Abstract
Pseudotypes of vesicular stomatitis virus (VSV) containing envelope glycoproteins provided by C3H mammary tumor virus (MTV) instead of the normal VSV G-proteins were prepared and used to assay the presence of an MTV receptor on cells. The assay was specific as demonstrated by competition studies with excess MTV particles and neutralization of the pseudotypes with anti-MTV serum or monoclonal antibodies directed against MTV gp52. The MTV receptor was abundantly present on mouse cells but hardly detectable on nonmurine cells, including the Chinese hamster cell line E36. Somatic cell hybrids between E36 cells and GRS/A spontaneous leukemia cells (GRSL cells) and between E36 and GRS/A primary mammary tumor cells were made. The hybrids retained all Chinese hamster chromosomes but segregated mouse chromosomes. From the analysis of the isoenzymes and chromosomes of the hybrid cell lines we conclude that the gene for the receptor (MTVR-1) is located on mouse chromosome 16.
Full text
PDF

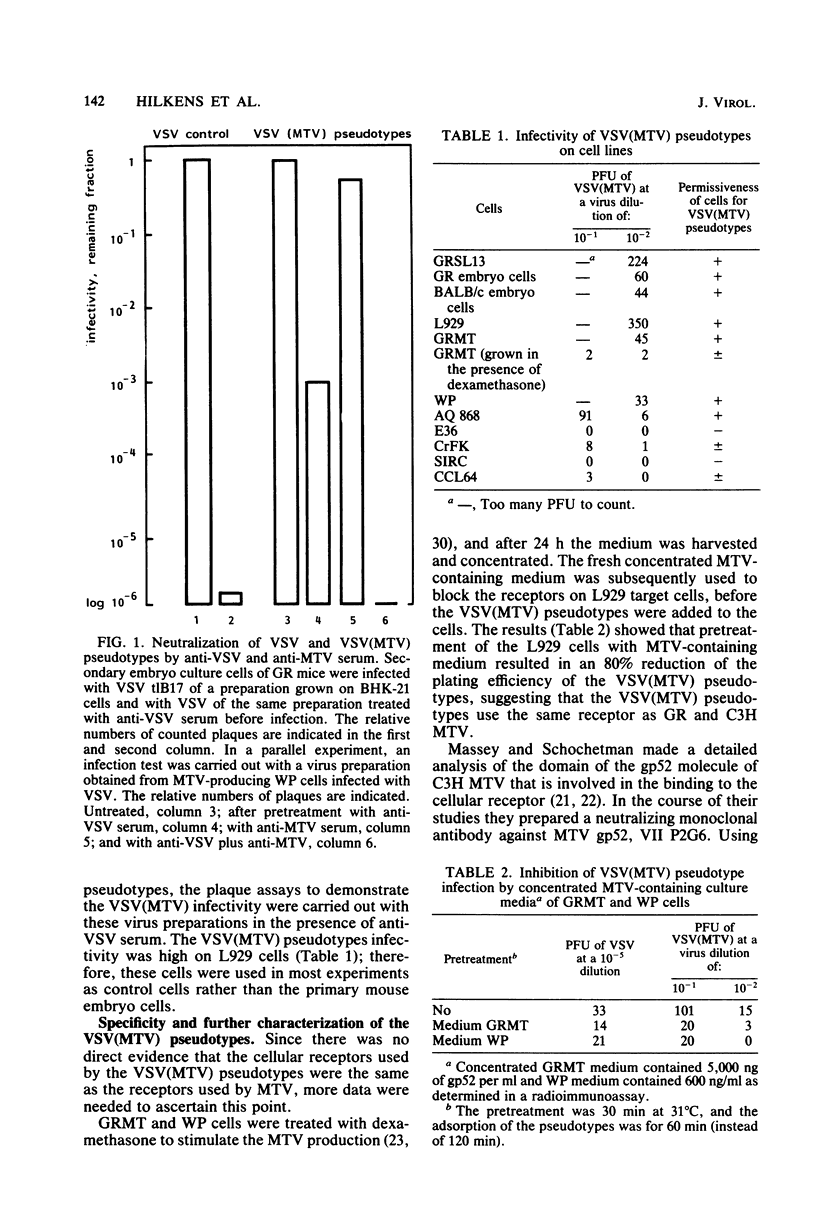
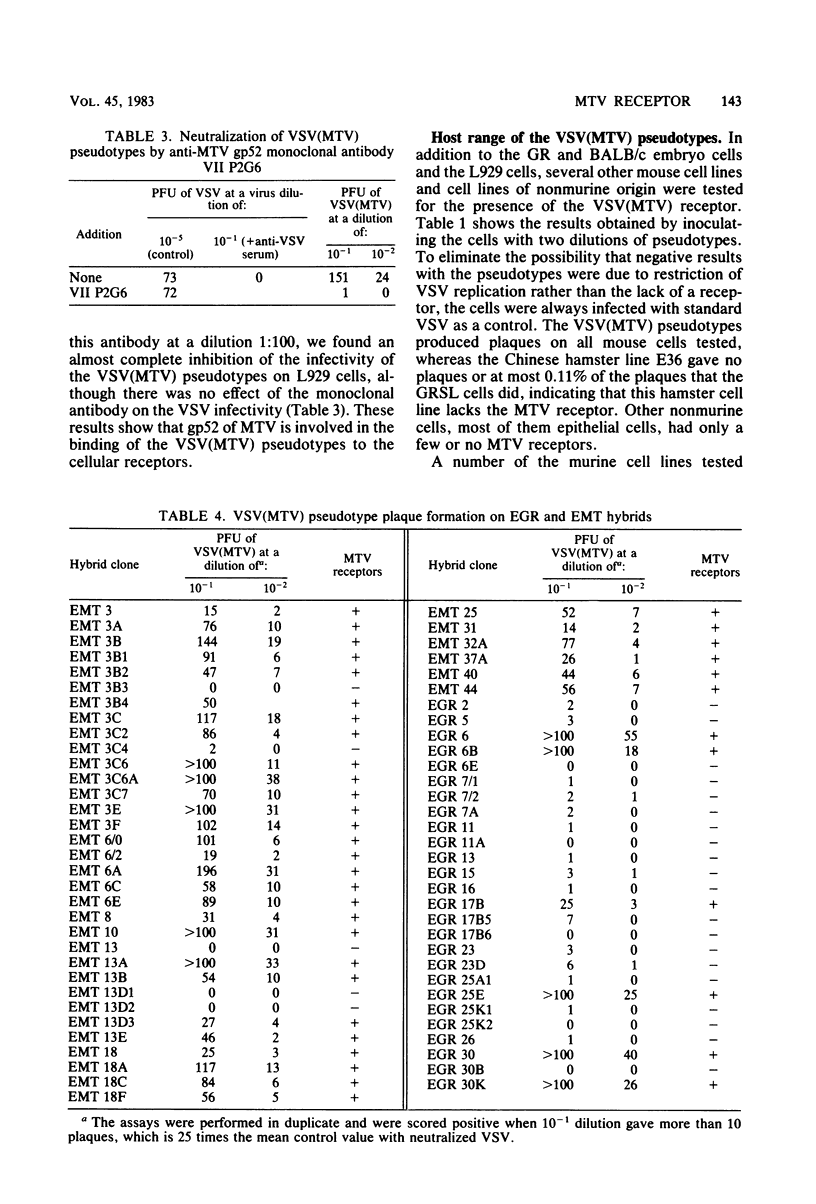
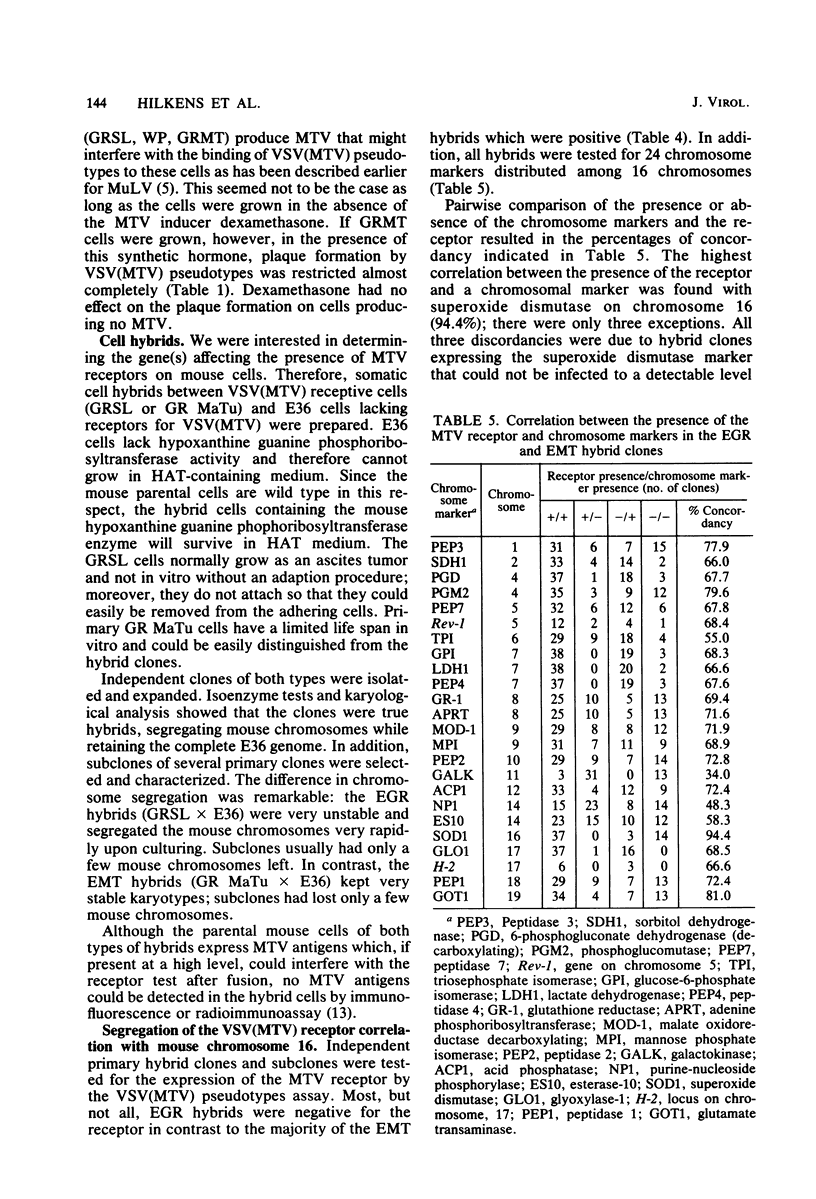



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altrock B. W., Arthur L. O., Massey R. J., Schochetman G. Common surface receptors on both mouse and rat cells distinguish different classes of mouse mammary tumor viruses. Virology. 1981 Mar;109(2):257–266. doi: 10.1016/0042-6822(81)90497-9. [DOI] [PubMed] [Google Scholar]
- Besmer P., Baltimore D. Mechanism of restriction of ecotropic and xenotropic murine leukemia viruses and formation of pseudotypes between the two viruses. J Virol. 1977 Mar;21(3):965–973. doi: 10.1128/jvi.21.3.965-973.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan J. C., East J. L., Bowen J. M., Massey R., Schochetman G. Monoclonal and polyclonal antibody studies of VSV(hrMMTV) pseudotypes. Virology. 1982 Jul 15;120(1):54–64. doi: 10.1016/0042-6822(82)90006-x. [DOI] [PubMed] [Google Scholar]
- Crandell R. A., Fabricant C. G., Nelson-Rees W. A. Development, characterization, and viral susceptibility of a feline (Felis catus) renal cell line (CRFK). In Vitro. 1973 Nov-Dec;9(3):176–185. doi: 10.1007/BF02618435. [DOI] [PubMed] [Google Scholar]
- DeLarco J., Todaro G. J. Membrane receptors for murine leukemia viruses: characterization using the purified viral envelope glycoprotein, gp71. Cell. 1976 Jul;8(3):365–371. doi: 10.1016/0092-8674(76)90148-3. [DOI] [PubMed] [Google Scholar]
- Gazdar A. F., Oie H., Lalley P., Moss W. W., Minna J. D. Identification of mouse chromosomes required for murine leukemia virus replication. Cell. 1977 Aug;11(4):949–956. doi: 10.1016/0092-8674(77)90306-3. [DOI] [PubMed] [Google Scholar]
- Gilbert S. F., Migeon B. R. D-valine as a selective agent for normal human and rodent epithelial cells in culture. Cell. 1975 May;5(1):11–17. doi: 10.1016/0092-8674(75)90086-0. [DOI] [PubMed] [Google Scholar]
- Gillin F. D., Roufa D. J., Beaudet A. L., Caskey C. T. 8-Azaguanine resistance in mammalian cells. I. Hypoxanthine-guanine phosphoribosyltransferase. Genetics. 1972 Oct;72(2):239–252. doi: 10.1093/genetics/72.2.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson I. C., Lieber M. M., Todaro G. J. Mink cell line Mv 1 Lu (CCL 64). Focus formation and the generation of "nonproducer" transformed cell lines with murine and feline sarcoma viruses. Virology. 1974 Jul;60(1):282–287. doi: 10.1016/0042-6822(74)90386-9. [DOI] [PubMed] [Google Scholar]
- Hilgers J., Haverman J., Nusse R., van Blitterswijk W. J., Cleton F. J., Hageman P. C., van Nie P., Calafat J. Immunologic, virologic, and genetic aspects of mammary tumor virus-induced cell-surface antigens: presence of these antigens and the Thy 1.2 antigen on murine mammary gland and tumor cells. J Natl Cancer Inst. 1975 Jun;54(6):1323–1333. doi: 10.1093/jnci/54.6.1323. [DOI] [PubMed] [Google Scholar]
- Hilgers J., Nowinski R. C., Geering G., Hardy W. Detection of avian and mammalian oncogenic RNA viruses (oncornaviruses) by immunofluorescence. Cancer Res. 1972 Jan;32(1):98–106. [PubMed] [Google Scholar]
- Hilkens J., Colombatti A., Strand M., Nichols E., Ruddle F. H., Hilgers J. Identification of a mouse gene required for binding of Rauscher MuLV envelope gp70. Somatic Cell Genet. 1979 Jan;5(1):39–49. doi: 10.1007/BF01538785. [DOI] [PubMed] [Google Scholar]
- Howard D. K., Colcher D., Teramoto Y. A., Young J. M., Schlom J. Characterization of mouse mammary tumor viruses propagated in heterologous cells. Cancer Res. 1977 Aug;37(8 Pt 1):2696–2704. [PubMed] [Google Scholar]
- Howard D. K., Schlom J. Isolation of host-range variants of mouse mammary tumor viruses that efficiently infect cells in vitro. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5718–5722. doi: 10.1073/pnas.75.11.5718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak C. A., Lawrence J. B., Ruddle F. H. A sequential staining technique for the chromosomal analysis of the interspecific mouse/hamster and mouse/human somatic cell hybrids. Exp Cell Res. 1977 Mar 1;105(1):109–117. doi: 10.1016/0014-4827(77)90156-2. [DOI] [PubMed] [Google Scholar]
- LEERHOY J. CYTOPATHIC EFFECT OF RUBELLA VIRUS IN A RABBIT-CORNEA CELL LINE. Science. 1965 Aug 6;149(3684):633–634. doi: 10.1126/science.149.3684.633. [DOI] [PubMed] [Google Scholar]
- Lasfargues E. Y., Vaidya A. B., Lasfargues J. C., Moore D. H. In vitro susceptibility of mink lung cells to the mouse mammary tumor virus. J Natl Cancer Inst. 1976 Aug;57(2):447–449. doi: 10.1093/jnci/57.2.447. [DOI] [PubMed] [Google Scholar]
- Massey R. J., Schochetman G. Topographical analysis of viral epitopes using monoclonal antibodies: mechanism of virus neutralization. Virology. 1981 Nov;115(1):20–32. doi: 10.1016/0042-6822(81)90085-4. [DOI] [PubMed] [Google Scholar]
- Massey R. J., Schochetman G. Viral epitopes and monoclonal antibodies: isolation of blocking antibodies that inhibit virus neutralization. Science. 1981 Jul 24;213(4506):447–449. doi: 10.1126/science.6264601. [DOI] [PubMed] [Google Scholar]
- Massey R., Arthur L. O., Long C. W., Schochetman G. C3H/HeN mammary tumor-bearing mice develop type-specific neutralizing antibodies and group-specific precipitating antibodies for the mouse mammary tumor virus. J Virol. 1980 Jan;33(1):123–128. doi: 10.1128/jvi.33.1.123-128.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath C. M. Replication of mammary tumor virus in tumor cell cultures: dependence on hormone-induced cellular organization. J Natl Cancer Inst. 1971 Aug;47(2):455–467. [PubMed] [Google Scholar]
- Moore D. H., Merryman C. F., Maurer P. H., Holben J. A. Comparative susceptibilities of hybrid mice to four mammary tumor viruses from two mouse strains. Cancer Res. 1978 Nov;38(11 Pt 1):3871–3878. [PubMed] [Google Scholar]
- Nichols E. A., Elsevier S. M., Ruddle F. H. A new electrophoretic technique for mouse, human, and Chinese hamster galactokinase. Cytogenet Cell Genet. 1974;13(3):275–278. doi: 10.1159/000130279. [DOI] [PubMed] [Google Scholar]
- Nichols E. A., Ruddle F. H. A review of enzyme polymorphism, linkage and electrophoretic conditions for mouse and somatic cell hybrids in starch gels. J Histochem Cytochem. 1973 Dec;21(12):1066–1081. doi: 10.1177/21.12.1066. [DOI] [PubMed] [Google Scholar]
- Nichols E. A., Ruddle F. H. Polymorphism and linkage of glutathione reductase in Mus musculus. Biochem Genet. 1975 Jun;13(5-6):323–329. doi: 10.1007/BF00485817. [DOI] [PubMed] [Google Scholar]
- Oie H. K., Gazdar A. F., Lalley P. A., Russell E. K., Minna J. D., DeLarco J., Todaro G. J., Francke U. Mouse chromosome 5 codes for ecotropic murine leukaemia virus cell-surface receptor. Nature. 1978 Jul 6;274(5666):60–62. doi: 10.1038/274060a0. [DOI] [PubMed] [Google Scholar]
- Owens R. B., Smith H. S., Hackett A. J. Epithelial cell cultures from normal glandular tissue of mice. J Natl Cancer Inst. 1974 Jul;53(1):261–269. doi: 10.1093/jnci/53.1.261. [DOI] [PubMed] [Google Scholar]
- Parks W. P., Scolnick E. M., Kozikowski E. H. Dexamethasone stimulation of murine mammary tumor virus expression: a tissue culture source of virus. Science. 1974 Apr 12;184(4133):158–160. doi: 10.1126/science.184.4133.158. [DOI] [PubMed] [Google Scholar]
- Rapp U. R., Marshall T. H. Cell surface receptors for endogenous mouse type C viral glycoproteins and epidermal growth factor: tissue distribution in vivo and possible participation in specific cell-cell interaction. J Supramol Struct. 1980;14(3):343–352. doi: 10.1002/jss.400140308. [DOI] [PubMed] [Google Scholar]
- Ruddle N. H., Conta B. S., Leinwand L., Kozak C., Ruddle F., Besmer P., Baltimore D. Assignment of the receptor for ecotropic murine leukemia virus to mouse chromosome 5. J Exp Med. 1978 Aug 1;148(2):451–465. doi: 10.1084/jem.148.2.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STOKER M., MACPHERSON I. SYRIAN HAMSTER FIBROBLAST CELL LINE BHK21 AND ITS DERIVATIVES. Nature. 1964 Sep 26;203:1355–1357. doi: 10.1038/2031355a0. [DOI] [PubMed] [Google Scholar]
- Schochetman G., Long C., Massey R. Generation of a mouse mammary tumor virus (MMTV) pseudotype of Kirsten sarcoma virus and restriction of MMTV gag expression in heterologous infected cells. Virology. 1979 Sep;97(2):342–353. doi: 10.1016/0042-6822(79)90345-3. [DOI] [PubMed] [Google Scholar]
- Shaw C. R., Prasad R. Starch gel electrophoresis of enzymes--a compilation of recipes. Biochem Genet. 1970 Apr;4(2):297–320. doi: 10.1007/BF00485780. [DOI] [PubMed] [Google Scholar]
- Steeves R., Lilly F. Interactions between host and viral genomes in mouse leukemia. Annu Rev Genet. 1977;11:277–296. doi: 10.1146/annurev.ge.11.120177.001425. [DOI] [PubMed] [Google Scholar]
- Womack J. E., Davisson M. T., Eicher E. M., Kendall D. A. Mapping of nucleoside phosphorylase (Np-1) and esterase 10 (Es-10) on mouse chromosome 14. Biochem Genet. 1977 Apr;15(3-4):347–355. doi: 10.1007/BF00484465. [DOI] [PubMed] [Google Scholar]
- Womack J. E., Sharp M. Comparative autosomal linkage in mammals: genetics of esterases in Mus musculus and Rattus norvegicus. Genetics. 1976 Apr;82(4):665–675. doi: 10.1093/genetics/82.4.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Závada J., Dickson C., Weiss R. Pseudotypes of vesicular stomatitis virus with envelope antigens provided by murine mammary tumor virus. Virology. 1977 Oct 1;82(1):221–231. doi: 10.1016/0042-6822(77)90045-9. [DOI] [PubMed] [Google Scholar]
- Závada J. VSV pseudotype particles with the coat of avian myeloblastosis virus. Nat New Biol. 1972 Nov 22;240(99):122–124. doi: 10.1038/newbio240122a0. [DOI] [PubMed] [Google Scholar]



