Abstract
Delivery of the hypoxia inducible vascular endothelial growth factor (RTP-VEGF) plasmid using a novel reducible disulfide poly(amido ethylenediamine) (SS-PAED) polymer carrier was studied in vitro and in vivo. In vitro transfection of primary rat cardiomyoblasts (H9C2) showed SS-PAED at a weighted ratio of 12:1 (polymer/DNA) mediates 16 fold higher expression of luciferase compared to an optimized bPEI control. FACS analysis revealed up to 57 ± 2% GFP positive H9C2s. The efficiency of plasmid delivery to H9C2 using SS-PAED was found to depend upon glutathione (GSH) levels inside the cell. SS-PAED mediated delivery of RTP-VEGF plasmid produced significantly higher levels of VEGF expression (up to 76 fold) under hypoxic conditions compared to normoxic conditions in both H9C2 and rat aortic smooth muscle cells (A7R5). Using SS-PAED, delivery of RTP-VEGF was investigated in a rabbit myocardial infarct model using 100 μg RTP-VEGF. Results showed up to 4 fold increase in VEGF protein expression in the region of the infarct compared to injections of SS-PAED/RTP-Luc. In conclusion, SS-PAED mediated therapeutic delivery improves the efficacy of ischemia-inducible VEGF gene therapy both in vitro and in vivo and therefore, has potential for the promotion of neo-vascular formation and improvement of tissue function in ischemic myocardium.
Keywords: Reducible polymer, VEGF, gene therapy, hypoxia, myoblasts
1. Introduction
The delivery of naturally occurring growth factors as an alternate to more invasive therapeutic interventions is central to the field of gene therapy. Delivery of vascular endothelial growth factor (VEGF) to ischemic tissues in order to promote neo-vascularization and improve organ function has been observed in different clinical trials [1]. Treatment of myocardial ischemia in initial gene delivery trials, for example, has shown promise [2]. Recently, a phase II multicenter clinical trial using delivery of naked VEGF, the Euroinject One trial, showed modest results, suggesting the need for a more effective method of packaging and delivering therapeutic DNA [3].
Biodegradable polymers have recently been of active interest in the field of nonviral gene delivery. In an effort to improve the efficiency of nucleic acid delivery without compromising carrier induced toxicity, researchers have proposed novel approaches. Hydrolyzable polyesters, for example, comprise a major class of biodegradable polymeric gene carriers including linear and hyperbranched poly(ester amine)s [4–8], poly[α-(4-aminobutyl)-L-glycolic acid [9], multiblock copolymers of poly(L-lysine) and poly(ethylene glycol) [10, 11], nonionic poly(ethylene glycol) and poly(lactic-co-glycolic) copolymers [12, 13], and degradable polyethylenimine (PEI) [14–16]. Acid labile cationic polymers through Schiff base formation [17] and polyphosphazines [18] have also been looked at. A more controlled degradation of these polymers, however, could be utilized for more efficient delivery of therapeutic genes into the cell.
Recently, a new class of biodegradable polymer, poly(amido amine)s (PAA) containing reducible disulfide bonds (SS-PAA) was reported. SS-PAAs are promising due to their: (1) facile synthesis, (2) stability in an aqueous environment for purification, (3) endosomal buffering capacity, (4) small particle formation when complexed with plasmid DNA (pDNA), (5) relative low toxicity and high mediated gene expression compared to PEI, (6) low serum interactions in vitro and (7) the ability to take advantage of the high physiological redox potential leading to better release of genetic material inside the cellular compartment [19–21].
Previously, we reported that reducible poly(amido ethylenimine) (SS-PAEI) synthesis produces polymers with a low polydispersity and stable complex formation with pDNA until introduced to a reducing agent or reductive environment in vitro [21]. In this paper, we report the use of a specific SS-PAEI, SS-PAED, in the delivery of RTP-VEGF plasmid to ischemic rabbit myocardium. First, we optimize delivery in H9C2 cells and show that effective SS-PAED mediated pDNA delivery is dependent upon the oxidation state of the disulfide bond inside the intracellular compartment. Therapeutic gene delivery of VEGF is then demostrated in vitro in both A7R5 and H9C2 cells. Finally we show that SS-PAED mediated delivery of RTP-VEGF promotes significant VEGF protein expression in ischemic rabbit myocardium.
2. Materials and Methods
2.1. Polymer Synthesis
Synthesis of disulfide reducible poly(amido ethylenimine) (SS-PAED) was performed as previously reported [21]. Briefly, Michael addition polymerization of ethylenediamine (EDA, Sigma) with cystamine bisacrylamide (Polysciences Inc., Warrington, PA) was conducted in a brown flask under an Argon atmosphere. The reaction was done at a 1:1 molar ratio in 10% aqueous MeOH at 50 ºC stirring for 48 hr. At this point, any remaining acrylamide functional groups were terminated by addition of excess EDA and allowed to stir for an additional 2 hrs. Polymer was isolated by ultrafiltration (MWCO 1000) and lyophilization then characterized accordingly.
2.2. Cell Culture and pDNA
Rat cardiomyoblasts (H9C2, ATCC) were cultured according to ATCC suggested specifications in Dubelcco’s Modified Eagle medium (DMEM) supplemented in 10% Fetal Bovine Serum (FBS), 1% antibiotics (penicillin-streptomycin, 10 000 U/mL) at 37 ºC in 5% CO2 humidified conditions. Rat aortic smooth muscle cells (A7R5, ATCC) were treated under the same conditions.
All plasmids were purified from overnight bacterial cultures by alkaline lysis and column purification using an Invitrogen Maxiprep kit (Carlsbad, CA). Concentration of pDNA solution was determined by UV absorbance at 260 nm and its optical density value at 260/280 was in the range of 1.8 to 2.0.
2.3. Luciferase Expression and MTT Assay
H9C2 cells were plated at 1.2 × 105 per well in 6-well plates and 2.4 × 104 per well in 24-well plates. Transfection experiments were conducted using SS-PAED at different polymer/pDNA weighted ratios ranging from 3:1 to 12:1 with hyperbranched PEI 25k (Sigma) at 0.75:1 w/w as the control. Complexes of polymer with a chicken beta actin promoter driven luciferase reporter plasmid (pβ-Luc) were prepared in a buffered solution (20 mM HEPES, pH 7.4, 5% glucose) and used for transfections as previously mentioned [21]. In short, 48 hrs post transfection, cells were washed with 1× PBS and lysed. Luciferase expression was determined using a luciferase assay system (Promega) on a Luminometer from Dynex Technologies, Inc. (Chantilly, VA). Results were standardized by the amount of protein present in the cell lysate.
For results measuring cell viability, an MTT assay (Sigma) was used. Cells were transfected in 24-well plates and quantification of produced formazan crystals was done after 24 hrs followed by removal of media and addition of 1 mL DMSO. Overall cellular toxicity was determined relative to untreated cells at an optical density of 570 nm.
2.4. Flow Cytometry
H9C2 cells were plated at a density of 1.2 × 105 cells per well in 6-well plates. Transfection experiments were conducted using SS-PAED at different polymer/pDNA ratios compared to a bPEI control as described above in Luciferase expression and MTT assay. In addition, naked pDNA and cells treated with transfection buffer only were used as negative controls. A CMV promoter driven GFP plasmid was used to analyze expression. Forty-eight hrs post transfection, the cells were trypsinized and washed twice with PBS then resuspended in a final volume of 1 mL PBS. GFP expression was analyzed using the BD FACScan analyzer (Becton Dickinson, San Jose CA) at a minimum of 1 × 104 cells gated per sample. Analysis was performed using Becton Dickinson CellQuest software.
2.5. GSH Inhibition
D,L-Buthionine sulfoxamine (BSO), a known GSH inhibitor, was used as previously described by Read et al [22]. H9C2 cells were plated 48 hrs prior to BSO treatment at 1.0 × 105 cells/well in 6-well plates. Briefly, cells were incubated in presence of 20–500 μM BSO 24 hr prior to transfection and maintained throughout the experiment. SS-PAED complexed with pβ-Luc at 12:1 w/w was compared to bPEI/pβ-Luc at 0.75:1 w/w. Cells were harvested 24 hr post-transfection and analyzed for luciferase expression per mg protein as mentioned above.
2.6. VEGF Expression Under Normoxia and Hypoxia
Regulated VEFG expression was measured by using a hypoxia inducible RTP-VEGF plasmid as previously described [23] on H9C2 and A7R5 cells. Cells were plated in 6-well plates at 1.2 × 105 cells per well for H9C2 and in 12-well plates at 1.0 × 105 cells per well for A7R5 24 hr prior to transfection. Four hours post-transfections, DMEM was replaced with 500 μL supplemented media and cells were incubated in either hypoxic or normoxic conditions for the remainder of the experiment. The hypoxia chamber used was a Modulator Incubator Chamber from Billups-Rothenburg Inc. (Del Mar, CA) using mixed gas (1% O2, 5% CO2, balanced N2). Purging and incubation of the hypoxia chamber was done according to manufacturers recommendations at 37 ºC. Media was collected at 24 and 48 hr time points. Therapeutic protein levels were analyzed using ELISA specific for human VEGF (R&D Systems, Minneapolis, MN).
2.7. Analysis of mRNA Expression
Total RNA was extracted from the transfected cells using a Qiagen RNeasy kit. The total RNA was measured by absorbance at 260 nm. A total of 300 ng RNA was reverse transcribed then amplified using a one-step RT-PCR system (Promega). The sequences of the specific oligonucleotide primers VEGF (654 bp) were forward: 5’ – GAT GAG ATC GAG TAC ATC TT – 3’ reverse: 5’ – CAC CGC CTC GGC TTG TCA CAT 3’, β-actin (354 bp) forward: 5’ – TGG AAT CCT GTG GCA TCC ATG AAA C – 3’ reverse: 5’ – TAA AAC GCA GCT CAG TAA CAG TCC G – 3’. The PCR reaction consisted of 94 ºC for 3 min, 30 cycles at 94 ºC for 30 s, 60 ºC for 30 s, and 68 ºC for 30 s, followed by an extension of 7 min at 68 ºC. PCR products were separated by electrophoresis in a 2% agarose gel stained with ethidium bromide.
2.8. Animals and Myocardial Infarct Protocol
All experiments were approved by the University of Utah Institutional Animal Care and Use Committee and followed the guidelines provided by the National Institutes of Health in Guide for the Care and Use of Laboratory Animals.
Surgical procedures were performed as previously reported [24]. In short, New Zealand White Rabbits (Western Oregon), weighing 2.5–3.5 kg, were sedated with ketamine (50 mg/kg, IM), and xylazine (8.8 mg/kg, IM), shaved and intubated. The left chest was sterilely prepped and draped setting up a proper surgical plane of anesthesia with isoflurane. Animals were maintained on a rodent ventilator (Harvard Apparatus, Cambridge, MA) with a respiratory rate of 60 and 40% FiO2. A left thoracotomy was performed through the third intercostal space, the lung was retracted and the pericardium was widely opened. A 5-0 prolene suture was placed around the proximal circumflex artery and a 10-s test occlusion was performed to determine the area of the ventricle at risk. Ischemia was confirmed by visual inspection and by electrocardiographic changes noted on continuous EKG monitoring. Polymer/pDNA complexes were prepared in a buffered solution (20 mM HEPES, pH 7.4, 5% glucose) and allowed to incubate 30 min before injections were made. Four injections, totaling 500 μL, were placed into the myocardium with a 30-gauge needle at the border zone surrounding the area of infarct. After a period of observation, the lung was re-inflated with positive end expiratory pressure produced by temporary outflow occlusion. Residual air was evacuated from the pleural space with an angiocatheter. The ribs were reapproximated with a single figure of eight stitch. The muscular layers were closed individually with a running absorbable stitch and the skin was closed with a running Prolene stitch. Rabbits were weaned from anesthesia and extubated when breathing spontaneously. Post-operative pain was controlled with buprenorphine (0.05 mg/kg, IM) q 12 h for 48 h.
Animals were sacrificed on post-operative day 4 by euthanasia injection.
2.9. VEGF Analysis from Animal Model
Tissue samples (~400 mg) were homogenized in 2.4 mL cocktail containing 1× Lysis buffer (Promega) and protease inhibitor using a MiniBeadbeater-96 system (BioSpec Products Inc, Bartlesville, OK) using 1.0 mm Zirconium beads incubating samples on ice between homogenization cycles. Homogenates were centrifuged at 3000 ×g for 4 min collecting the upper clear supernatant fraction. VEGF protein concentration was determined by ELISA standardized by protein concentration as described above.
Blood was collected at the time of euthanasia and centrifuged at 6000 rpm for 20 min. Serum and all tissue homogenate were kept at −70 Cº until the time of analysis.
2.10. Statistical Analysis
Statistical calculations were carried out using GraphPad Prism 3.0 for Windows. Results were reported as mean ± standard error of the mean. For comparison of gene expression as a result of delivery in vitro, stastistical significance was obtained using unpaired, two-tailed, Student’s t-test with 95% or greater confidence. In vivo results were analyzed using one-way ANOVA and a Tukey’s multiple comparison post-test determining significance at a 95% confidence interval.
3. Results and Discussion
3.1. Optimized Reporter Gene Expression in Cardiomyoblast Culture
Reducible SS-PAEIs were previously shown to exhibit nearly 20 fold higher gene expression compared to bPEI in a number of cells lines including rat aortic smooth muscle cells, mouse embryonic fibroblasts, and bovine aortic endothelial cells while maintaining low toxicity [21]. Figure 1 shows the backbone repeating structure of SS-PAED containing secondary amines, a reducible disulfide bond and amide bonds formed as a result of Michael addition chemistry. Also shown is the mechanism of pDNA release upon transfection into the cell. Even though the exact mechanism of gene expression from a resultant transfection is not clear, we hypothesize that after polymer/pDNA complexes are internalized, complexed material is released from the endosomal compartment due to the “proton sponge effect” [25] followed by a complete reduction of the disulfide bonds in the intracellular space leading to pDNA release.
Fig. 1.
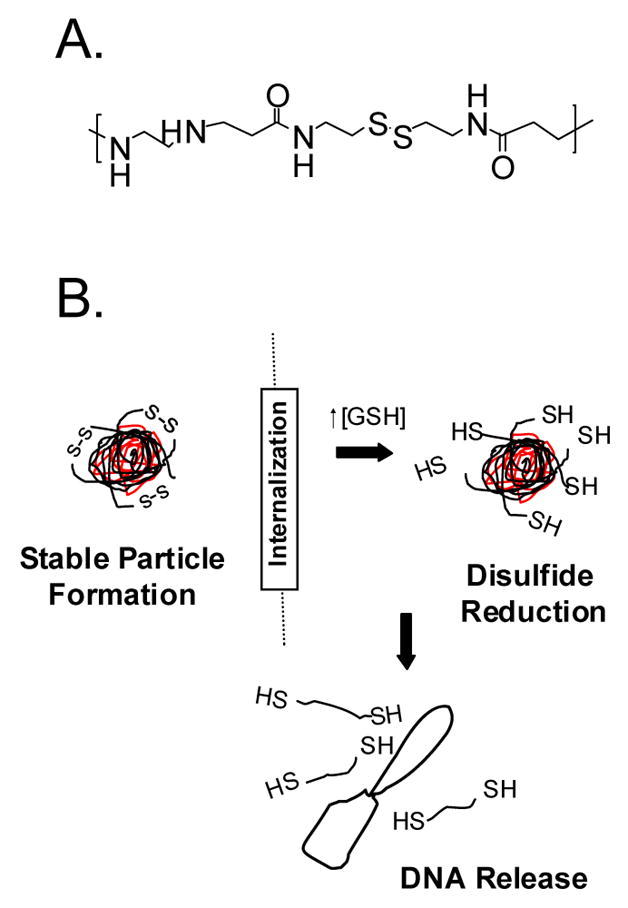
Chemical structure of SS-PAED where (A) is the proposed repeating unit containing both amines for pDNA interactions and a reducible disulfide bond along the polymer backbone and (B) shows the ideal characteristics of the polymer as a carrier for pDNA delivery. Initially, polymer/pDNA interactions under optimized conditions form stable nanoparticles. Upon transfection and endosomal release, glutathione reduces the disulfide bonds on the polymer backbone to release pDNA for nuclear uptake.
H9C2 cells were chosen for in vitro optimization of SS-PAED due to their close resemblance to myocardial cells. Cell transfections were performed using a luciferase reporter plasmid, a toxicity assay, and a plasmid driving green fluorescent protein (GFP) expression in order to show transfection efficacy on a population of myoblast cells. Figure 2 shows the reporter gene expression of SS-PAED on H9C2 cells compared to the relative cell viability with increasing polymer/pDNA weighted ratios. At 12:1 w/w, cell viability was 99 ± 1% while exhibiting 6 fold higher expression compared to 3:1 w/w and 16 fold higher expression compared to the bPEI control (88 ± 4% viability). Ratios higher than 12:1 w/w gradually showed higher toxicity which led to the decision to use 12:1 (SS-PAED:pDNA) for all future in vitro experiments.
Fig. 2.
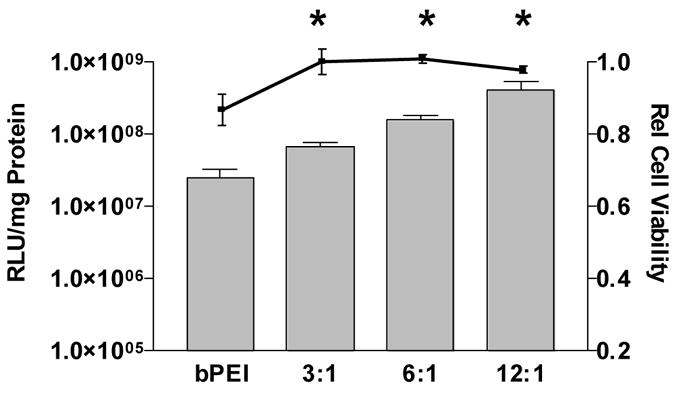
Reporter gene expression on H9C2 cells (bar graph, n = 3 ± SEM) where viability is represented by the scatter plot (n = 5, reported as the mean ± SEM) where * p < 0.05 for both expression and viability of bPEI (0.75:1 w/w) mediated delivery compared to each SS-PAED/pDNA w/w ratio.
In order to assess the transfection efficiency of the SS-PAED/pDNA system, CMV-GFP was used for fluorescence activated cell sorting (FACS) analysis. Figure 3 shows the percentage of GFP positive cells transfected compared to bPEI, naked pDNA and untreated cells as controls. Naked CMV-GFP delivery shows significantly lower transfection as previously reported in human endothelial cells and cardiomyoblasts [26, 27]. Characterization of SS-PAED mediated transfection positively transfects up to 57 ± 2% of H9C2s at 12:1 w/w compared to bPEI at 11 ± 1%. While reducible polylysine polymers dependent upon histidin content have been reported to produce as much as ~57% positive GFP expression in human prostate cancer cells (PC-3) [25], to our knowledge, this is the highest level of GFP expression mediated by a non-viral carrier system reported in cardiac cells to date. The fact that such expression is obtained in a primary cardiac cell line reveals the potential for in vivo cardiovascular therapy. Additional improvements to SS-PAED may increase tissue specificity and in vivo stability in order to narrow the difference in transfection efficiency between viral and non-viral gene carriers.
Fig. 3.
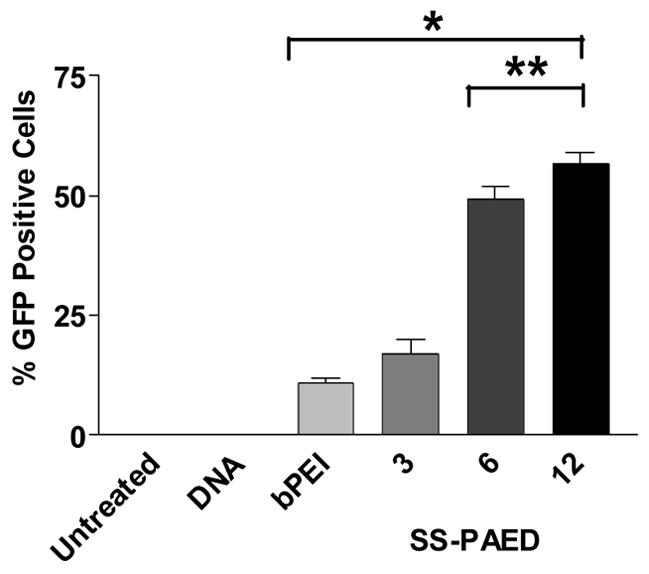
Population of H9C2 cells transfected in 6-well plates using SS-PAED/CMV-GFP at different ratios compared to bPEI/CMV-GFP, naked DNA and untreated controls. Cells were analyzed at a minimum of 1 × 104 cells gated per sample (n = 3 reported as the mean ± SEM, *p < 0.05, **p < 0.001).
3.2. SS-PAED Mediated Gene Delivery is Dependent on Glutathione
Seymour and coworkers showed that the intracellular levels of glutathione (GSH) affected nucleic acid delivery of their reducible polycations [22]. Previous work in mouse fibroblast cells also showed that upon internalization of SS-PAEI complexed with fluorescent labeled pDNA, complexes showed a wider dispersion of pDNA as compared to a non-reducible bPEI control. This higher level of dispersion is most likely due to a reduction of the disulfide bonds in the backbone of the polymer and consequent release of pDNA [21]. To further substantiate the importance of disulfide bond reduction on delivering nucleic acids inside the cell, a GSH inhibitor, D,L-buthionine sulfoxamine (BSO), was added to H9C2 cells 24 hr prior to transfection. Results displayed a trend where a decrease in luciferase expression was evident as BSO concentrations increased (Fig. 4). At 500 μM BSO concentrations, SS-PAED mediated transfections showed a 4.6 fold decline compared to transfections without the GSH inhibitor present. Lower gene expression at higher BSO concentrations suggests the inability of SS-PAED/pDNA complexes to completely dissociate therefore creating an extra rate limiting step in nuclear localization of transfected pDNA. This rate limiting step was suggested in our previous work using confocal microscopy as well as work published by other groups [21, 28–30]. It should also be noted that the decline in gene expression for SS-PAED mediated delivery should not be associated with toxicity since bPEI mediated delivery revealed no significant change in gene expression over the range of BSO used.
Fig. 4.
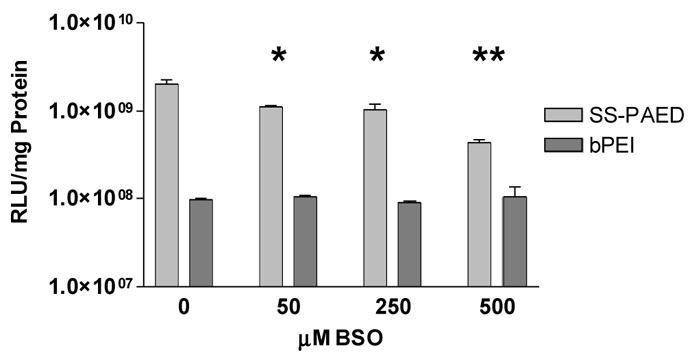
Cells were plated on 6-well plates 24 hr prior to transfection. Results show reporter gene expression on H9C2 cells receiving increasing amounts of GSH inhibitor (BSO). Samples were analyzed 24 hrs after transfection (n = 3 reported as the mean ± SEM, *p ≤ 0.01, **p < 0.001 compared to the BSO untreated SS-PAED control).
3.3. Delivery of Inducible VEGF to Cardiomyoblasts
Recently it was reported that transplanted H9C2 cells could aid in the functional improvement in ischemic rat hearts [31], which suggests different applications for SS-PAED mediated delivery of pDNA.
Two different cell types were used to look at the ability of SS-PAED to successfully deliver therapeutic pDNA under both normoxic and hypoxic conditions. Previous work from our lab using the hypoxia inducible RTP801 promoter driven human VEGF plasmid displayed up to 4 fold increase in VEGF expression under hypoxic conditions as compared to normoxic conditions on a variety of cells [32]. The ability to enhance gene expression in a local ischemic environment allows for a potentially more safe and effective gene delivery system. Figure 5 shows the effect of hypoxia on RTP-Luc reporter plasmid transfection mediated by SS-PAED or bPEI to H9C2 cells. Results using both polymers showed 3 and 3.5 fold increase in reporter expression for SS-PAED and bPEI respectively under conditions of hypoxia. Similar levels of reporter gene up-regulation were obtained using A7R5 cells (data not shown).
Fig. 5.
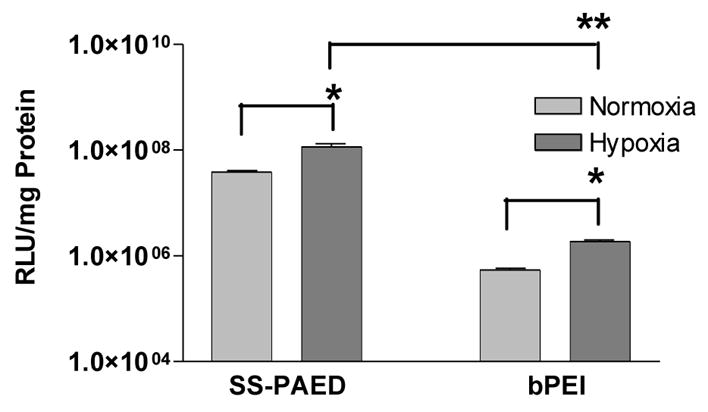
Reporter RTP-Luc gene expression under normoxic and hypoxic conditions 48 hrs post transfection on H9C2 cells in 6-well plates. Optimal polymer w/w ratios were used for the experiment (n = 3, reported as the mean ± SEM, *p ≤ 0.01, **p < 0.001).
VEGF expression was then looked at for both cell lines (Fig. 6). Media was taken 24 and 48 hrs after transfection and analyzed for human VEGF protein expression by ELISA. A difference in overall VEGF protein up-regulation under hypoxic conditions was found between A7R5 and H9C2 cells. Figure 6A shows a modest 2 fold increase in VEGF expression for A7R5s under hypoxic conditions, similar to results using a reporter plasmid. H9C2 cells, however, produced higher levels of VEGF expression under hypoxia displaying over 60 fold difference between normoxia and hypoxic conditions (67 and 76 fold increase after 24 and 48 hrs, Fig. 6B) as compared to similar experiments using reporter gene expression as displayed in Figure 5. Experiments using bPEI as a carrier showed 22 fold increase in VEGF protein under hypoxic conditions (Fig. 6C) suggesting, as in Figure 5, that this effect is not polymer dependent. Delivery of SS-PAED/CMV-GFP did not produce any detectable levels of VEGF under normoxia or hypoxic conditions.
Fig. 6.
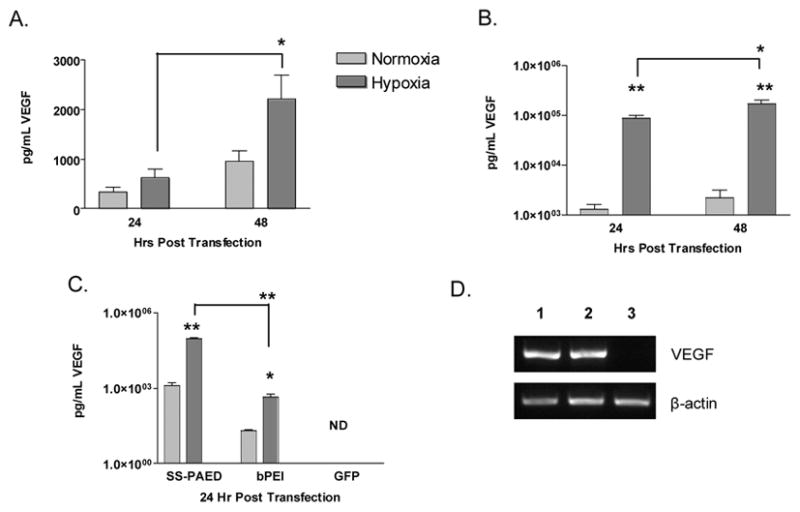
VEGF expression on (A) A7R5 (n = 4) and (B) H9C2 (n = 6) cells in 6-well plates at different time points after transfections with SS-PAED/RTP-VEGF under normoxic and hypoxic conditions. (C) Comparison of VEGF expression in H9C2 cells using SS-PAED to bPEI and SS-PAED mediated transfections delivering CMV-GFP (ND - no detection, all data reported as the mean ± SEM, *p < 0.05, **p < 0.001). (D) RT-PCR results on human VEGF mRNA where 1- normoxia, 2- hypoxia and 3- hypoxia delivering CMV-GFP in H9C2 cells.
Such an observation using cardiomyoblasts under hypoxic conditions was reported previously by Wu et al showing increased VEGF protein was likely due to the protein kinase C pathway. They used H9C2 cells and primary cardiac myocytes from neonatal rats under hypoxic conditions to demonstrate the dependence of VEGF protein expression upon staurosporine, a known inhibitor of the protein kinase C pathway [33]. This evidence is further supported in the literature by other groups who have shown VEGF expression to depend upon this same pathway [34, 35].
The possibility of a post-translational modification of VEGF was further evidenced by RT-PCR. Figure 6D shows that there is not a significant over-expression of human VEGF mRNA under hypoxic conditions. The bands for lanes 1 and 2 for human VEGF expression more closely represent luciferase mRNA expression under normoxia and hypoxia as evidenced by the results in Figure 4 respectively. Semi-quantitative densitometry calculations using Quantity One visualizing software (BioRad, Hercules, CA) suggested only a modest 10% increase in mRNA which does not correlate to the over 60 fold increase in expression. Additional results looking at endogenous rat VEGF mRNA expression did not show any significant difference between RTP-VEGF and CMV-GFP transfected groups under hypoxic conditions (data not shown).
3.4. In Vivo Delivery of SS-PAED/RTP-VEGF in Ischemic Myocardium
In an attempt to optimize VEGF gene expression using SS-PAED in vivo, a rabbit infarct model was used. Previous work in our lab using water soluble lipopolymer (WSLP) produced significantly higher gene expression than naked pDNA and bPEI/pDNA in the same model [36]. WSLP also mediated higher transgene expression in ischemic rabbit myocardium as apposed to healthy myocardium using the hypoxia inducible pEpo-SV-VEGF plasmid [23]. We used WSLP as the positive control for our in vivo experiments.
In the Euroinject One trial, humans were injected with 500 μg of naked VEGF165 pDNA into the myocardium [3]. Previous work in our lab was performed by injecting 200 μg pDNA complexed with WSLP into rabbit myocardium [23, 36]. We initially decided to perform dose response injections with our SS-PAED/RTP-VEGF system starting at 100 μg RTP-VEGF and then increasing the dose until VEGF expression was apparent.
Figure 7 shows the results of injections made for SS-PAED at different w/w ratios compared to the positive control, WSLP delivering RTP-VEGF. Ischemic heart tissue was collected 4 days after injections were made and kept at −70 ºC until the time of analysis of protein expression. For analysis, samples taken from the peripheral region of the infarct were homogenized and then human VEGF expression was determined by ELISA normalized by protein concentration. All four polymer/RTP-VEGF groups showed higher VEGF concentrations than the SS-PAED/RTP-Luc control injected at 12:1 w/w. The initial dosing regimen of 100 μg pDNA was statistical significant compared to the control group. SS-PAED/RTP-VEGF at 12:1 w/w showed the highest overall VEGF expression, nearly 4 fold higher than the RTP-Luc control and 2 fold higher than the WSLP positive control at half the transfection amount.
Fig. 7.
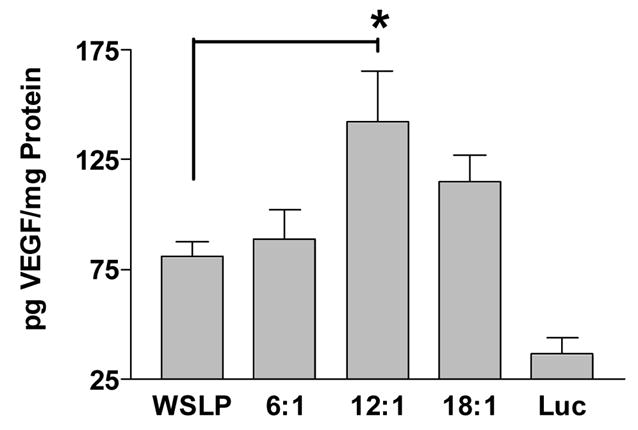
VEGF expression in rabbit myocardial infarct as determined by ELISA. Samples were compared as amount of VEGF protein expressed per mg protein (n ≥ 4, reported as the mean ± SEM, *p < 0.05). All polymer/RTP-VEGF injections showed p < 0.05 significance compared to the SS-PAED/RTP-Luc control at 12:1 w/w. There was no detectable increase in VEGF protein expression in the serum for any group analyzed.
All blood samples taken at the time of euthanasia did not show any detectable increase of VEGF protein in the serum.
In conclusion, myocardial ischemia leads to cell damage, tissue scarring, loss of contractile function and death. It has long been thought that delivering genes to ischemic myocardium could lead to prevention of these adverse effects by inducing tissue regeneration [37]. VEGF gene delivery has produced reduction of myocardial infarct size through cardiomyocyte regeneration, enhancement of cardiomyocyte viability and neo-vascular proliferation [38, 39]. It is our working hypothesis that we can use nonviral delivery of VEGF in order to obtain the same positive therapeutic effects without the concerns associated with alternative forms of therapy.
We believe this is the first report to use reducible polymers for therapeutic gene delivery of VEGF in vivo. The results reported herein show that SS-PAED is an efficient nonviral gene delivery agent that not only is nontoxic but mediates high levels of gene expression in vitro and in vivo. We anticipate further dosing and efficacy studies in the future in order to more fully understand the potential of reducible polymers in gene delivery.
Acknowledgments
This work was supported by NIH grants HL 071541 and HL 065477.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Brewster LP, Brey EM, Greisler HP. Cardiovascular gene delivery: The good road is awaiting. Adv Drug Deliv Rev. 2006;58(4):604–629. doi: 10.1016/j.addr.2006.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Markkanen JE, Rissanen TT, Kivela A, Yla-Herttuala S. Growth factor-induced therapeutic angiogenesis and arteriogenesis in the heart--gene therapy. Cardiovasc Res. 2005;65(3):656–664. doi: 10.1016/j.cardiores.2004.10.030. [DOI] [PubMed] [Google Scholar]
- 3.Kastrup J, Jorgensen E, Ruck A, Tagil K, Glogar D, Ruzyllo W, Botker HE, Dudek D, Drvota V, Hesse B, Thuesen L, Blomberg P, Gyongyosi M, Sylven C. Direct intramyocardial plasmid vascular endothelial growth factor-A165 gene therapy in patients with stable severe angina pectoris A randomized double-blind placebo-controlled study: the Euroinject One trial. J Am Coll Cardiol. 2005;45(7):982–988. doi: 10.1016/j.jacc.2004.12.068. [DOI] [PubMed] [Google Scholar]
- 4.Lynn DM, Anderson DG, Putnam D, Langer R. Accelerated discovery of synthetic transfection vectors: parallel synthesis and screening of a degradable polymer library. J Am Chem Soc. 2001;123(33):8155–8156. doi: 10.1021/ja016288p. [DOI] [PubMed] [Google Scholar]
- 5.Zhong Z, Song Y, Engbersen JF, Lok MC, Hennink WE, Feijen J. A versatile family of degradable non-viral gene carriers based on hyperbranched poly(ester amine)s. J Control Release. 2005;109(1–3):317–329. doi: 10.1016/j.jconrel.2005.06.022. [DOI] [PubMed] [Google Scholar]
- 6.Greenland JR, Liu H, Berry D, Anderson DG, Kim WK, Irvine DJ, Langer R, Letvin NL. Beta-amino ester polymers facilitate in vivo DNA transfection and adjuvant plasmid DNA immunization. Mol Ther. 2005;12(1):164–170. doi: 10.1016/j.ymthe.2005.01.021. [DOI] [PubMed] [Google Scholar]
- 7.Liu Y, Wu D, Ma Y, Tang G, Wang S, He C, Chung T, Goh S. Novel poly(amino ester)s obtained from Michael addition polymerizations of trifunctional amine monomers with diacrylates: safe and efficient DNA carriers. Chem Commun (Camb) 2003;(20):2630–2631. doi: 10.1039/b309487a. [DOI] [PubMed] [Google Scholar]
- 8.Lim Y, Kim SM, Lee Y, Lee W, Yang T, Lee M, Suh H, Park J. Cationic hyperbranched poly(amino ester): a novel class of DNA condensing molecule with cationic surface, biodegradable three-dimensional structure, and tertiary amine groups in the interior. J Am Chem Soc. 2001;123(10):2460–2461. doi: 10.1021/ja005715g. [DOI] [PubMed] [Google Scholar]
- 9.Lim YB, Han SO, Kong HU, Lee Y, Park JS, Jeong B, Kim SW. Biodegradable polyester, poly[alpha-(4-aminobutyl)-L-glycolic acid], as a non-toxic gene carrier. Pharm Res. 2000;17(7):811–816. doi: 10.1023/a:1007552007765. [DOI] [PubMed] [Google Scholar]
- 10.Bikram M, Ahn CH, Chae SY, Lee M, Yockman JW, Kim SW. Biodegradable Poly(ethylene glycol)-co-poly(L-lysine)-g-histidine Multiblock Copolymers for Nonviral Gene Delivery. Macromolecules. 2004;37(5):1903–1916. [Google Scholar]
- 11.Ahn CH, Chae SY, Bae YH, Kim SW. Synthesis of biodegradable multi-block copolymers of poly(L-lysine) and poly(ethylene glycol) as a non-viral gene carrier. J Control Release. 2004;97(3):567–574. doi: 10.1016/j.jconrel.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 12.Jeong JH, Kim SW, Park TG. Biodegradable triblock copolymer of PLGA-PEG-PLGA enhances gene transfection efficiency. Pharm Res. 2004;21(1):50–54. doi: 10.1023/b:pham.0000012151.05441.bf. [DOI] [PubMed] [Google Scholar]
- 13.Chang CW, Choi D, Kim WJ, Yockman JW, Christensen LV, Kim YH, Kim SW. Non-ionic amphophilic biodegradable PEG-PLGA-PEG copolymer enhances gene delivery efficiency in rat skeletal muscle. J Control Release. doi: 10.1016/j.jconrel.2006.11.025. In press. [DOI] [PubMed] [Google Scholar]
- 14.Forrest ML, Koerber JT, Pack DW. A degradable polyethylenimine derivative with low toxicity for highly efficient gene delivery. Bioconjug Chem. 2003;14(5):934–940. doi: 10.1021/bc034014g. [DOI] [PubMed] [Google Scholar]
- 15.Ahn CH, Chae SY, Bae YH, Kim SW. Biodegradable poly(ethylenimine) for plasmid DNA delivery. J Control Release. 2002;80(1–3):273–282. doi: 10.1016/s0168-3659(01)00547-8. [DOI] [PubMed] [Google Scholar]
- 16.Park MR, Han KO, Han IK, Cho MH, Nah JW, Choi YJ, Cho CS. Degradable polyethylenimine-alt-poly(ethylene glycol) copolymers as novel gene carriers. J Control Release. 2005;105(3):367–380. doi: 10.1016/j.jconrel.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 17.Kim YH, Park JH, Lee M, Kim YH, Park TG, Kim SW. Polyethylenimine with acid-labile linkages as a biodegradable gene carrier. J Control Release. 2005;103(1):209–219. doi: 10.1016/j.jconrel.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 18.Luten J, van Steenis JH, van Someren R, Kemmink J, Schuurmans-Nieuwenbroek NM, Koning GA, Crommelin DJ, van Nostrum CF, Hennink WE. Water-soluble biodegradable cationic polyphosphazenes for gene delivery. J Control Release. 2003;89(3):483–497. doi: 10.1016/s0168-3659(03)00127-5. [DOI] [PubMed] [Google Scholar]
- 19.Emilitri E, Ranucci E, Ferruti P. New poly(amidoamine)s containing disulfide linkages in their main chain. J Polym Sci, Part A: Polym Chem. 2005;43:1404–1416. [Google Scholar]
- 20.Lin C, Zhong Z, Lok MC, Jiang X, Hennink WE, Feijen J, Engbersen JF. Linear poly(amido amine)s with secondary and tertiary amino groups and variable amounts of disulfide linkages: Synthesis and in vitro gene transfer properties. J Control Release. 2006;116(2):130–137. doi: 10.1016/j.jconrel.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 21.Christensen LV, Chang CW, Kim WJ, Kim SW, Zhong Z, Lin C, Engbersen JF, Feijen J. Reducible Poly(amido ethylenimine)s for Triggered Intracellular Gene Delivery. Bioconjug Chem. 2006;17(5):1233–1240. doi: 10.1021/bc0602026. [DOI] [PubMed] [Google Scholar]
- 22.Read ML, Singh S, Ahmed Z, Stevenson M, Briggs SS, Oupicky D, Barrett LB, Spice R, Kendall M, Berry M, Preece JA, Logan A, Seymour LW. A versatile reducible polycation-based system for efficient delivery of a broad range of nucleic acids. Nucl Acids Res. 2005;33(9):e86. doi: 10.1093/nar/gni085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee M, Rentz J, Bikram M, Han S, Bull DA, Kim SW. Hypoxia-inducible VEGF gene delivery to ischemic myocardium using water-soluble lipopolymer. Gene Ther. 2003;10(18):1535–1542. doi: 10.1038/sj.gt.3302034. [DOI] [PubMed] [Google Scholar]
- 24.Bull DA, Bailey SH, Rentz JJ, Zebrack JS, Lee M, Litwin SE, Kim SW. Effect of Terplex/VEGF-165 gene therapy on left ventricular function and structure following myocardial infarction. VEGF gene therapy for myocardial infarction. J Control Release. 2003;93(2):175–181. doi: 10.1016/j.jconrel.2003.06.002. [DOI] [PubMed] [Google Scholar]
- 25.Boussif O, Lezoualc'h F, Zanta MA, Mergny MD, Scherman D, Demeneix B, Behr JP. A versatile vector for gene and oligonucleotide transfer into cells in culture and in vivo: polyethylenimine. Proc Natl Acad Sci U S A. 1995;92(16):7297–7301. doi: 10.1073/pnas.92.16.7297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu Y, Reineke TM. Poly(glycoamidoamine)s for Gene Delivery: Stability of Polyplexes and Efficacy with Cardiomyoblast Cells. Bioconjug Chem. 2006;17(1):101–108. doi: 10.1021/bc050275+. [DOI] [PubMed] [Google Scholar]
- 27.Green JJ, Shi J, Chiu E, Leshchiner ES, Langer R, Anderson DG. Biodegradable Polymeric Vectors for Gene Delivery to Human Endothelial Cells. Bioconjug Chem. 2006;17(5):1162–1169. doi: 10.1021/bc0600968. [DOI] [PubMed] [Google Scholar]
- 28.Godbey WT, Wu KK, Mikos AG. Tracking the intracellular path of poly(ethylenimine)/DNA complexes for gene delivery. Proc Natl Acad Sci U S A. 1999;96(9):5177–5181. doi: 10.1073/pnas.96.9.5177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gosselin MA, Guo W, Lee RJ. Efficient gene transfer using reversibly cross-linked low molecular weight polyethylenimine. Bioconjug Chem. 2001;12(6):989–994. doi: 10.1021/bc0100455. [DOI] [PubMed] [Google Scholar]
- 30.Oupicky D, Carlisle RC, Seymour LW. Triggered intracellular activation of disulfide crosslinked polyelectrolyte gene delivery complexes with extended systemic circulation in vivo. Gene Ther. 2001;8(9):713–724. doi: 10.1038/sj.gt.3301446. [DOI] [PubMed] [Google Scholar]
- 31.Kutschka I, Chen IY, Kofidis T, Arai T, von Degenfeld G, Sheikh AY, Hendry SL, Pearl J, Hoyt G, Sista R, Yang PC, Blau HM, Gambhir SS, Robbins RC. Collagen matrices enhance survival of transplanted cardiomyoblasts and contribute to functional improvement of ischemic rat hearts. Circulation. 2006;114(1 Suppl):I167–173. doi: 10.1161/CIRCULATIONAHA.105.001297. [DOI] [PubMed] [Google Scholar]
- 32.Lee M, Bikram M, Oh S, Bull DA, Kim SW. Sp1-dependent regulation of the RTP801 promoter and its application to hypoxia-inducible VEGF plasmid for ischemic disease. Pharm Res. 2004;21(5):736–741. doi: 10.1023/b:pham.0000026421.09367.b3. [DOI] [PubMed] [Google Scholar]
- 33.Wu G, Mannam AP, Wu J, Kirbis S, Shie JL, Chen C, Laham RJ, Sellke FW, Li J. Hypoxia induces myocyte-dependent COX-2 regulation in endothelial cells: role of VEGF. Am J Physiol Heart Circ Physiol. 2003;285(6):H2420–2429. doi: 10.1152/ajpheart.00187.2003. [DOI] [PubMed] [Google Scholar]
- 34.Finkenzeller G, Marme D, Weich HA, Hug H. Platelet-derived growth factor-induced transcription of the vascular endothelial growth factor gene is mediated by protein kinase C. Cancer Res. 1992;52(17):4821–4823. [PubMed] [Google Scholar]
- 35.Kieser A, Weich HA, Brandner G, Marme D, Kolch W. Mutant p53 potentiates protein kinase C induction of vascular endothelial growth factor expression. Oncogene. 1994;9(3):963–969. [PubMed] [Google Scholar]
- 36.Lee M, Rentz J, Han SO, Bull DA, Kim SW. Water-Soluble lipopololymer as an effiecient carrier for gene delivery to myocardium. Gene Therapy. 2003;10:585–593. doi: 10.1038/sj.gt.3301938. [DOI] [PubMed] [Google Scholar]
- 37.Hughes GC, Annex BH. Angiogenic therapy for coronary artery and peripheral arterial disease. Expert Rev Cardiovasc Ther. 2005;3(3):521–535. doi: 10.1586/14779072.3.3.521. [DOI] [PubMed] [Google Scholar]
- 38.Ferrarini M, Arsic N, Recchia FA, Zentilin L, Zacchigna S, Xu X, Linke A, Giacca M, Hintze TH. Adeno-associated virus-mediated transduction of VEGF165 improves cardiac tissue viability and functional recovery after permanent coronary occlusion in conscious dogs. Circ Res. 2006;98(7):954–961. doi: 10.1161/01.RES.0000217342.83731.89. [DOI] [PubMed] [Google Scholar]
- 39.Vera Janavel G, Crottogini A, Cabeza Meckert P, Cuniberti L, Mele A, Papouchado M, Fernandez N, Bercovich A, Criscuolo M, Melo C, Laguens R. Plasmid-mediated VEGF gene transfer induces cardiomyogenesis and reduces myocardial infarct size in sheep. Gene Ther. 2006;13(15):1133–1142. doi: 10.1038/sj.gt.3302708. [DOI] [PubMed] [Google Scholar]


