Abstract
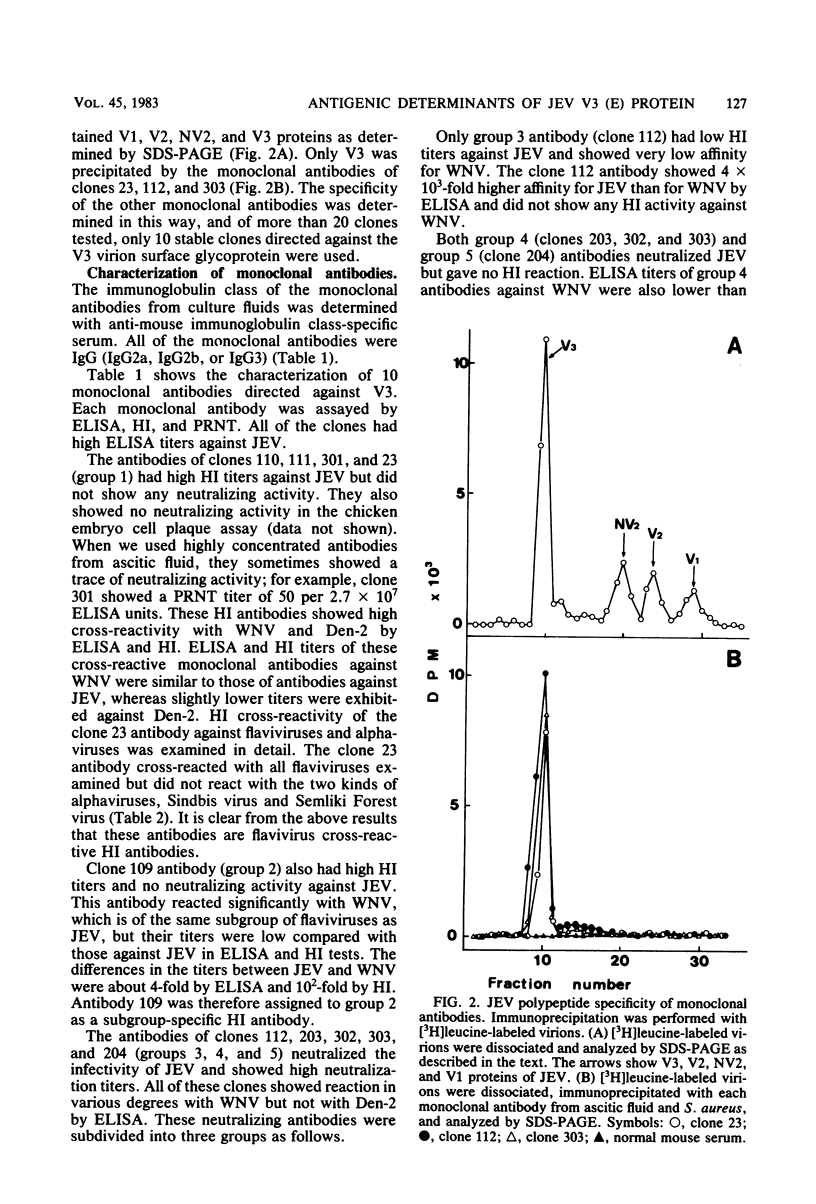
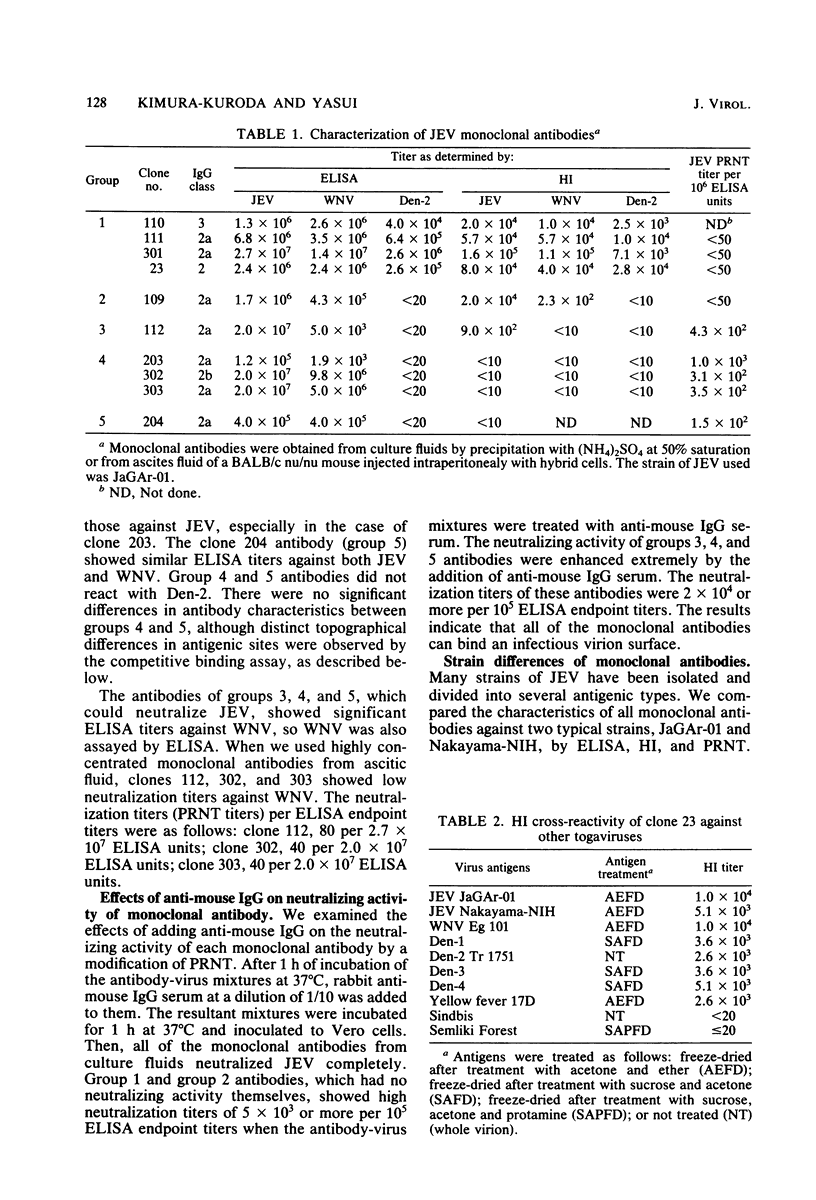
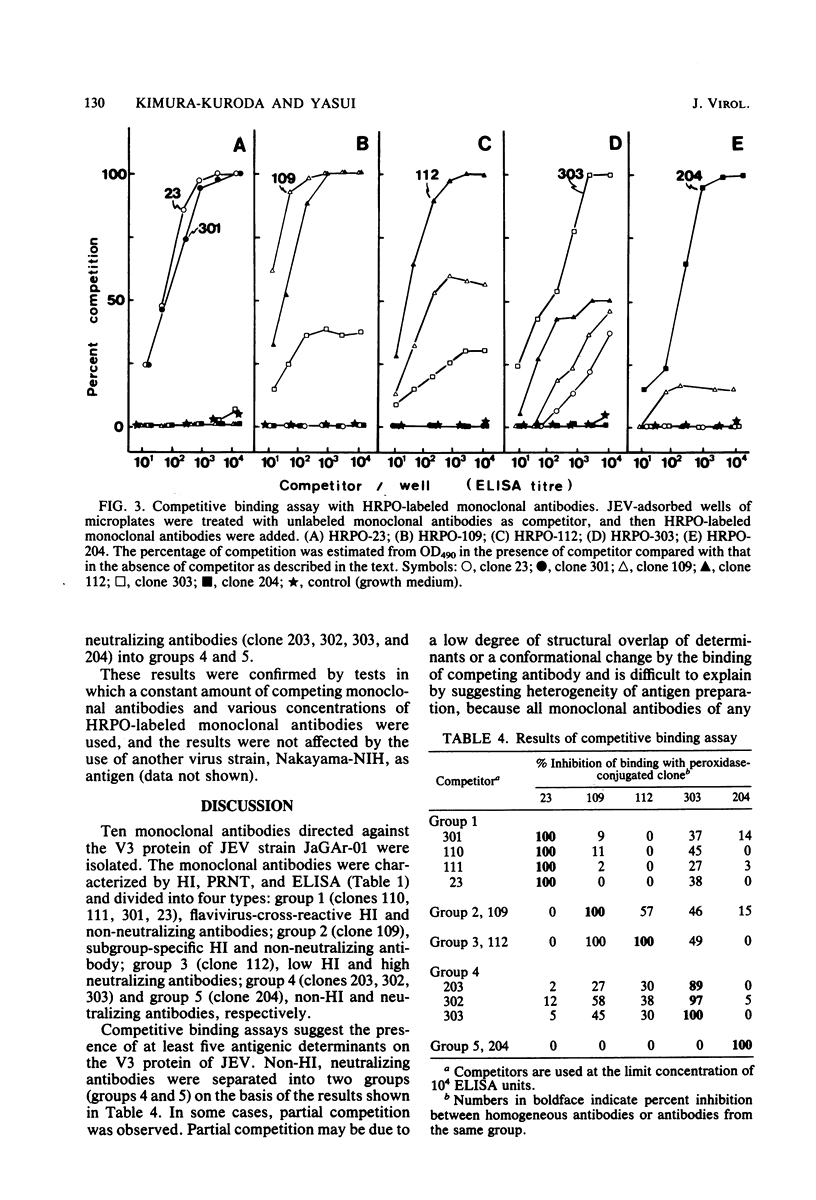
Ten monoclonal antibodies directed against envelope glycoprotein V3 (E) of Japanese encephalitis virus were obtained. They were characterized by hemagglutination inhibition (HI), neutralization, and enzyme-linked immunosorbent assay and divided into four types: flavivirus-cross-reactive HI and non-neutralizing antibody (group 1), subgroup-specific HI and non-neutralizing antibody (group 2), low HI and neutralizing antibody (group 3), and non-HI and neutralizing antibody (groups 4 and 5, respectively). Competitive binding assays were performed to analyze the topography of antigenic determinants by enzyme-linked immunosorbent assay. The results of the competitive binding assay separated non-HI and neutralizing antibody into groups 4 and 5, respectively, and demonstrated the existence of at least five distinct antigenic determinants on V3. The site of group 1 was distinct from any other site. The sites of groups 2 and 3 seemed to be located close together. Our results suggest the following relationship between HI and neutralization: (i) The HI sites are separated from the neutralization sites, and (ii) there are two distinct HI sites, one of which is flavivirus cross-reactive, the other subgroup specific.
Full text
PDF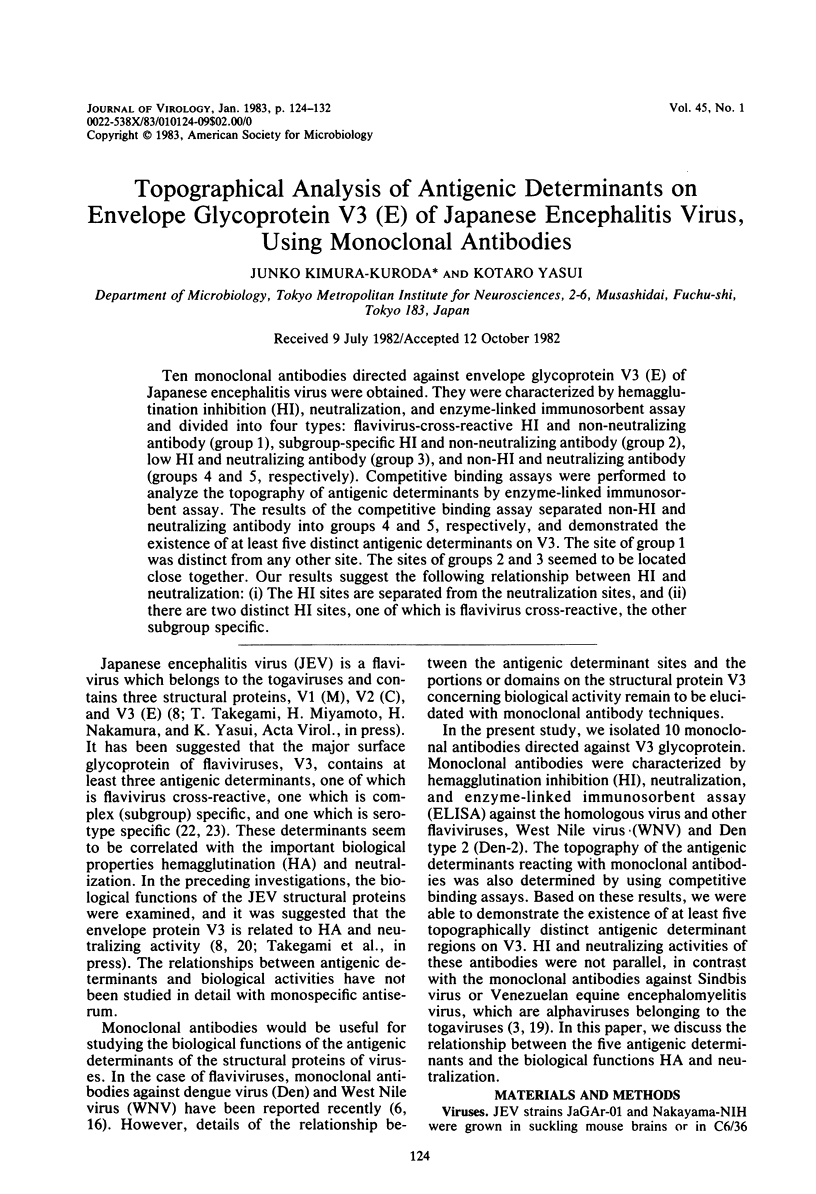




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Breschkin A. M., Ahern J., White D. O. Antigenic determinants of influenza virus hemagglutinin. VIII. Topography of the antigenic regions of influenza virus hemagglutinin determined by competitive radioimmunoassay with monoclonal antibodies. Virology. 1981 Aug;113(1):130–140. doi: 10.1016/0042-6822(81)90142-2. [DOI] [PubMed] [Google Scholar]
- CLARKE D. H., CASALS J. Techniques for hemagglutination and hemagglutination-inhibition with arthropod-borne viruses. Am J Trop Med Hyg. 1958 Sep;7(5):561–573. doi: 10.4269/ajtmh.1958.7.561. [DOI] [PubMed] [Google Scholar]
- Chanas A. C., Gould E. A., Clegg J. C., Varma M. G. Monoclonal antibodies to Sindbis virus glycoprotein E1 can neutralize, enhance infectivity, and independently inhibit haemagglutination or haemolysis. J Gen Virol. 1982 Jan;58(Pt 1):37–46. doi: 10.1099/0022-1317-58-1-37. [DOI] [PubMed] [Google Scholar]
- De Madrid A. T., Porterfield J. S. The flaviviruses (group B arboviruses): a cross-neutralization study. J Gen Virol. 1974 Apr;23(1):91–96. doi: 10.1099/0022-1317-23-1-91. [DOI] [PubMed] [Google Scholar]
- Dittmar D., Haines H. G., Castro A. Monoclonal antibodies specific for dengue virus type 3. J Clin Microbiol. 1980 Jul;12(1):74–78. doi: 10.1128/jcm.12.1.74-78.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendal A. P., Phillips D. J., Webster R. G., Galland G. G., Reimer C. B. Effect of test system on the ability of monoclonal antibodies to detect antigenic drift in influenza A(H1N1) virus haemagglutinins. J Gen Virol. 1981 Jun;54(Pt 2):253–261. doi: 10.1099/0022-1317-54-2-253. [DOI] [PubMed] [Google Scholar]
- Kitano T., Suzuki K., Yamaguchi T. Morphological, chemical, and biological characterization of Japanese encephalitis virus virion and its hemagglutinin. J Virol. 1974 Sep;14(3):631–639. doi: 10.1128/jvi.14.3.631-639.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köhler G., Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975 Aug 7;256(5517):495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lubeck M. D., Gerhard W. Topological mapping antigenic sites on the influenza A/PR/8/34 virus hemagglutinin using monoclonal antibodies. Virology. 1981 Aug;113(1):64–72. doi: 10.1016/0042-6822(81)90136-7. [DOI] [PubMed] [Google Scholar]
- Lubeck M., Gerhard W. Conformational changes at topologically distinct antigenic sites on the influenza A/PR/8/34 virus HA molecule are induced by the binding of monoclonal antibodies. Virology. 1982 Apr 15;118(1):1–7. doi: 10.1016/0042-6822(82)90313-0. [DOI] [PubMed] [Google Scholar]
- Massey R. J., Schochetman G. Topographical analysis of viral epitopes using monoclonal antibodies: mechanism of virus neutralization. Virology. 1981 Nov;115(1):20–32. doi: 10.1016/0042-6822(81)90085-4. [DOI] [PubMed] [Google Scholar]
- Nakane P. K., Kawaoi A. Peroxidase-labeled antibody. A new method of conjugation. J Histochem Cytochem. 1974 Dec;22(12):1084–1091. doi: 10.1177/22.12.1084. [DOI] [PubMed] [Google Scholar]
- Peiris J. S., Porterfield J. S., Roehrig J. T. Monoclonal antibodies against the flavivirus West Nile. J Gen Virol. 1982 Feb;58(Pt 2):283–289. doi: 10.1099/0022-1317-58-2-283. [DOI] [PubMed] [Google Scholar]
- Pinter A., Honnen W. J., Tung J. S., O'Donnell P. V., Hämmerling U. Structural domains of endogenous murine leukemia virus gp70s containing specific antigenic determinants defined by monoclonal antibodies. Virology. 1982 Jan 30;116(2):499–516. doi: 10.1016/0042-6822(82)90143-x. [DOI] [PubMed] [Google Scholar]
- Roehrig J. T., Day J. W., Kinney R. M. Antigenic analysis of the surface glycoproteins of a Venezuelan equine encephalomyelitis virus (TC-83) using monoclonal antibodies. Virology. 1982 Apr 30;118(2):269–278. doi: 10.1016/0042-6822(82)90346-4. [DOI] [PubMed] [Google Scholar]
- Shapiro D., Brandt W. E., Cardiff R. D., Russell P. K. The proteins of Japanese encephalitis virus. Virology. 1971 Apr;44(1):108–124. doi: 10.1016/0042-6822(71)90158-9. [DOI] [PubMed] [Google Scholar]
- Stone M. R., Nowinski R. C. Topological mapping of murine leukemia virus proteins by competition-binding assays with monoclonal antibodies. Virology. 1980 Jan 30;100(2):370–381. doi: 10.1016/0042-6822(80)90528-0. [DOI] [PubMed] [Google Scholar]
- Trent D. W. Antigenic characterization of flavivirus structural proteins separated by isoelectric focusing. J Virol. 1977 Jun;22(3):608–618. doi: 10.1128/jvi.22.3.608-618.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trent D. W., Harvey C. L., Qureshi A., LeStourgeon D. Solid-phase radioimmunoassay for antibodies to flavivirus structural and nonstructural proteins. Infect Immun. 1976 May;13(5):1325–1333. doi: 10.1128/iai.13.5.1325-1333.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasui K., Nozima T., Homma R., Ueda S. Extraction of active fragment from Japanese encephalitis viral receptor of susceptible cells. Acta Virol. 1971 Jan;15(1):7–18. [PubMed] [Google Scholar]



