Abstract
Background:
Modifying the balance between resorption and apposition through selectively injuring the cortical plate of the alveolus has been an approach to speed tooth movement and is referred to as periodontally accelerated osteogenic orthodontics. The aim of this study was to investigate the alveolar response to corticotomy as a function of time and proximity to the surgical injury in a rat model.
Methods:
Maxillary buccal and lingual cortical plates were injured in 36 healthy adult rats adjacent to the upper left first molars. Twenty-four animals were euthanized at 3, 7, or 11 weeks. In one group, the maxillae were removed and stripped of soft tissues, and histomorphometric analysis was performed to study alveolar spongiosa and periodontal ligament (PDL) modeling dynamics. Catabolic activity was analyzed with tartrate-resistant acid phosphatase–positive osteoclasts and preosteoclasts. Anabolic actions were measured using a fluorescent vital bone stain series followed by sacrifice at 30 and 51 days. To further analyze the new bone formation, a separate group of animals were fed with calcein fluorescent stain and processed for non-decalcified fluorescent stain histology.
Results:
At 3 weeks, the surgery group had significantly (P <0.05) less calcified spongiosa bone surface, greater periodontal ligament surface, higher osteoclast number, and greater lamina dura apposition width. The catabolic activity (osteoclast count) and anabolic activity (apposition rate) were three-fold greater, calcified spongiosa decreased by two-fold, and PDL surface increased by two-fold. Surgical injury to the alveolus that induced a significant increase in tissue turnover by week 3 dissipated to a steady state by postoperative week 11. The impact of the injury was localized to the area immediately adjacent to the decortication injury.
Conclusion:
Selective alveolar decortication induced increased turnover of alveolar spongiosa, and the activity was localized; dramatic escalation of demineralization-remineralization dynamics is the likely biologic mechanism underlying rapid tooth movement following selective alveolar decortication.
Keywords: Bone formation, osteoclast, tooth movement
Turnover of bone is related to maturation, skeletal maintenance, and mineral metabolism and takes place in bone-forming and -resorbing phases defined as anabolic and catabolic modeling, respectively.1 Orthodontic tooth movement is influenced by increased alveolar bone metabolism and decreased bone density, and the bone turnover rate determines the quantity and quality of orthodontic tooth movement.2 High bone turnover significantly increases the rate of tooth movement, whereas slower tooth movement was found in animals with less turnover.3-5 Catabolic activity mediated by osteoclasts is the limiting factor in the rate of tooth movement in which the periodontal ligament (PDL) plays a crucial role.1 The theoretical linear rate of resorption for osteoclasts during cortical bone remodeling is estimated to be 0.6 mm/month through cortical bone;6 however, a clinical study7 in adults demonstrated that the linear rate of resorption at the PDL interface of mandibular molars, i.e., the lamina dura, during translation is significantly less. Vascular access of osteoclasts to the PDL–bone interface is limited when the PDL is compressed, suggesting that modeling of the cortical lamina dura is more difficult during tooth movement. Comparison of rates of mandibular and maxillary molar movement in the same patient showed that maxillary molars moved twice as fast as mandibular molars because of the increased surface area of resisting bone in the maxilla that is primarily of a trabecular nature.1 Collectively, these findings suggest that the osteoclastic activity to resorb the cortical lamina dura of the alveolar bone during bone turnover is the key element in defining the kinetics of tooth movement.
Modifying the balance between resorption and apposition, and thereby “by-passing” the waiting time for the alveolar cortex to resorb and, thus, moving the teeth faster without causing irreversible damage to periodontium, has been the focus of many research projects. Corticotomy, or the intentional injury of cortical bone, although first described in 1892 as a surgical approach to correct malocclusion with incisions to the cortical alveolar bone to splint teeth into new positions, received attention when a series of articles describing different approaches toward treating orthodontic patients was published in 1959.8-10 In these landmark articles, “corticotomy” was reintroduced as a surgical procedure to facilitate subsequent orthodontic treatment penetrating the buccal and palatal cortical layers at different points while leaving the spongiosa intact. Vertical cortical scarring incisions were performed interproximally and extended well beyond the dental apices. A subapical horizontal osteotomy was added to the corticotomies, thereby connecting the vertical incisions to facilitate what was characterized as bony “block movement.” The horizontal osteotomy was done at 10 mm supra-apical to the anterior teeth and penetrated the buccal cortical plate, the lingual plate, and the interposed medullary bone. This technique involved a combined corticotomy/osteotomy procedure and resulted in a shorter treatment time (6 to 12 weeks).8-10 This technique was later adapted to a dog model11 and used in clinical cases.12-14 Generson et al.14 described rapid orthodontic treatment for open bite malocclusion in 1978 using alveolar decortication without subapical osteotomy. Treatment of a large group of adult patients using this modified surgical procedure was reported in 1991 and was referred to as “corticotomy-facilitated orthodontics.”15 The only minor modification of this approach involved making a fissure through the cortical bone surrounding teeth so that the teeth would be embedded within a block of bone that is connected to adjacent blocks only through the medullary bone. It was speculated that the achieved rapid orthodontic movement was due to the bony “blocks” movement. The investigator described the teeth with braces acting as handles that moved these “blocks” and stated that tooth movement should be completed in 3 to 4 months before the fusion of the bony “blocks.” However, no biomechanic or biologic studies were performed to test the validity of the bony-block concept.
In 2001, Wilcko et al.16 published case reports of two patients with severely crowded dental arches and revisited the original technique with some modifications. Both patients were treated using a non-extraction modified corticotomy-facilitated orthodontic technique, an approach that they defined as accelerated osteogenic orthodontics (AOO). The AOO technique benefited from the past work; however, it included some unique modifications. Similar to the original approach, AOO involves full-thickness labial and lingual alveolar flaps, but decortication surgical scarring barely reaches into the medullary bone adjacent to the roots of the teeth intended for movement, thereby posing little threat to tooth vitality. Thicker portions of the exposed alveolar cortex are traumatized with bur marks (dots) or cuts to enhance bleeding; an allograft of bioabsorbable grafting material is applied directly over the bleeding bone, and the flaps are sutured into place. Tooth movement is initiated within 1 week after surgery, and the fixed orthodontic appliances are activated every 2 weeks thereafter until the malocclusion is resolved.17 The investigators suggested that the design of corticotomy and perforations was intended to maximize the trauma to the alveolus and to promote ample bleeding compared to creating blocks of bone. No luxation of teeth was performed following the corticotomy procedure, and no clinically significant periodontal problems were identified during the active treatment time. Clinically, no disruption of the vitality of teeth was observed, no alveolar crest height changes occurred, and no significant apical root resorption was detected on the periapical radiographs.
Wilcko et al.16,17 further speculated that the dynamics of the physiologic tooth movement in patients who underwent selective decortication might be due to a demineralization–remineralization process rather than bony block movement. They suggested that this process would manifest as a part of the regional acceleratory phenomenon (RAP) that involves the alveolar bone after being exposed to injury (corticotomy) and during active tooth movement. Because RAP refers to reorganizing activity and the cascade of physiologic healing events that occur in tissues adjacent to the site of injury,18,19 orthodontic force application alone is a stimulant sufficient to trigger a mild RAP activity. But when tooth movement is combined with selective decortication, RAP is maximized. RAP results in a decrease in regional bone densities (osteopenia) in healthy tissues, whereas the volume of bone matrix remains constant. In human long bones, RAP begins within a few days of surgery, typically peaks at 1 to 2 months, and may take from 6 to 24 months to subside completely.18-22 Simply elevating a mucoperiosteal flap induced a RAP response in the rat mandible.23 RAP led to a five-fold increase in new trabecular bone formation adjacent to the corticotomy cut in the rabbit tibia, and was so localized that there was no influence on the contralateral pole of the bipolar head of the tibia.24 The initial phase of RAP is characterized by an increase in cortical bone porosity and dramatic turnover of trabecular bone surfaces due to increased osteoclastic activity; these events are contributing factors for the increased mobility of the teeth after periodontal surgery.23
Collectively, anecdotal and clinical reports and limited scientific evidence suggest that the physiologic healing events following alveolar surgery, i.e., calcium depletion, diminished bone density, and RAP, would result in rapid tooth movement. However, the biology underlying these changes is not clear. Therefore, it was the aim of this study to investigate the alveolar response to corticotomy as a function of time and proximity to the surgical injury. Specifically, spatial and temporal differences in catabolic and anabolic activities between control and experimental dento-alveolar arches were compared in rats.
MATERIALS AND METHODS
Animal Model and Corticotomy
Thirty-six healthy adult Charles River Laboratory - caesarean derived (CRL-CD) male rats with a body weight of 400 to 450 g were obtained.§ The animals were acclimated to the Laboratory Animal Science Center animal care facilities at Boston University for ≥2 days, fed rat chow and water ad libitum, and weighed daily. The study protocol was approved by the Institutional Animal Care and Use Committee at Boston University Medical Center. To control for inter-animal variability, a split-mouth design was used with the contralateral side as control. General anesthesia was provided with intraperitoneal injection of ketamine∥ (8 mg/kg) combined with xylazine¶ (5 mg/kg).
Maxillary buccal and lingual full-thickness, triangular-shaped periosteal flaps were elevated adjacent to the upper left first molar. Injury to the buccal and lingual cortical plates was performed using a 1/2-round bur in a surgical handpiece under sterile water irrigation. The decortication injuries consisted of 10 dot-shaped bur marks ∼0.2 mm in diameter: five bur marks on the lingual aspect and five bur marks on the buccal aspect (Fig. 1A). The periosteal flaps were repositioned after the surgery and secured with a single 6-0 size silk suture. Following postanesthesia recovery, all animals were permitted to move freely in their cages. All animals were housed in the same manner and were provided rat chow and water ad libitum. Twenty-four of the 36 animals were eventually euthanized at 3, 7, or 11 weeks using an overdose of carbon dioxide.
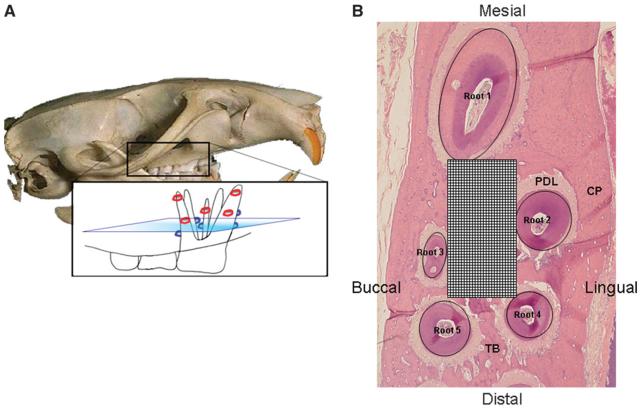
Figure 1.
Surgical procedure and analysis. A) The corticotomy procedure. Red and blue circles indicate surgical bur cuts on buccal and lingual surfaces, respectfully. B) Histomorphometric analysis was performed for bone and PDL modeling dynamics and osteoclast count (hematoxylin and eosin; original magnification: ×2.5). CP = cortical plate; TB = trabecular bone.
Histopathology of Corticotomy
To study the tissue response to the corticotomy procedure, 18 animals were divided into three groups of six based on the 3-, 7-, or 11-week time points. After the euthanasia, maxillae were removed, stripped of soft tissues, and prepared for routine histology. Samples were decalcified, embedded in paraffin, and transversely sectioned (5-μm thick) through the first, second, and third-molar roots using a microtome, producing eight slide sections from apex to crown per hemi-arch. Slides at three levels (coronal, mid, and apical) were stained with hematoxylin and eosin and examined for morphology as well as the catabolic activity. All reagents used in these assays were purchased.#
Histomorphometric analysis was performed for alveolar spongiosa and PDL modeling dynamics and osteoclast count (Fig. 1B). First, the images of the first molars were captured at ×2.5 and ×10 magnifications. Then, a standardized grid of 15 mm2 was used for histomorphometric analysis of the area defined by and centered within the boundaries of the five roots of the first molar on the surgical and the control sides; the grid was drawn using a software program.** Within the standardized grid, the trabecular bone surface and the total PDL surface were recorded in square millimeters. The PDL width was also recorded for each root per quadrant (mesial, palatal, lingual, and buccal) with five measurements per PDL quadrant. Study variables of trabecular bone surface and PDL surface were measured on each of the eight slides per specimen twice by two examiners (JDS and JWT) and then averaged.
Catabolic Response to Corticotomy
To study the catabolic activity in alveolar bone, slides at three levels were stained with tartrate-resistant acid phosphatase (TRAP) following the previously described protocol25 and using a commercially available kit.†† First, images of the first and third molars were captured at ×40 magnification. Then, catabolic activity was measured within the PDL and trabecular bone by recording the number of TRAP-stained osteoclasts and preosteoclasts within the specified grids. For trabecular bone, osteoclasts and preosteoclasts were counted within the standardized grid of 15 mm2 centered within the geometric center of the four distal roots of the first molar and within the geometric center of the three roots of the third molar. To record the osteoclasts and preosteoclasts within the PDL, each region (mesial, palatal, lingual, and buccal) was examined with the aid of three 0.02-mm2 rectangular (cementum, middle, and bone) grids. The PDL measurements were made in first and third molars. Cell counts were performed on each of the eight slides per specimen and were measured twice by each of the two examiners and then averaged.
Anabolic Activity in Response to Corticotomy
To study the time-course of anabolic activity of corticotomy as a function of new bone deposition, we injected 12 animals subcutaneously with fluorescent vital bone stains. Six of the animals in this group received injections each week after surgery in sequence with calcein, then tetracycline, and finally alizarin red starting at postoperative day 7. The rest of the animals (n = 6) received the calcein-tetracycline-alizarin red sequence initiated at postoperative day 28. The animals were euthanized 2 days after the final injection, i.e., at day 30 or 51. Maxillae were harvested, stripped, and then processed for non-decalcified fluorescent stain histology. Histomorphometric analysis of the lamina dura bone apposition rate adjacent to each first-molar root was assessed with the aid of analysis software. Bone apposition width was measured by the new lamina dura bone thickness showing the three vital stains; the width variable was expressed as average thickness over the 3-week period. Bone apposition length was measured by the length of three stains as a percentage of overall root perimeter and expressed as average length over the 3-week period.
To further analyze the new bone formation after the corticotomy procedure, six animals were fed with calcein fluorescent stain‡‡ ad libitum in the drinking water postdecortication and processed for non-decalcified fluorescent stain histology as described above. Histomorphometric analysis for new bone formation was performed at 3, 7, and 11 weeks using a 10,000-pixel grid centered within the geometric center of the four distal roots of the first molar and within the geometric center of the three roots of the third molar. New trabecular bone formation was measured using the analysis software.
Statistical Analysis
Parametric statistical testing was performed on bone and PDL surface data as well as lamina dura width and length data using the one-way analysis of variance and Tukey post hoc comparison tests; the Kruskal-Wallis test was used for non-parametric testing of osteoclast counts, and the significance level was set at 95% probability.
RESULTS
Trabecular Bone Surface and PDL Surface
In this first study on the kinetics of the tissue response to the corticotomy procedure, we chose three time points (3, 7, and 11 weeks) to determine the dynamics of tissue turnover over time. Histopathologic evaluation of the slides stained with hematoxylin and eosin demonstrated that at 3 weeks, the surgery group had significantly (P <0.05) less trabecular bone surface (4.4 ± 1.8 mm2) compared to the control side and all other groups (6.16 to 9.04 mm2) (Figs. 2A and 2B). In parallel to the reduction in the trabecular bone surface area, there was a significantly greater PDL surface (7.2 ± 0.9 mm2) in the surgery group at the third week compared to all other groups (2.06 to 5.30 mm2). By 7 weeks, there was no statistically significant difference between the surgery and control sites, and by 11 weeks after the surgery, the tissue architecture was completely restored and returned to baseline (Fig. 2C). The difference between the surgery sites at 3 weeks compared to 7 and 11 weeks was statistically significant (P <0.05), demonstrating that the smallest bone area and largest periodontal ligament space were present at 3 weeks after the surgery (Fig. 2D).
Figure 2.
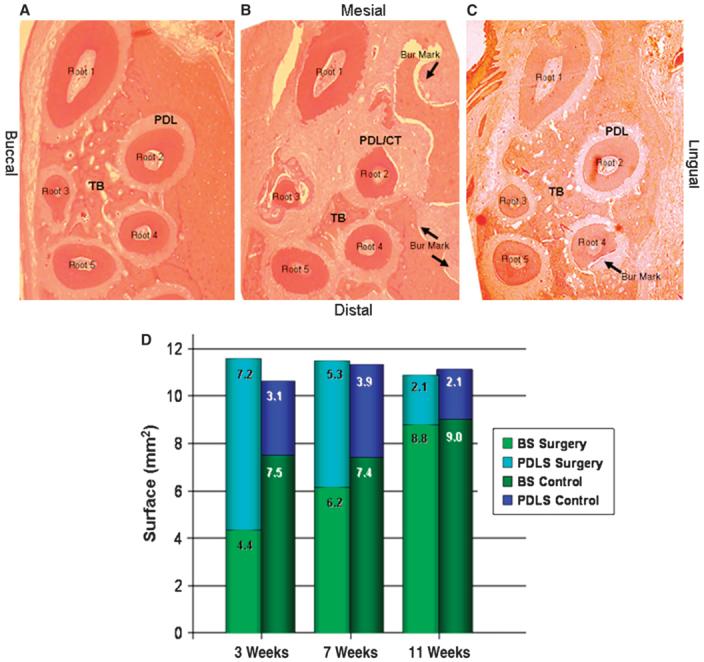
Histopathologic analysis of tissue response. The contralateral control sites (A) had significantly more trabecular bone surface compared to the surgery group at 3 weeks (B). There was a significantly greater PDL surface in the surgery group at the third week compared to the control group. C) Eleven weeks after surgery, the tissue architecture was completely restored and returned to baseline. (Hematoxylin and eosin; original magnification: A through C, ×2.5.) CT = connective tissue; TB = trabecular bone. Arrows indicate the bur marks; root 1 through root 5 denote the roots of the first maxillary molar. D) The surface area of the trabecular bone (BS) and the PDL (PDLS; the extended PDL area into the connective tissue that replaced the resorbing bone) were inversely correlated; the smallest amount of trabecular bone surface was observed at the 3-week analysis. By the end of 11 weeks, the proportional distribution of the BS and the PDLS at the surgery site was similar to the contralateral side.
Osteoclastic Activity and Bone Resorption
The histologic observation that there was an early and fast bone resorption led us to hypothesize that this phenomenon should be accompanied by increased osteoclastic activity. Catabolic activity, as measured by the number of tartrate resistant acid phosphatase (TRAP)-positive osteoclasts and preosteoclasts, was significantly increased in the surgery group by the end of 3 weeks compared to the control sites (Figs. 3A and 3B; P <0.05). When medullary osteoclasts were counted and compared among the groups at 3 weeks, the surgery sites had significantly (P <0.05) higher counts (55.9 ± 13.9) compared to the control sites (30.0 ± 6.2) and all other groups (28.1 to 30.1), except the surgery group at 7 weeks (33.3 ± 2.1; P = 0.09). The TRAP-positive osteoclast and preosteoclast numbers returned to baseline levels by 11 weeks (Fig. 3C).
Figure 3.
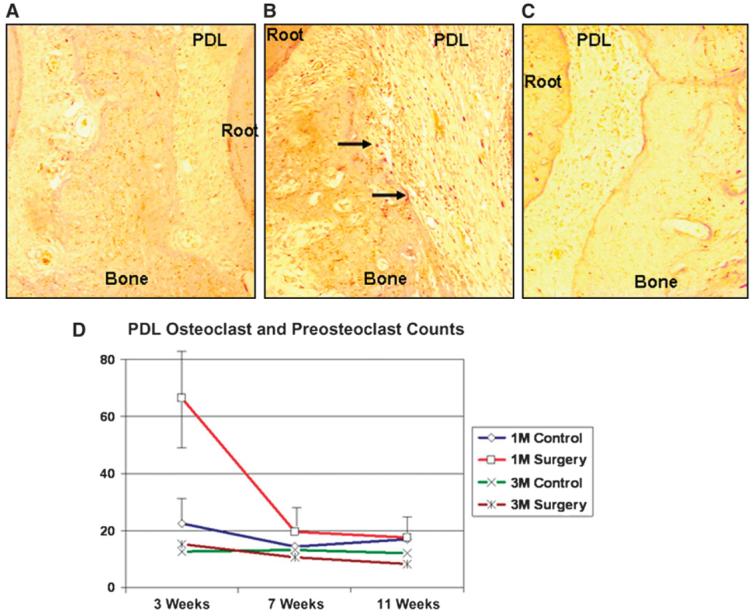
Osteoclastic activity and bone resorption. The number of TRAP-positive osteoclasts and preosteoclasts was significantly lower in the control group (A) compared to the surgery group (B) by the end of 3 weeks. C) Eleven weeks after the surgery, the TRAP-positive cell counts and their distribution at the surgery site were similar to baseline and the contralateral control sides. Arrows indicate TRAP-positive osteoclasts. (TRAP; original magnification: A through C, ×20.) D) The first-molar (lM) PDL of the surgery group at 3 weeks had significantly greater osteoclastic activity than the 3-week control and the other lM groups, as well as the third-molar (3M) areas at 3, 7, and 11 weeks. M = molar.
To study the extent of the bone-resorptive activity of osteoclasts and preosteoclasts, we also compared the PDL space around the first molars (the area of injury) to the third molars (area distant from the injury site). Osteoclastic activity in the PDL space of the first molar was greater in the surgery group at 3 weeks (66.3 ± 17.6) compared to the control group at 3 weeks (22.5 ± 9.8; P = 0.000) as well as all other first-molar groups (14.5 to 19.5). Moreover, the PDL activity level surrounding the first molar at 3 weeks was significantly higher than all third-molar groups (8.5 to 15.3, P <0.05) at 3, 7, and 11 weeks, demonstrating the site specificity of the decortication or RAP activity (Fig. 3D).
Bone Apposition Width and Length
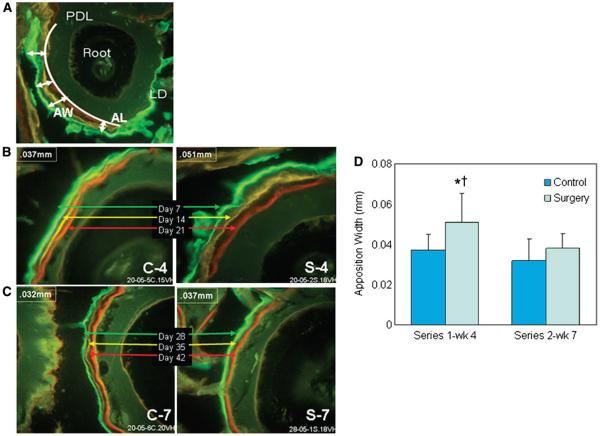
To study the bone turnover as a dynamic phenomenon, we analyzed the lamina dura bone apposition in non-decalcified histologic preparations using fluorescent dyes to mark the weekly activity. Injections were made every 7 days, allowing very little time for apposition to occur. Except for the surgery side during the first injection series, the appositional rate between fluorescent stain markers for all animal subjects was very low. Because the time between injections of fluorescent stains was minimal, overall apposition during a 23-day period (including all three stains) was used instead of standard histomorphometric measures. Based on our observation that 11 weeks represented complete restoration of the bone architecture, we chose to study the bone apposition by injecting the first group of animals at the end of 1, 2, and 3 weeks and sacrificing them at day 30 and injecting the second group of animals at the end of 4, 5, and 6 weeks and sacrificing them at day 51 (Fig. 4). Lamina dura apposition width around the first molar was significantly greater following surgery at 4 weeks postdecortication (0.051 ± 0.025 mm) compared to the 4-week control (0.037 ± 0.014 mm; P <0.001). Similar to our initial observations using conventional histology on decalcified samples, there were no significant differences between surgery and control sites at other time points, whereas the apposition at 4 weeks was significantly greater than in the surgery group at 7 weeks (0.038 ± 0.013 mm; P <0.001). No differences were observed in apposition length of the lamina dura as a percentage of first-molar root perimeters.
Figure 4.
Lamina dura apposition width. A) Following injection of calcein (green), tetracycline (yellow), and alizarin red (red), apposition length (AL; curved line) and apposition width (AW; arrows) of the lamina dura (LD) surrounding the root were measured. B) After series 1 injections at 7, 14, and 21 days and sacrifice at 30 days, lamina dura width around the first molar was significantly greater on the decortication side (S-4) compared to the control (C-4). C) After series 2 injections at 28, 35, and 42 days and sacrifice at 51 days, there were no significant differences between decortication (S-7) and control sides (C-7), whereas the apposition width at 4 weeks was significantly higher than the surgery group at 7 weeks. (Original magnification: A through C, ×20.) D) Apposition width of lamina dura as a function of time in surgery and control groups (mean ± SEM). *P <0.001 compared to control at week 4; †P <0.001 compared to surgery at week 7. wk = week.
New Trabecular Bone Formation
Anabolic activity was further studied by feeding animals with calcein fluorescent stain and processing the samples for non-decalcified fluorescent stain histology at 3, 7, and 11 weeks. Data demonstrated that the percentage of new bone apposition in the first-molar area was significantly greater in the postdecortication group at 3 weeks (59.7%) compared to the control group at 3 weeks (24.5%, P <0.05) and the surgery group at 7 and 11 weeks (Fig. 5).
Figure 5.
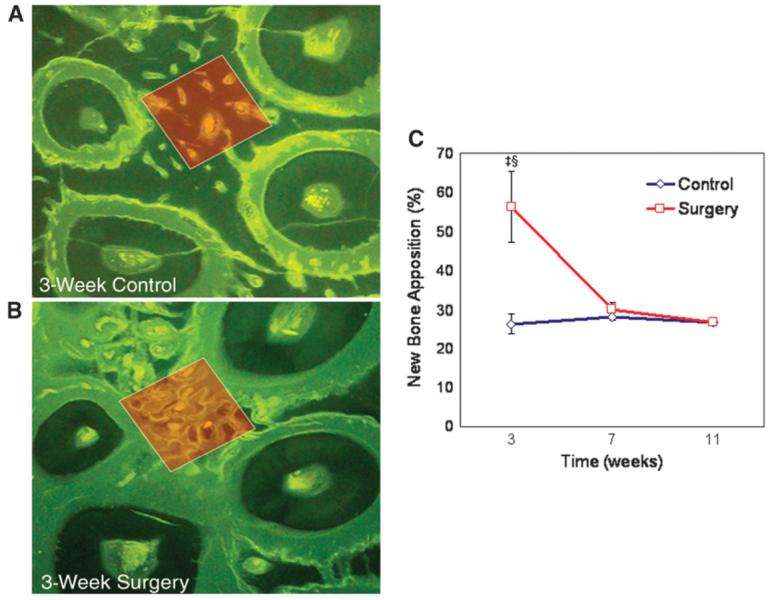
New trabecular bone formation. The percentage of new bone apposition in the first-molar area was significantly lower in the control sites (A) compared to the postdecortication group at 3 weeks (B) (calcein ad libitum; original magnification: ×10). C) Anabolic modeling of alveolar trabecular bone adjacent to the decortication site increased by ∼1.5 times at 3 weeks; this change was diminished by 7 weeks to the level of the control side and was stabilized by 11 weeks (mean ± SEM). ‡P <0.01 compared to control at week 3; §P <0.01 compared to surgery at weeks 7 and 11.
Similar to the catabolic activity, anabolic activity of the spongiosa was also delimited to the site of decortication injury. The RAP activity in trabecular bone was demonstrated at 3 weeks when the first-molar surgical site (59.7%) was compared to the first-molar control (24.5%) and to the third-molar area on the surgery side (17.8%) and the control side (22.9%). Hence, anabolic modeling of alveolar trabecular bone adjacent to the decortication site increased by ∼2.4 times at 3 weeks compared to control and had 2.6 to 3.4 times greater anabolic modeling activity compared to the third-molar area.
DISCUSSION
The goal of this study was to analyze the alveolar and periodontal response to decortication surgery as a function of time and proximity to the injury based on the hypothesis that selective alveolar decortication leads to osteopenia. Extrapolation of the results helps to explain how selective alveolar decortication facilitates clinical orthodontic treatment when applied in conjunction with tooth movement. The experimental design did not include tooth movement; therefore, in effect, this study clarified the dynamics of periodontium change as a consequence of decortication-type injury or intentional wounding. Results from tooth movement experimentation were not included in this study because application of biomechanic force impacts PDL and bone change dynamics so broadly as to make it difficult to describe in a single publication. In the present study, we used a combination of vital stains, TRAP staining, and histomorphometric techniques to analyze the anabolic and catabolic activities involved in bone turnover in response to selective alveolar decortication. The results demonstrated that selective alveolar decortication injury provided an overwhelming activating stimulus for the catabolic activation (resorption response) and the anabolic activation (formation response) in the periodontium. Over the time points chosen in this study, 3 weeks postdecortication represented the peak in both aspects of bone-modeling behavior; the catabolic activity (osteoclast count) and anabolic activity (apposition width or rate) were three-fold higher. At 3 weeks, the calcified spongiosa content of the alveolar bone adjacent to the injury had decreased by two-fold and PDL surface had increased by two-fold. Overall, surgical injury to the alveolus induced a dramatic increase in tissue turnover by week 3, which dissipated to steady state by 11 weeks postoperatively. Another key finding was that the increased bone metabolism was localized to the area immediately adjacent to the injury, which supports the suggestion that RAP is responsible for the observed tissue response to selective alveolar decortication.
A previous study1 showed that because bone is a mineralized tissue, changes in the metabolic fraction of the alveolus, i.e., the trabecular bone, represent surface changes because external osseous activities take place along vascularized periosteal surfaces via uncoupled anabolic and catabolic modeling events. In the current study, we evaluated both of these events in spongiosa of the rat maxilla adjacent to alveolar decortication. At 3 weeks post-surgery, catabolic activity increased significantly; osteoclast counts in the first-molar medullary space increased 1.9 times compared to controls and were 1.7 to 2.0 times greater than the other time points. Meanwhile, osteoclast counts in the PDL area were 3.0 to 4.6 times higher than in the other groups. Histomorphometric analysis of the first-molar spongiosa showed 1.6 times less calcified trabecular bone surface on the surgery side at 3 weeks compared to control. Increased anabolic modeling was also found; serial vital stain injections showed lamina dura surface apposition was 1.3 to 1.6 times greater by postdecortication week 4. Likewise, calcein bone label added to the drinking water showed anabolic modeling of alveolar trabecular bone adjacent to the decortication site at 3 weeks had increased 2.4 times compared to control. Thus, the changes and their sequence that we observed in trabecular bone and the lamina dura support the notion that bone turnover in response to alveolar corticotomy does not involve a linear/sequential series of events, and, at least in healthy trabecular bone, simultaneous stimulation of anabolic and catabolic activities takes place. Because the alveolar trabecular bone is the milieu of orthodontic tooth movement, the immediate application of this knowledge to clinical dentistry suggests that the biologic mechanisms underlying turnover of the spongiosa bone may have a profound influence on orthodontic tooth movement.
This study was designed to look specifically at PDL, lamina dura, and alveolar spongiosa to gain a global orthodontic perspective on bone turnover dynamics following wounding. To the best of our knowledge, our results provided the first histologic and systematic evidence that corticotomy-type wounding increases alveolar metabolism and spongy bone turnover. These results support the rapid tooth movement concept hypothesized by Wilcko et al.16 of demineralization and remineralization events rather than bone moving in blocks as the explanation for rapid movement. Previously, “block movement” was believed to be the mechanism behind the corticotomy-facilitated orthodontic tooth movement where the teeth acted as handles that move these static blocks.15 However, our findings demonstrated that the injury to the bone, even in the absence of any tooth movement, led to the increased physiologic activity in bone. Our findings are also consistent with previous reports24,26 in which increased anabolic turnover of long bone spongiosa immediately adjacent to corticotomy incision in the head of the rabbit tibia was demonstrated at 4 weeks. It was demonstrated that a periodontium undergoing increased osseous turnover resulted in greater and more rapid tooth movement; that same environment has been described as favoring the rapid tooth movement.2 Our data showed that the surgical injury to the alveolus in the rat induced a dramatic increase in anabolic and catabolic modeling by the third week after the corticotomy; the impact of surgery dissipated considerably by postoperative week 7, stabilizing to a steady state by postoperative week 11.
Frost18,19 described increased tissue turnover secondary to the magnitude and proximity of injury in spatial-temporal terms (RAP). The RAP that occurs adjacent to the PDL triggered by the orthodontic force alone is known as “undermining resorption,” but the level of expression is only mild to moderate; orthodontic tooth movement combined with selective alveolar decortication would induce a profound level of RAP activity. The argument that cortical bone injury is not necessary if elevating a mucoperiosteal flap induces RAP23 is not valid; the expressed level of the RAP response would not be pervasive enough to enable rapid tooth movement. It was shown that RAP resulted in osteopenia at a specific region, and bone matrix volume did not change.18-22 The RAP principle emphasizing spatial proximity to the injury was demonstrated in the connective tissue/PDL areas in the present study. The first-molar PDL osteoclast count for the surgery side at 3 weeks was three times greater than the control at 3 weeks, and it also was significantly greater than all other first-molar groups; at 3 weeks post-surgery, PDL was also 4.3 to 7.8 times greater than the osteoclast counts around the third-molar sites. These results support the principles of RAP and suggest negligible metabolic change across the dental arch or more than one tooth away.
Rodent models are useful tools to study the tissue biology and response to clinical applications in dental research. A recent review27 discussed the potential applications and limitations of these models, suggesting that every animal study should be justified with the objective of the study while the limitations of these models, their applicability to the clinical practice, are kept under consideration. The obvious advantage of using animal models is the ability to study the biologic phenomena that are impossible to replicate in the human model for obvious ethical reasons. Therefore, we chose to study the dynamics of selective alveolar decortication in an animal model. Surely, the bone turnover rate of a rat is different than that of a human, and the orthodontic tooth movement dynamics are not identical, as reviewed in a recent work.28 However, the anabolic and catabolic phases of bone remodeling are parallel to those seen in humans.29 Therefore, the information gathered from the rat model can be applicable to the osseous response to a similar injury in humans. Nevertheless, this limitation has to be kept in mind when transferring the information gathered from the experimental rat model to humans.
CONCLUSIONS
This study demonstrated that spongy bone turnover is extensive and pervasive after selective alveolar decortication. The biologic mechanisms underlying the corticotomy-assisted rapid tooth movement are not clearly elucidated, but our results suggest a broad periodontium-mediated phenomenon. PDL activity is clearly enhanced by decortication injury, but the spongiosa likely plays a dominant role in rapid tooth movement. Studies should follow to demonstrate tooth movement and periodontal dynamics after selective alveolar decortication has been performed and tissue response has been evaluated. Nevertheless, the time span that we analyzed in this study will form the basis of understanding the biologic kinetics of the corticotomy.
ACKNOWLEDGMENTS
This work was supported, in part, by grant DE016191 from the National Institutes of Health/National Institute of Dental and Craniofacial Research, Bethesda, Maryland. The authors report no conflicts of interest related to this study.
Footnotes
Charles River Laboratories, Wilmington, MA.
AnaSed, Ben Venue Laboratories, Bedford, OH.
Ketacet, Fort Dodge Animal Health, Fort Dodge, IA.
Sigma Aldrich, St. Louis, MO.
Olympus MicroSuite FIVE, Olympus, Center Valley, PA.
Sigma Aldrich.
AnaSpec, San Jose, CA.
REFERENCES
- 1.Roberts WE, Huja S, Roberts JA. Bone modeling: Biomechanics, molecular mechanisms, and clinical perspectives. Semin Orthod. 2004;10:123–161. [Google Scholar]
- 2.Verna C, Dalstra M, Melsen B. The rate and the type of orthodontic tooth movement is influenced by bone turnover in a rat model. Eur J Orthod. 2000;22:343–352. doi: 10.1093/ejo/22.4.343. [DOI] [PubMed] [Google Scholar]
- 3.Midgett RJ, Shaye R, Fruge JF., Jr. The effect of altered bone metabolism on orthodontic tooth movement. Am J Orthod. 1981;80:256–262. doi: 10.1016/0002-9416(81)90289-x. [DOI] [PubMed] [Google Scholar]
- 4.Goldie RS, King GJ. Root resorption and tooth movement in orthodontically treated, calcium-deficient, and lactating rats. Am J Orthod. 1984;85:424–430. doi: 10.1016/0002-9416(84)90163-5. [DOI] [PubMed] [Google Scholar]
- 5.Engström C, Granström G, Thilander B. Effect of orthodontic force on periodontal tissue metabolism. A histologic and biochemical study in normal and hypocalcemic young rats. Am J Orthod Dentofacial Orthop. 1988;93:486–495. doi: 10.1016/0889-5406(88)90077-7. [DOI] [PubMed] [Google Scholar]
- 6.Roberts WE, Goodwin WC, Jr., Heiner SR. Cellular response to orthodontic force. Dent Clin North Am. 1981;25:3–17. [PubMed] [Google Scholar]
- 7.Roberts WE, Arbuckle GR, Analoui M. Rate of mesial translation of mandibular molars using implant-anchored mechanics. Angle Orthod. 1996;66:331–338. doi: 10.1043/0003-3219(1996)066<0331:ROMTOM>2.3.CO;2. [DOI] [PubMed] [Google Scholar]
- 8.Kole H. Surgical operations on the alveolar ridge to correct occlusal abnormalities. Oral Surg Oral Med Oral Pathol. 1959;12:515–529. doi: 10.1016/0030-4220(59)90153-7. [DOI] [PubMed] [Google Scholar]
- 9.Kole H. Surgical operations on the alveolar ridge to correct occlusal abnormalities. Oral Surg Oral Med Oral Pathol. 1959;12:413–420. doi: 10.1016/0030-4220(59)90051-9. [DOI] [PubMed] [Google Scholar]
- 10.Kole H. Surgical operations on the alveolar ridge to correct occlusal abnormalities. Oral Surg Oral Med Oral Pathol. 1959;12:277–288. doi: 10.1016/0030-4220(59)90177-x. [DOI] [PubMed] [Google Scholar]
- 11.Düker J. Experimental animal research into segmental alveolar movement after corticotomy. J Maxillofac Surg. 1975;3:81–84. doi: 10.1016/s0301-0503(75)80022-1. [DOI] [PubMed] [Google Scholar]
- 12.Anholm JM, Crites DA, Hoff R, Rathbun WE. Corticotomy-facilitated orthodontics. CDA J. 1986;14:7–11. [PubMed] [Google Scholar]
- 13.Gantes B, Rathbun E, Anholm M. Effects on the periodontium following corticotomy-facilitated orthodontics. Case reports. J Periodontol. 1990;61:234–238. doi: 10.1902/jop.1990.61.4.234. [DOI] [PubMed] [Google Scholar]
- 14.Generson RM, Porter JM, Zell A, Stratigos GT. Combined surgical and orthodontic management of anterior open bite using corticotomy. J Oral Surg. 1978;36:216–219. [PubMed] [Google Scholar]
- 15.Suya H. Corticotomy in orthodontics. In: Hosl E, Baldauf A, editors. Mechanical and Biological Basics in Orthodontic Therapy. Hütlig Buch; Heidelberg, Germany: 1991. pp. 207–226. [Google Scholar]
- 16.Wilcko WM, Wilcko T, Bouquot JE, Ferguson DJ. Rapid orthodontics with alveolar reshaping: Two case reports of decrowding. Int J Periodontics Restorative Dent. 2001;21:9–19. [PubMed] [Google Scholar]
- 17.Wilcko WM, Ferguson DJ, Bouguot JE, Wilcko MT. Rapid orthodontic decrowding with alveolar augmentation: Case report. World J Orthod. 2003;4:197–205. [Google Scholar]
- 18.Frost HM. The biology of fracture healing. An overview for clinicians. Part II. Clin Orthop Relat Res. 1989;248:294–309. [PubMed] [Google Scholar]
- 19.Frost HM. The biology of fracture healing. An overview for clinicians. Part I. Clin Orthop Relat Res. 1989;248:283–293. [PubMed] [Google Scholar]
- 20.Henrikson PA. Periodontal disease and calcium deficiency. An experimental study in the dog. Acta Odontol Scand. 1968;26(Suppl 50):1–132. [PubMed] [Google Scholar]
- 21.Krook L, Lutwak L, Henrikson PA, et al. Reversibility of nutritional osteoporosis: Physicochemical data on bones from an experimental study in dogs. J Nutr. 1971;101:233–246. doi: 10.1093/jn/101.2.233. [DOI] [PubMed] [Google Scholar]
- 22.Krook L, Whalen JP, Lesser GV, Berens DL. Experimental studies on osteoporosis. Methods Achiev Exp Pathol. 1975;7:72–108. [PubMed] [Google Scholar]
- 23.Yaffe A, Fine N, Binderman I. Regional accelerated phenomenon in the mandible following mucoperiosteal flap surgery. J Periodontol. 1994;65:79–83. doi: 10.1902/jop.1994.65.1.79. [DOI] [PubMed] [Google Scholar]
- 24.Bogoch E, Gschwend N, Rahn B, Moran E, Perren S. Healing of cancellous bone osteotomy in rabbits – Part I: Regulation of bone volume and the regional acceleratory phenomenon in normal bone. J Orthop Res. 1993;11:285–291. doi: 10.1002/jor.1100110216. [DOI] [PubMed] [Google Scholar]
- 25.Barka T, Anderson PJ. Histochemical methods for acid phosphatase using hexazonium pararosanilin as coupler. J Histochem Cytochem. 1962;10:741–753. [Google Scholar]
- 26.Bogoch E, Gschwend N, Rahn B, Moran E, Perren S. Healing of cancellous bone osteotomy in rabbits – Part II: Local reversal of arthritis-induced osteopenia after osteotomy. J Orthop Res. 1993;11:292–298. doi: 10.1002/jor.1100110217. [DOI] [PubMed] [Google Scholar]
- 27.Graves DT, Fine D, Teng YT, Van Dyke TE, Hajishengallis G. The use of rodent models to investigate host-bacteria interactions related to periodontal diseases. J Clin Periodontol. 2008;35:89–105. doi: 10.1111/j.1600-051X.2007.01172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ren Y, Maltha JC, Kuijpers-Jagtman AM. The rat as a model for orthodontic tooth movement – A critical review and a proposed solution. Eur J Orthod. 2004;26:483–490. doi: 10.1093/ejo/26.5.483. [DOI] [PubMed] [Google Scholar]
- 29.Meikle MC. Remodeling the dentofacial skeleton: The biological basis of orthodontics and dentofacial orthopedics. J Dent Res. 2007;86:12–24. doi: 10.1177/154405910708600103. [DOI] [PubMed] [Google Scholar]