Taylor, R.S., Fialka, I., Jones, S.M., Huber, L.A., and Howell, K.E. (1997). 2D mapping of the endogenous proteins of the rat hepatocyte Golgi complex cleared of proteins in transit. Electrophoresis (in press). [DOI] [PubMed]
- Thastrup O, Cullen PJ, Drobak BK, Hanley MR, Dawson AP. Thapsigargin, a tumor promoter, discharges intracellular Ca2+ stores by specific inhibition of the endoplasmic reticulum Ca(2+)-ATPase. Proc Natl Acad Sci USA. 1990;87:2466–2470. doi: 10.1073/pnas.87.7.2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tooze J, Kern HF, Fuller SD, Howell KE. Condensation-sorting events in the rough endoplasmic reticulum of exocrine pancreatic cells. J Cell Biol. 1989;109:35–50. doi: 10.1083/jcb.109.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vegh K, Spiegler P, Chamberlain C, Mommaerts WF. The molecular size of the calcium-transport ATPase of sarcotubular vesicles estimated from radiation inactivation. Biochim Biophys Acta. 1968;163:266–268. doi: 10.1016/0005-2736(68)90106-5. [DOI] [PubMed] [Google Scholar]
- Verostek MF, Trimble RB. Mannosyltransferase activities in membranes from various yeast strains. Glycobiology. 1995;5:671–681. doi: 10.1093/glycob/5.7.671. [DOI] [PubMed] [Google Scholar]
- Virk SS, Kirk CJ, Shears SB. Ca2+ transport and Ca2+-dependent ATP hydrolysis by Golgi vesicles from lactating rat mammary glands. Biochem J. 1985;226:741–748. doi: 10.1042/bj2260741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K, Osborn M. The reliability of molecular weight determinations by dodecyl sulfate polyacrylamide gel electrophoresis. J Biol Chem. 1969;244:4406–4412. [PubMed] [Google Scholar]
- Weibel ER. International Review of Cytology. Vol. 26. G.H. Bourne and J.F. Danielli, New York: Academic Press; 1969. Stereological principles for morphometry in electron microscopic cytology; pp. 235–299. [DOI] [PubMed] [Google Scholar]
- Weibel ER, Staubli W, Gnagi HR, Hess FA. Correlated morphometric and biochemical studies on the liver cell. J Cell Biol. 1969;42:68–91. doi: 10.1083/jcb.42.1.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West DW. Energy-dependent calcium sequestration activity in a Golgi apparatus fraction derived from lactating rat mammary glands. Biochim Biophys Acta. 1981;673:374–386. doi: 10.1016/0304-4165(81)90469-4. [DOI] [PubMed] [Google Scholar]
- Wrighton S, Maurel P, Shuetz E, Watkins P, Young B, Guzelian PS. Identification of the cytochrome P-450 induced by macrolide antibiotics in rat liver as the glucocorticoid responsive cytochrome P-450p. Biochemistry. 1985;24:2171–2178. doi: 10.1021/bi00330a010. [DOI] [PubMed] [Google Scholar]
- Wu KD, Lee WS, Wey J, Bungard D, Lytton J. Localization and quantification of endoplasmic reticulum Ca(2+)-ATPase isoform transcripts. Am J Phys. 1995;269:C775–C784. doi: 10.1152/ajpcell.1995.269.3.C775. [DOI] [PubMed] [Google Scholar]