Abstract
Background
The importance of theory in underpinning interventions to promote effective professional practice is gaining recognition. The Medical Research Council framework for complex interventions has assisted in promoting awareness and adoption of theory into study design. Human error theory has previously been used by high risk industries but its relevance to healthcare settings and patient safety requires further investigation. This study used this theory as a framework to explore non‐prescription medicine supply from community pharmacies. The relevance to other healthcare settings and behaviours is discussed.
Method
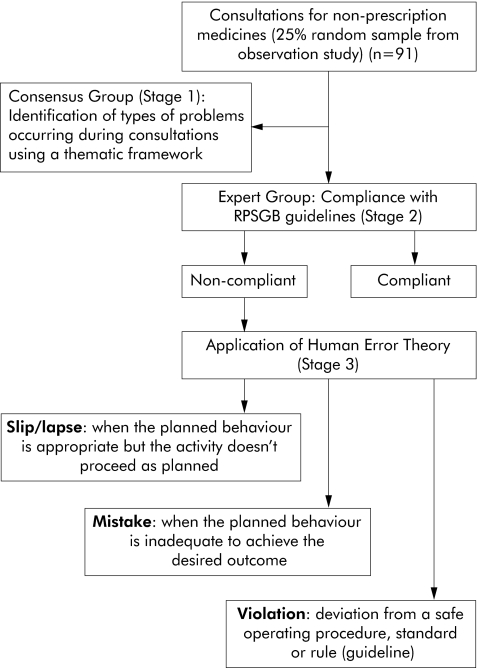
A 25% random sample was made of 364 observed consultations for non‐prescription medicines. Each of the 91 consultations was assessed by two groups: a consensus group (stage 1) to identify common problems with the consultation process, and an expert group (stages 2 and 3) to apply human error theory to these consultations. Paired assessors (most of whom were pharmacists) categorised the perceived problems occurring in each consultation (stage 1). During stage 2 paired assessors from an expert group (comprising patient safety experts, community pharmacists and psychologists) considered whether each consultation was compliant with professional guidelines for the supply of pharmacy medicines. Each non‐compliant consultation identified during stage 2 was then categorised as a slip/lapse, mistake, or violation using human error theory (stage 3).
Results
During stage 1 most consultations (n = 75, 83%) were deemed deficient in information exchange. At stage 2, paired assessors varied in attributing non‐compliance to specific error types. Where agreement was achieved, the error type most often selected was “violation” (n = 27, 51.9%, stage 3). Consultations involving product requests were less likely to be guideline compliant than symptom presentations (OR 0.30, 95% CI 0.10 to 0.95, p = 0.05).
Conclusions
The large proportion of consultations classified as violations suggests that either pharmacy staff are unaware of professional guidelines and thus do not follow them (therefore these acts would not be violations), or that they knowingly violate the guidelines due to reasons that need further research. The methods presented here could be used in other healthcare settings to explore healthcare professional behaviour and to develop strategies to promote patient safety and effective professional practice.
Keywords: non‐prescription medicines, evidence based practice, human error theory, community pharmacy services
Patient safety has attracted considerable attention in recent years.1,2 Medication errors are one of the most commonly occurring errors in health care, yet most evidence for their occurrence has been derived from hospital settings despite the majority of prescribing and medicine supply occurring in the primary care. This imbalance is currently being addressed in the UK through developments such as the National Patient Safety Agency (www.npsa.org.uk) and the Patient Safety Research Network (www.ihs.man.ac.uk/PSRN).
At the same time, many governments are promoting the greater availability of medicines to the public to reduce national drug expenditure. In the UK the reclassification of medicines from prescription only (POM) to pharmacy (P) or general sales list (GSL) status has provided the public with greater direct access to a growing range of medicines. Medicines with P and GSL status (also known as non‐prescription medicines) are sold from community pharmacies either by, or under the supervision of, a pharmacist. [GSL medicines can also be sold from non‐pharmacy outlets]. As with prescribed medication, non‐prescription medicines should be supplied in accordance with agreed guidelines to ensure public safety. Furthermore, drugs that have been reclassified recently tend to be more potent than those reclassified in earlier years, so their inappropriate supply and use may have even greater implications for patient safety. The Royal Pharmaceutical Society of Great Britain (RPSGB) has published guidelines in their Code of Ethics3 indicating how medicines should be supplied from community pharmacies in Great Britain. The guidelines relate to the process of supply and not specifically to clinical practice or specific drugs. For example, the guidelines recommend that “sufficient information” is obtained during consultations “to enable a suitable product to be recommended”, and that advice should be given. The guidelines also require the involvement of a pharmacist in consultations where appropriate, and that particular care is taken with specific patient groups (such as the elderly) and specific drugs (such as newly reclassified drugs). The extent to which current practice complies with these guidelines is unknown, but there is evidence that non‐prescription medicines are sometimes supplied or used inappropriately.4,5,6,7 WWHAM (Who is it for; What are the symptoms; How long have the symptoms been present; Any other medication being taken; Medication tried already) is a mnemonic that is also used as a guideline for medicine counter assistants to derive information during consultations.8 The use of WWHAM is significantly associated with appropriate outcomes during consultations for non‐prescription medicines.9 Although a survey found that most medicine counter assistants report using WWHAM and perceived its use to be important or very important (Watson, unpublished data), consultations involving the same medicine counter assistants achieved a median WWHAM score of 2—that is, only two of the possible five WWHAM questions were asked or information elicited. There is evidence that medicine counter assistants use WWHAM as a matter of rote rather than in an informed way, tailored to individual consultations.10
The importance of theory in underpinning interventions to promote effective professional practice is gaining increasing recognition.11 The Medical Research Council framework for the development and evaluation of randomised controlled trials for complex interventions to improve health12 has assisted in promoting awareness and adoption of theory into study design.
Human error theory13 has been used by high risk industries such as North Sea Oil companies and aviation to identify causes of error and to develop strategies to reduce their frequency as well as the consequences of their occurrence. This theory looks at the process that generates the error rather than the individual who commits the error. It classifies errors or “unsafe acts” as slips and lapses, mistakes, and violations (fig 1). Slips and lapses occur when the planned action is appropriate but doesn't go according to plan. Slips are errors that can be seen—for example, when the plan is to select drug A from the shelf but drug B is picked up instead. Lapses are usually “internal” errors which often occur due to memory failure—for example, the plan is to remind a customer about the maximum dose of an analgesic but the staff member forgets to do so. Mistakes occur when the planned behaviour is inadequate to achieve the desired goal—for example, a medicine counter assistant may recommend an antifungal for the treatment of vaginal itch but the symptoms are due to a sexually acquired infection, not vaginal candidiasis. Violations occur when an individual knows the rule or guideline that they should follow in a given situation but chooses not to follow this plan. In the context of a community pharmacy, a violation could be the sale of a pharmacy only medicine when the pharmacist is not on the premises. The most important distinction here is between violations (which are intentional), and non‐intentional errors (slips, lapses and mistakes). Clearly these two types of errors are likely to have different causes and solutions.
Figure 1 Applying the Royal Pharmaceutical Society of Great Britain (RPSGB) guidelines and human error theory to consultations for non‐prescription medicines.
There is growing recognition of the relevance of human error theory to healthcare settings14,15 yet, to date, there has been little evaluation of this theory in primary care in general, and medication use in particular. The results of earlier work showed that pharmacy staff are safety conscious and risk averse.10 Human error theory incorporates these themes and was selected as a potentially suitable theoretical framework to explore consultations for non‐prescription medicines in community pharmacies.
The aims of this study were (1) to identify problems associated with the supply of non‐prescription medicines from community pharmacies; (2) to explore whether consultations for non‐prescription medicines were compliant with RPSGB guidelines; and (3) to categorise non‐compliant consultations using human error theory.
Methods
In 2002 a study comprising observation of the supply of non‐prescription medicines by a pharmacist observer and semi‐structured interviews of pharmacists and pharmacy support staff was conducted in nine community pharmacies across Grampian, Scotland.16 In summary, 364 consultations were observed and written notes made. For the purpose of this current study, a 25% random sample (n = 91) was taken (using blind selection of random numbers17) to enable the completion of the scheduled tasks within the allocated time. The study comprised three stages.
Types of problems occurring during consultations (stage 1)
The purpose of stage 1 was solely to identify the general types of problems that occurred during consultations for non‐prescription medicines. A Consensus Group of local assessors was convened, of whom five were pharmacists, one was a clinical psychologist, and one a health service researcher with extensive pharmacy practice research experience. The 91 consultations were grouped by pharmacy and each assessor was assigned consultations from up to three pharmacies. Each consultation was assessed independently by the pharmacist who was the observer in the original study (who assessed all 91 consultations) and one other group member, to identify perceived problems. Each assessor used a thematic framework to categorise the identified problems (table 1). The themes were derived from the observation study and interviews and were discussed, refined, and agreed by the group prior to the rating exercise. The extent of agreement between the paired assessors was calculated; the percentage agreement for consultations was also presented for consultations where kappa could not be calculated. [N.B. Kappa is calculated across the diagonal—that is, from a 2×2 or 3×3 table. Where there is no diagonal—for example, with a 2×1 table—Kappa cannot be calculated. This situation arises when there is no variation across one axis of the table.]
Table 1 Categorisation of types of problems occurring during consultations (stage 1, n = 91).
| Average % agreement across pharmacies* | Pharmacy | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | ||
| Average % agreement between paired raters by pharmacy* | ||||||||||
| Information/advice problem | 82.7 | 85.7 | 87.5 | 86.7 | 85.7 | 100.0 | 70.0 | 73.3 | 100.0 | 55.6 |
| Information/advice problem due to: | ||||||||||
| Inadequate questioning skills | 48.0 | 75.0 | 87.5 | 86.7 | 57.1 | 40.0 | 45.0 | 20.0 | 10.0 | 11.1 |
| Inadequate listening skills | 5.7 | 12.5 | 6.7 | 7.1 | 5.0 | 20.0 | ||||
| Non‐verbal communication | 1.0 | 3.6 | 5.0 | |||||||
| Communication problems between staff and pharmacist due to: | ||||||||||
| Inadequate questioning skills | 0.4 | 3.6 | ||||||||
| Inadequate listening skills | 0 | |||||||||
| Non‐verbal communication | 1.5 | 10.0 | 3.3 | |||||||
| Lack of staff/pharmacist confidence | 2.1 | 3.6 | 6.7 | 3.6 | 5.0 | |||||
| Lack of staff/pharmacist knowledge | 7.1 | 12.5 | 13.3 | 17.9 | 10.0 | 10.0 | ||||
| Customer response neutral | 2.6 | 3.3 | 7.1 | 13.3 | ||||||
| Customer response negative | 3.4 | 3.6 | 20.0 | 6.6 | ||||||
| Total number of consultations | 91 | 14 | 4 | 15 | 14 | 5 | 10 | 15 | 5 | 9 |
*Average agreement between pairs of raters for each pharmacy.
Assessing compliance with RPSGB guidelines (stage 2)
For stages 2 and 3 a second group was convened, referred to as the Expert Group. This group comprised 10 participants who had expertise either in pharmacy or patient safety including health (1) and industrial psychologists (2); community pharmacists (2); health risk management specialists (anaesthetists (2), clinical pharmacologist (1)) (3); and academic pharmacists (2). The group met once in February 2004 and their three hour meeting comprised two stages (fig 1). The first objective was to assess which consultations for non‐prescription medicines were compliant with the RPSGB guidelines.3 WWHAM was used to assess whether “sufficient information” (as specified in the RPSGB guidelines) was elicited during these consultations. The second objective was to categorise non‐compliant consultations according to human error theory.
The 91 consultations were randomly allocated to one of 10 sets labelled A–J and a duplicate of each set was made. The consultations were not categorised before the allocation process—for example, they were not grouped together on the basis of the type of presentation (product request, request for advice). Each group member was assigned two sets of consultations and asked to assess each consultation in terms of compliance with the RPSGB guidelines for the sale of pharmacy medicines. Group members were supplied with full details of the RPSGB guidelines in order to make this evaluation. Two participants assessed each set of consultations independently. The assessors in each pair had different expertise—for example, no consultation was assessed by two community pharmacists or two anaesthetists.
Categorising consultations using human error theory (stage 3)
During stage 3, consultations that were deemed non‐compliant by the paired assessors were categorised by the same assessors using human error theory. Recognised definitions of unsafe acts (errors) were presented to the group (fig 1).13 Each assessor was required to indicate whether the error was most likely to be a slip, lapse, mistake or violation, and to rate their confidence using a scale of 0–10 where 0 was “confident that non‐compliance was not due to this error” and 10 was “confident that non‐compliance was due to this specific error”.
Analysis of data
The Kappa statistic was used to measure inter‐rater agreement during stages 1 and 2 using SPSS Version 11.5. The 1% significance level was used to ensure scientific rigour due to the large number of tests performed. The association between guideline compliance and type of consultation was assessed using logistic regression and expressed as odds ratios and 95% confidence intervals.
Results
Stage 1: Identifying problems associated with non‐prescription medicine consultations
The number of consultations from each pharmacy ranged from 4 to 15. On average, 83% (n = 75) of consultations were deemed deficient in information collection or advice provision and in 48% the problem was deemed to be due to inadequate questioning (table 1). Lack of knowledge (either pharmacist or pharmacy staff) was identified as a problem in 7% of consultations. Statistically significant agreement (p<0.01) was shown between the raters with 65 of the consultations. Kappa could not be calculated for 15 consultations; however, 14 of these showed 100% agreement between raters.
Stage 2
The Expert Group meeting was attended by nine members. One of the invited risk management specialists could not attend but completed the rating exercise by post. One of the attendees left the meeting early and did not return rating forms for either of their consultation sets.
Of the 10 consultation sets, eight were rated by two group members comprising 73 consultations, 20 of which were rated as guideline compliant by one or both assessors. Although statistically significant (p<0.01), inter‐rater agreement was shown with only one consultation; the median percentage agreement values shown in table 2 provide a better indication of the extent of agreement between raters—that is, the median percentage agreement between raters was high.
Table 2 Assessment of compliance of consultations with RPSGB guidelines (stage 2).
| Consultation set† | Inter‐rater agreement Median (%) [IQR] | No of consultations where inter‐rater agreement (kappa) calculated | Kappa values |
|---|---|---|---|
| A | 75 [66.6–100] | 3 | 1.00*, −0.50, 1.00 |
| B | 66.6 [41.7–87.5] | 2 | 1.00, 0.50 |
| E | 75 [50–100] | 6 | 1.00 (n = 4), 0.00, 0.20 |
| F | 75 [75–100] | 8 | 1.00 (n = 3), 0.50 (n = 4), 0.40 |
| G | 100 [100–100] | 7 | 1.00 (n = 6), 0.5 |
| H | 75 [37.5–100] | 3 | 0.00, 0.20, 0.50 |
| I | 75 [50–87.5] | 6 | 1.00, 0.50 (n = 2), −0.33 (n = 2), 0.20 |
| J | 100 [93.8–100] | 9 | 1.00 (n = 8), 0.50 |
IQR, interquartile range.
*p<0.001.
† Consultation sets C and D excluded as rated by one rater only. Nine consultations in each set except set J (n = 10).
Of the 53 consultations deemed non‐compliant by both raters, 27 (51.9%) were categorised as violations by both raters. Medicine counter assistants were the only members of staff involved in 46 (50.5%) of the consultations. Consultations deemed to be non‐compliant by both raters were significantly more likely to involve only a medicine counter assistant than any other member of staff (or combination of staff) (OR 2.57 (95% CI 1.00 to 6.59, p = 0.05)). A maximum score of 10 (complete confidence that the error is due to a violation) was assigned by both assessors to 22 (81.5%) of these 27 consultations. There was considerable variation between raters in their categorisation of the 26 remaining consultations deemed to involve unintentional errors (that is, non‐violations). No agreement was shown for these consultations in terms of error type. No further statistical analyses were undertaken due to the small numbers involved. Examples of consultations are presented in table 3.
Table 3 Examples of consultations.
| Consultation | Description | Comment |
|---|---|---|
| Set B, Consultation 22 | A female customer aged 40–49 years requests Nurofen (ibuprofen). The medicine counter assistant sells this product without further discussion. | This consultation was rated as a violation, with both assessors assigning a rating of 10. This was a violation because no information was gathered about who would be using the product, nor the indication for use. |
| Set F, Consultation 49 | A female customer aged 30–39 years asks for a tube of Canesten. The medicine counter assistant sells this product without asking any questions or eliciting any information. | This consultation was rated as a violation, with both assessors assigning a rating of 10. This was a violation because no information was gathered about who would be using neither the product nor the indication for use. |
| Set H, Consultation 57 | A female customer aged 60–69 years asks for eardrops. The medicine counter assistant asks whether this is for earwax, which the customer confirms. The customer complains of pain and says that she usually uses Cerumol. The medicine counter assistant says that because there is pain she should not sell Cerumol because there may be an infection. The customer explains that she often has earwax and has previously been told to use Cerumol. The medicine counter assistant repeats what she said about pain. The customer says she is going away on holiday and won't be able to see the GP before she leaves. She asks whether Cerumol is in stock. The medicine counter assistant says yes and places the packet on the counter. The customer is persistent about buying the product so the medicine counter assistant refers to the pharmacist who comes to the counter. The pharmacist states that she is not happy to sell the product and is not prepared to do so. The customer is not happy and leaves saying “I'll just get it somewhere else”. | This consultation was rated fully guideline compliant by both assessors. |
Of the 18 consultations rated by one assessor only (from sets C and D), three were rated as guideline compliant. Fourteen of the non‐compliant consultations were categorised as a violation and the remaining consultation had missing data—that is, no categorisation was provided by the assessor.
Of the 91 consultations originally sampled, 19 (20.9%) involved symptom presentations, 67 (73.6%) were product requests, and five (5.5%) were unclassified. The extent to which consultations were deemed to be guideline compliant is presented in table 4. Consultations involving product requests were significantly less likely to be rated as compliant than those involving symptom presentations by one (OR 0.30, 95% CI 0.10 to 0.95, p = 0.05) or both raters (OR 0.08, 95% CI 0.008 to 0.83, p = 0.03).
Table 4 Percentage compliance with guideline recommendations.
| Guideline relating to: | Compliant Median % [IQR] | Non‐compliant Median % [IQR] | Missing Median % [IQR] |
|---|---|---|---|
| Advice/symptom presentation: ensuring information gathered to make suitable recommendation | 77.8 [66.7–91.7] | 11.1 [5.6–25.0] | 0 [0–11.8] |
| Product request: ensuring information gathered to ensure request is appropriate for customer's needs | 26.3 [19.5–47.2] | 73.7 [50.0–77.8] | 0 [0–5.6] |
| Specific patient groups: individuals who may require additional care such as the elderly | 63.2 [59.5–77.8] | 27.8 [16.7–37.9] | 0 [0–13.9] |
| Specific drug groups: drugs or therapeutic categories that may require additional care with supply and or use such as drugs which may be abused | 63.2 [38.9–88.9] | 26.3 [5.6–44.4] | 0 [0–8.2] |
IQR, interquartile range.
Discussion
Main findings
Stage 1
Most consultations were deemed deficient due to inadequate information gathering or advice provision. Similar findings have been shown with community pharmacists and simulated patients consultations.18 Previous research has highlighted the difficulties with providing too much or too little advice to customers and patients in pharmacy settings.19,20
In the context of human error theory, suboptimal communication could be due to violation if a medicine counter assistant was aware that specific information should be elicited from and provided to a customer and chose not to obtain or give this information (that is, failure to apply a normally good rule). In these circumstances, further exploration of the medicine counter assistant's reason for committing the violation would be necessary. External factors could influence their decision to commit the violation, including time constraints and customer pressure. Suboptimal communication could also be classified as a different type of rule based mistake from a violation—namely, that the rules of how to engage and communicate with customers are inadequate or that the rule has been misapplied in a particular context. This could result from latent conditions within the organisation (the pharmacy or company)—for example, lack of appropriate training to enable staff to undertake their duties.
Stages 2 and 3
Few consultations were deemed compliant with the RPSGB guidelines for the supply of pharmacy medicines and half of all non‐compliant consultations were categorised as violations. With human error theory, a violation occurs when an individual deliberately and knowingly chooses not to follow a guideline or rule.13 There is little doubt that it is easier to identify a violation than a slip, lapse or mistake. These latter types of error are cognitive failures and are either due to actions not going as planned (slips/lapses) or plans being inadequate to achieve the objective (mistakes).13 In order to establish whether a slip or lapse has occurred, it would be necessary to know what plan had been formulated in the mind of the medicine counter assistant, whether this plan was adequate in the first place, and whether it was acted on appropriately if it was adequate. A violation, on the other hand, is more easily identified, particularly if standard practices or formal rules and procedures are available as a point of comparison. More detailed knowledge is required of the action plans of medicine counter assistants in terms of their knowledge and their skills before more accurate assessments of error types can be made, and this is currently being investigated by the research team.
Most errors occurred with product requests; these consultations may make it more difficult to follow guidelines. Medicine counter assistants' awareness of customer resistance to questioning and perceived limited ability in information gathering and advice provision have been reported previously.21,22 Responding to product requests is more difficult than consultations involving symptom presentations22 with regard to eliciting sufficient information to assist decision making.
Methodological limitations
The sole data source for this analysis was written observation notes made by the researcher (observer) for each consultation during the observation study. These notes were limited in detail and assessment of their validity was not possible. The decision to use human error theory to categorise the incidents was made after these data had been collected, so the notes were unbiased with regard to the hypothesis being tested in this study—that is, that the consultations could be categorised using human error theory. The observer was unaware of human error theory at the time of the original data collection. A random 25% sample of observed consultations was used to enable the Expert Group to complete stages 2 and 3 within the 3 hour duration of the meeting.
The Expert Group were experts in their core disciplines (for example, community pharmacists, health psychologists). For many of them, human error theory was a new concept and their ability to categorise the consultations might have varied. Likewise, non‐pharmacist group members were unfamiliar with the RPSGB's Code of Ethics and community pharmacy activities in general, so they were unlikely to be influenced by common interpretations of the guidelines and perhaps' were more strict in their interpretation of the guidelines. As such, it was perhaps unsurprising that there was considerable variation across assessors in their strength of confidence with their selection of error category for non‐compliant consultations. This may have been attributable to the definitions of error categories used and/or the examples used to illustrate them. However, the use of mixed pairs of assessors in terms of expertise may have given a more balanced decision for each consultation than using pairs of assessors with the same expertise. No assessment was made of the validity of categorising the consultations during stages 2 and 3, and this omission would need to be addressed in future studies.
In these consultations we assumed that pharmacy staff intended to perform according to the standards of best practice—that is, the RPSGB Code of Ethics. Most consultations for non‐prescription medicines involve medicine counter assistants who should have completed an accredited medicine counter assistant qualification within the first 3 years of their employment. The guidelines presented in the Code of Ethics are for pharmacists and, as such, medicine counter assistants and other support staff may be unaware of them or may not acknowledge them as being relevant to their everyday practice. These guidelines represent the professional standards with which pharmacists in Britain must comply. Even if pharmacy support staff have no specific knowledge of the RPSGB guidelines, the pharmacist has a professional duty to ensure that their staff are compliant with them. Pharmacists should therefore reinforce the behaviour of their support staff to comply with these guidelines. Although no data were collected during the observation study with regard to pharmacy staff knowledge of these guidelines, a subsequent study (Watson, unpublished) found that while only 20% of support staff reported having read the RPSGB guidelines, the majority reported using WWHAM. In community pharmacy a violation would occur if the member of pharmacy staff involved in the consultation opted not to comply with the RPSGB guidelines for the supply of pharmacy medicines or did not elicit sufficient information (e.g. WWHAM) during the consultation. If the member of staff was unaware of these guidelines, then their “non‐compliance” could not be categorised as a violation and would lead to misclassification of the error in this study. For the purpose of this study it was assumed that the members of staff were aware of the WWHAM guidelines (in terms of “sufficient information”) which would be used to comply with the RPSGB guidelines.
The member of staff involved with the consultations might have had additional information about the customer which was not apparent to the observer but which may have influenced their behaviour and outcome of the consultation. For example, a customer might be well known to a medicine counter assistant as a regular purchaser of a particular product with the suitability of the product for the customer having been established during the initial consultation.
This study shows that many consultations for non‐prescription medicines do not comply with current professional guidelines. The reasons for non‐compliance are likely to be many. If non‐compliance was intentional, then further exploration is required of why pharmacy staff choose to violate these professional rules. This might reflect their attitude towards them or a lack of understanding of their relevance to the supply of non‐prescription medicines. It may be that staff do not feel able to comply with the RPSGB guidelines because of factors beyond their personal control—for example, when dealing with demanding customers, pressures of time, or understaffing. These issues could be considered to be error enforcing conditions and need to be addressed through organisational interventions targeted at training or improving staffing levels. However, these questions can only be addressed with further research using theoretical approaches such as human error theory to explore the influences on staff and their decision making in this context.
There are similarities between these types of consultations and the patient pressure experienced by some general practitioners (GPs) to prescribe a particular treatment. GPs have reported that their decision to prescribe is influenced by the desire to maintain good relationships with their patients,23 and similar concerns may influence the supply of non‐prescription medicines by pharmacy staff. Patients who have previously received a prescribed medicine for a particular condition may be more likely to demand or expect similar treatment in the future,24 whether appropriate or not, and similar behaviour may occur in the community pharmacy setting. Changes in cultural views have been highlighted as necessary to address patient demand for antibiotic prescriptions,25 and these may also be necessary to change customers' requests for particular non‐prescription medicines. Furthermore, customers who present in pharmacies may not give their full “agenda” with pharmacy staff, as has been shown with some GP consultations.26 Patient satisfaction is influenced by doctor‐patient communication27 and it is likely that customer satisfaction will also be affected by pharmacy staff's communication skills.
Historically, human error theory has been used by high risk industries to explore the cause(s) of adverse events after they had occurred. In the current study the theory was used to identify problems associated with consultations for non‐prescription medicines which may not necessarily have resulted in adverse events. Human error theory might be useful in developing strategies to enhance the appropriate supply of these drugs. However, this study generated additional questions about this behaviour and the application of this theory which need to be addressed before the full worth of this theoretical approach can be confirmed.
Relevance to other healthcare professionals and settings
Although this study was related to the supply of non‐prescription medicines from community pharmacies, similar methods could be used to explore healthcare provision by healthcare professionals in other settings. For example, GP prescribing behaviour could be explored in relation to compliance with national service framework recommendations and whether non‐compliance is intentional (violation) or due to lack of (or incorrect knowledge of) the guidelines (knowledge based mistake). This study shows how theory was used to explore existing behaviour in order to identify possible directions for future research.
Acknowledgements
The authors thank the pharmacists and support staff who participated in the observation study from where the consultations were generated, the members of both groups who participated in the study, and Dr Amanda J Lee who provided statistical advice.
Footnotes
* These guidelines refer to pharmacy medicines; however, for this study, the guidelines were applied to consultations involving pharmacy and general sales list medicines.
Dr M Watson is funded by a Special Medical Research Council Training Fellowship in Health Services Research. The costs of the study were met by funds from the Department of General Practice and Primary Care, University of Aberdeen.
Competing interests: none declared.
References
- 1.Institute of Medicine To err is human. Building a safer health system. Washington: National Academy Press, 1999
- 2.Department of Health Organisation with a memory. London: Department of Health, 2000
- 3.Royal Pharmaceutical Society of Great Britain Medicines, ethics and practice. Report No 29. London: RPSGB, 2005
- 4.Sinclair H K, Bond C, Hannaford P. Over‐the‐counter ibuprofen: how and why is it used? Int J Pharm Pract 20008121–127. [Google Scholar]
- 5.Ferris D, Nyirjesy P, Sobel J.et al Over‐the‐counter antifungal drug misuse associated with patient‐diagnosed vulvovaginal candidiasis.Obstet Gynaecol 200299419–424. [DOI] [PubMed] [Google Scholar]
- 6.Watson M C, Bond C M, Grimshaw J M.et al Educational strategies to promote evidence‐based community pharmacy practice: a cluster randomised controlled trial (RCT).Fam Pract 200219529–536. [DOI] [PubMed] [Google Scholar]
- 7.Anon Can your pharmacist cope? Which? 200410–13.
- 8.Anon NPA launches training with a W‐WHAM.Pharm J 198924340 [Google Scholar]
- 9.Watson M C, Bond C M, Grimshaw J M.et al Factors predicting the guideline compliant supply (or non‐supply) of non‐prescription medicines in the community pharmacy setting.QSHC 20061553–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Watson M C, Bond C M. The evidence based supply of non‐prescription medicines: barriers and beliefs. IJPP 20041265–72. [Google Scholar]
- 11.Eccles M, Grimshaw J, Walker A.et al Changing the behaviour of healthcare professionals: the use of theory in promoting the uptake of research findings.J Clin Epidemiol 200558107–112. [DOI] [PubMed] [Google Scholar]
- 12.MRC A framework for development and evaluation of RCTs for complex interventions to improve health. London: MRC, 2000
- 13.Reason J.Human error. Cambridge: Cambridge University Press, 1990
- 14.Reason J. Human error: models and management.BMJ 2000320768–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bogner M S.Human error in medicine. 1st ed. Hillsdale, New Jersey: Lawrence Erlbaum Associates, 1994
- 16.Watson M C, Bond C M.Identifying barriers to evidence based practice in the community pharmacy setting: an ethnographic study. Report No CZG/2/74 2002
- 17.Kirkwood B R.Essentials of medical statistics. Oxford: Blackwell Scientific Publications, 1988
- 18.Rutter P M, Horsley E, Brown D T. Evaluation of community pharmacists' recommendations to standardized patient scenarios.Ann Pharmacother 2004381080–1085. [DOI] [PubMed] [Google Scholar]
- 19.Pilnick A. ‘Why didn't you say just that?' Dealing with issues of asymmetry, knowledge and competence in the pharmacist/client encounter.Sociol Health Illness 19982029–51. [Google Scholar]
- 20.Airaksinen M, Ahonen R, Enlund H. The “Questions to ask about your medicines” campaign. An evaluation of pharmacists and the public's response. Med Care 199836422–427. [DOI] [PubMed] [Google Scholar]
- 21.Bissell P, Ward P, Noyce P. Appropriateness measurement: application to advice‐giving in community pharmacies.Soc Sci Med 200051343–359. [DOI] [PubMed] [Google Scholar]
- 22.Seston E, Nicolson M, Hassell K.et al “Not just someone stood behind the counter”: The views and experiences of medicines counter assistants.J Soc Admin Pharmacy 200118122–128. [Google Scholar]
- 23.Stevenson F A, Greenfield S M, Jones M.et al GPs' perceptions of patient influence on prescribing.Fam Pract 199916255–261. [DOI] [PubMed] [Google Scholar]
- 24.Britten N, Ukoumunne O. The influence of patients' hopes of receiving a prescription on doctors' perceptions and the decision to prescribe: a questionnaire survey.BMJ 19973151506–1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Avorn J, Solomon D H. Cultural and economic factors that (mis)shape antibiotic use: the nonpharmacologic basis of therapeutics.Ann Intern Med 2000133128–135. [DOI] [PubMed] [Google Scholar]
- 26.Barry C, Bradley C P, Britten N.et al Patients' unvoiced agendas in general practice consultations: qualitative study.BMJ 20003201246–1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Williams S, Weinman J, Dale J. Doctor‐patient communication and patient satisfaction: a review.Fam Pract 199815480–492. [DOI] [PubMed] [Google Scholar]