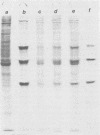
Abstract
Inhibition of host cellular protein synthesis by vesicular stomatitis virus (VSV) has been suggested to be primarily the result of competition for ribosomes between cellular and viral mRNAs (H. F. Lodish and M. Porter, J. Virol., 36:719-733, 1980; Lodish and Porter, J. Virol. 38:504-517, 1981). This hypothesis was investigated by regulating the extent of VSV mRNA synthesis through the use of defective interfering particles. Although intracellular VSV mRNA concentrations decreased by as much as a factor of 14 at high multiplicities of infection of defective interfering particles, the inhibition of host cell protein synthesis by VSV decreased by a maximum of only 10%. The data also indicated that under these conditions the protein-synthesizing capacity of the cells was not exhausted. We concluded that competition for cellular ribosomes could not have been the major factor in the inhibition of host cell protein synthesis by VSV. This conclusion was further supported by inhibition data obtained with VSV mutants. The ts G22 mutant, defective in replication but not in primary transcription, inhibited host protein synthesis at the nonpermissive temperature (39 degrees C) to the same extent as did wild-type virus, even though it generated only 30 to 50% of the amount of viral mRNA as did wild-type virus. Conversely, in infections with the R1 mutant, which did not inhibit host cell protein synthesis, the amount of total and polysome-bound viral mRNA was indistinguishable from that obtained in infections by wild-type virus.
Full text
PDF



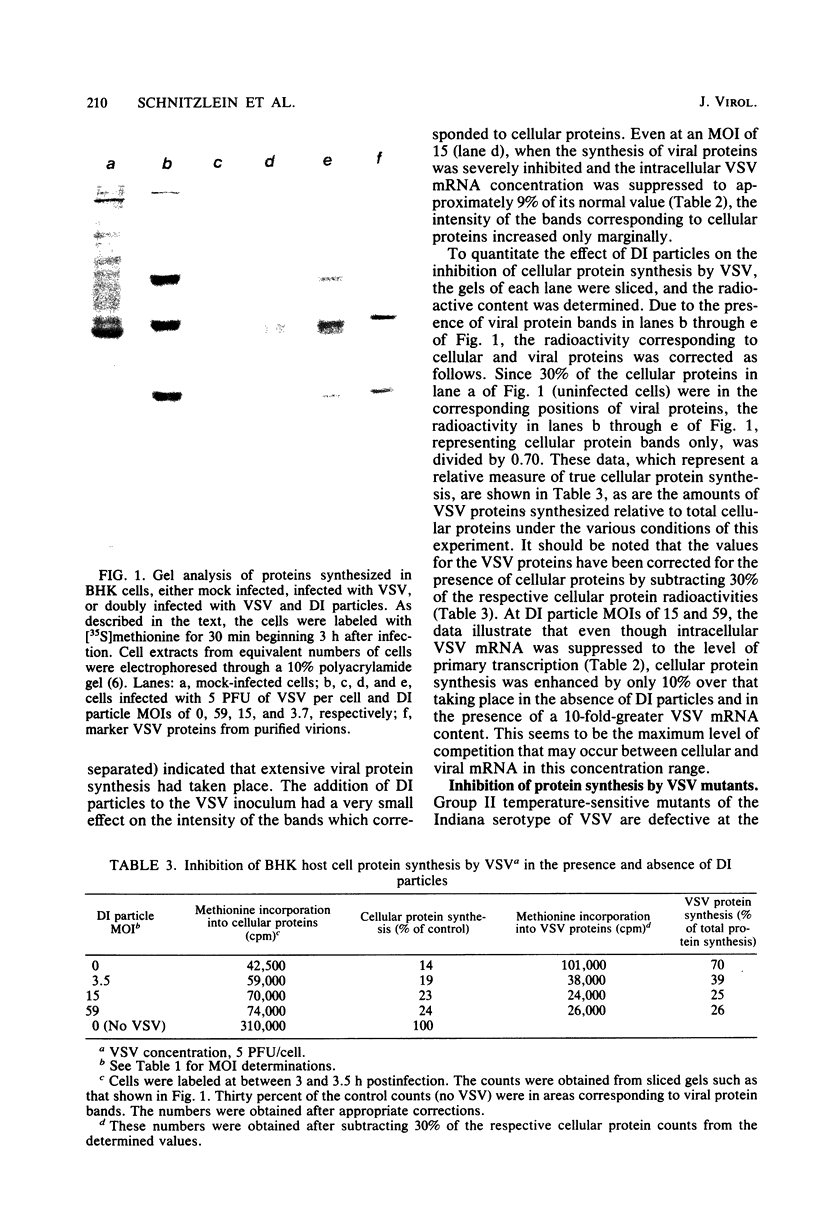
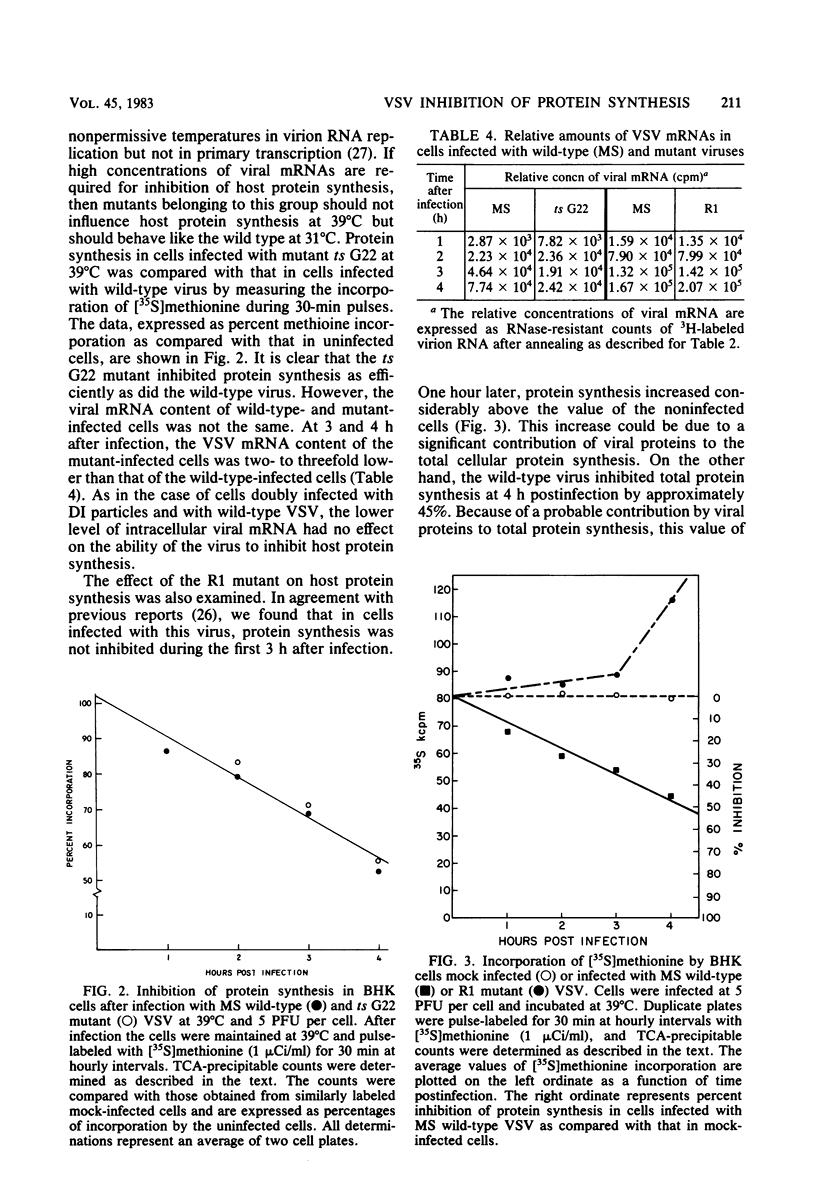



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BELLETT A. J., COOPER P. D. Some properties of the transmissible interfering component of vesicular stomatitis virus preparations. J Gen Microbiol. 1959 Dec;21:498–509. doi: 10.1099/00221287-21-3-498. [DOI] [PubMed] [Google Scholar]
- Baxt B., Bablanian R. Mechansims of vesicular stomatitis virus-induced cytopathic effects. II. Inhibition of macromolecular synthesis induced by infectious and defective-interfering particles. Virology. 1976 Jul 15;72(2):383–392. doi: 10.1016/0042-6822(76)90167-7. [DOI] [PubMed] [Google Scholar]
- Bay P. H., Reichmann M. E. UV inactivation of the biological activity of defective interfering particles generated by vesicular stomatitis virus. J Virol. 1979 Dec;32(3):876–884. doi: 10.1128/jvi.32.3.876-884.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang A. S., Manders E. K. Ribonucleic acid synthesis of vesicular stomatitis virus. IV. Transcription by standard virus in the presence of defective interfering particles. J Virol. 1972 Jun;9(6):909–916. doi: 10.1128/jvi.9.6.909-916.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jen G., Thach R. E. Inhibition of host translation in encephalomyocarditis virus-infected L cells: a novel mechanism. J Virol. 1982 Jul;43(1):250–261. doi: 10.1128/jvi.43.1.250-261.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leamnson R. N., Reichmann M. E. The RNA of defective vesicular stomatitis virus particles in relation to viral cistrons. J Mol Biol. 1974 Jan 5;85(4):551–568. doi: 10.1016/0022-2836(74)90315-5. [DOI] [PubMed] [Google Scholar]
- Lewis J. B., Esche H., Smart J. E., Stillman B. W., Harter M. L., Mathews M. B. Organization and expression of the left third of the genome of adenovirus. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 1):493–508. doi: 10.1101/sqb.1980.044.01.052. [DOI] [PubMed] [Google Scholar]
- Lodish H. F., Froshauer S. Rates of initiation of protein synthesis by two purified species of vesicular stomatitis virus messenger RNA. J Biol Chem. 1977 Dec 25;252(24):8804–8811. [PubMed] [Google Scholar]
- Lodish H. F., Porter M. Translational control of protein synthesis after infection by vesicular stomatitis virus. J Virol. 1980 Dec;36(3):719–733. doi: 10.1128/jvi.36.3.719-733.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodish H. F., Porter M. Vesicular stomatitis virus mRNA and inhibition of translation of cellular mRNA--is there a P function in vesicular stomatitis virus? J Virol. 1981 May;38(2):504–517. doi: 10.1128/jvi.38.2.504-517.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marvaldi J., Sekellick M. J., Marcus P. I., Lucas-Lenard J. Inhibition of mouse L cell protein synthesis by ultraviolet-irradiated vesicular stomatitis virus requires viral transcription. Virology. 1978 Jan;84(1):127–133. doi: 10.1016/0042-6822(78)90224-6. [DOI] [PubMed] [Google Scholar]
- McAllister P. E., Wagner R. R. Differential inhibition of host protein synthesis in L cells infected with RNA - temperature-sensitive mutants of vesicular stomatitis virus. J Virol. 1976 May;18(2):550–558. doi: 10.1128/jvi.18.2.550-558.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGowan J. J., Wagner R. R. Inhibition of cellular DNA synthesis by vesicular stomatitis virus. J Virol. 1981 Apr;38(1):356–367. doi: 10.1128/jvi.38.1.356-367.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McSharry J. J., Choppin P. W. Biological properties of the VSV glycoprotein. 1. Effects of the isolated glycoprotein on host macromolecular synthesis. Virology. 1978 Jan;84(1):172–182. doi: 10.1016/0042-6822(78)90229-5. [DOI] [PubMed] [Google Scholar]
- Nuss D. L., Oppermann H., Koch G. Selective blockage of initiation of host protein synthesis in RNA-virus-infected cells. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1258–1262. doi: 10.1073/pnas.72.4.1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrault J., Holland J. J. Absence of transcriptase activity or transcription-inhibiting ability in defective interfering particles of vesicular stomatitis virus. Virology. 1972 Oct;50(1):150–170. [PubMed] [Google Scholar]
- Pringle C. R. Genetic characteristics of conditional lethal mutants of vesicular stomatitis virus induced by 5-fluorouracil, 5-azacytidine, and ethyl methane sulfonate. J Virol. 1970 May;5(5):559–567. doi: 10.1128/jvi.5.5.559-567.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichmann M. E., Bishop D. H., Brown F., Crick J., Holland J. J., Kang C. Y., Lazzarini R., Moyer S., Perrault J., Prevec L. Proposal for a uniform nomenclature for defective interfering viruses of vesicular stomatitis virus. J Virol. 1980 Jun;34(3):792–794. doi: 10.1128/jvi.34.3.792-794.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichmann M. E., Schnitzlein W. M. Defective interfering particles of rhabdoviruses. Curr Top Microbiol Immunol. 1979;86:123–168. doi: 10.1007/978-3-642-67341-2_4. [DOI] [PubMed] [Google Scholar]
- Schnitzlein W. M., Reichmann M. E. Inhibition of vesicular stomatitis virus replication by adenosine. Virology. 1980 May;103(1):123–137. doi: 10.1016/0042-6822(80)90131-2. [DOI] [PubMed] [Google Scholar]
- Schnitzlein W. M., Reichmann M. E. The size and the cistronic origin of defective vesicular stomatitis virus particle RNAs in relation to homotypic and heterotypic interference. J Mol Biol. 1976 Mar 5;101(3):307–325. doi: 10.1016/0022-2836(76)90150-9. [DOI] [PubMed] [Google Scholar]
- Sekellick M. J., Marcus P. I. Viral interference by defective particles of vesicular stomatitis virus measured in individual cells. Virology. 1980 Jul 15;104(1):247–252. doi: 10.1016/0042-6822(80)90385-2. [DOI] [PubMed] [Google Scholar]
- Shmookler R. J., Buss J., Green M. H. Properties of the polyoma virus transcription complex obtained from mouse nuclei. Virology. 1974 Jan;57(1):122–127. doi: 10.1016/0042-6822(74)90113-5. [DOI] [PubMed] [Google Scholar]
- Stampfer M., Baltimore D., Huang A. S. Absence of interference during high-multiplicity infection by clonally purified vesicular stomatitis virus. J Virol. 1971 Mar;7(3):409–411. doi: 10.1128/jvi.7.3.409-411.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanners C. P., Francoeur A. M., Lam T. Analysis of VSV mutant with attenuated cytopathogenicity: mutation in viral function, P, for inhibition of protein synthesis. Cell. 1977 Jun;11(2):273–281. doi: 10.1016/0092-8674(77)90044-7. [DOI] [PubMed] [Google Scholar]
- Unger J. T., Reichmann M. E. RNA synthesis in temperature-sensitive mutants of vesicular stomatitis virus. J Virol. 1973 Sep;12(3):570–578. doi: 10.1128/jvi.12.3.570-578.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weck P. K., Wagner R. R. Inhibition of RNA synthesis in mouse myeloma cells infected with vesicular stomatitis virus. J Virol. 1978 Mar;25(3):770–780. doi: 10.1128/jvi.25.3.770-780.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wertz G. W., Youngner J. S. Inhibition of protein synthesis in L cells infected with vesicular stomatitis virus. J Virol. 1972 Jan;9(1):85–89. doi: 10.1128/jvi.9.1.85-89.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]