Abstract
Emergency ultrasonography is an appealing imaging modality in paediatric emergencies, given its non‐invasive nature and potential as an adjunct to physical examination in a setting where rapid decisions need to be made. This review of a case series describes the applications, versatility, and limitations of emergency physician led ultrasonography in a paediatric resuscitation room in a sub‐Saharan African setting.
Keywords: paediatric emergency medicine, ultrasound, sub‐Saharan Africa
Emergency ultrasonography (EU) is the practice of ultrasonographic examination, interpretation, and clinical correlation in an emergency setting where immediate diagnosis is necessary to improve or expedite patient care. Emergency physicians (EP) recognise the importance of early decision making and timely interventions necessary to prevent avoidable mortality or morbidity. This is especially relevant in the seriously ill and injured child where there is potential for rapid deterioration. For this reason, the use of real time ultrasonography to supplement clinical assessment has become increasingly popular in emergency medicine practice. EU is relatively new and how it can be integrated into EP clinical training and practice are yet to be defined. Of importance are issues concerning the indications for its use, user accreditation, and cost effectiveness. There have been few reports on the routine practice of EP led ultrasonography in the early management of the critically ill child. Evidence supporting its use will be necessary to answer questions regarding crossing specialty boundaries.
The aim of this article is to demonstrate the applications, versatility, and limitations of EU as performed by a trained medical "non‐radiologist" in the resuscitation room of a paediatric ED in a sub‐Saharan African setting. It is intended for specialists involved in the early clinical care of the critically sick or injured child, whether in a resource poor or developed country. We assume the reader has a basic knowledge of the principles and techniques of EU.
Medical "non‐radiologist" training
There were 536 supervised paediatric ultrasound scans made during a 6 month period of apprenticeship (by SA) with a consultant radiologist (SK) covering the cranium, eye, chest, cardiac, abdominal, pelvic, and musculoskeletal systems in acute, sub‐acute, and chronic conditions. Following this initial training, approximately 20 scans per week were performed and interpreted in the paediatric ED (by SA) using a portable ultrasound device (Sonosite 180 plus 4–7 MHz). The clinical correlation of performed scans was validated by an inpatient team of consultant paediatricians and paediatric surgeons. After 18 months of EU experience, two paediatricians were trained in EU (by SA), and they are now proficient in its use. The opportunities for training others in EU, such as medical students, doctors, and radiographers are considerable in Malawi given the varied case mix and considerable throughput of sick patients. In addition, the characteristics of the device itself (portable, computer connectivity, inbuilt memory, and video output) allows for simultaneous or large group training.
METHODS
In Malawi, a sub‐Saharan African country of 12 million people, there are four tertiary hospitals, 40 district general hospitals, 510 primary healthcare facilities, and one trained consultant radiologist (SK). The Queen Elizabeth Central Hospital is the tertiary centre serving the southern region of the country, with a catchment population of up to two million people. Its paediatric emergency department (ED) receives approximately 75 000 acutely sick and injured children a year, of whom 1000 will require resuscitation room care. On a typical day, two EU scans will be required in the resuscitation room. The cases described in this article represent the commonest paediatric emergency situations in which EU was found to be most useful over a 2 year period (April 2003 to April 2005) in the resuscitation area of the ED. The EP (SA) performed the scans, with review and interpretation of the images by the EP, radiologist (SK) and paediatrician (EM). A summary table of indications for EU in children in a sub‐Saharan setting is included in table 1.
Table 1 Indications for emergency ultrasound in children in a sub‐Saharan setting (Malawi).
| Symptom | Potential diagnosis | |||
|---|---|---|---|---|
| Airway | Stridor (+ neck swelling) | Retropharyngeal abscess, collar stud abscess with retropharyngeal extension, relationship of neck swelling to airway | ||
| Breathing | Cyanosis | Complex congenital cyanotic heart disease | ||
| Respiratory failure | Massive pleural effusion (+/− EU guided chest drain insertion) | |||
| Heart failure | Dilated cardiomyopathy, rheumatic heart disease, endocarditis, intracavity masses | |||
| Circulation | Shock | Tamponade (+/− EU guided pericardiocentesis), abdominal haemorrhage (free fluid +/− solid organ injury), cardiac filling pressures | ||
| Neurological disability | Coma/convulsions | Cerebral abscess (open fontanelle), subdural empyema (open fontanelle), raised intra‐cranial pressure (optic nerve sheath diameter measurement) |
CASES IN WHICH EU WAS FOUND TO BE USEFUL
Airway: stridor
EU is useful in assessing neck swellings by showing their echogenicity, shape, size, continuity, and relationship with the airway, carotid sheath, thyroid, peritonsillar region, and retropharyngeal and parapharyngeal spaces. Localised "superficial" pyogenic adenopathy can usually be diagnosed clinically; however, determining a "deeper" infection in the neck often requires computerised tomography (CT) scanning, a facility not readily available in our setting, hence the importance of ultrasound in the detection of a retropharyngeal abscess.
A child with a large neck swelling in the left anterior triangle who presented with difficulty in swallowing and breathing is shown (fig 1A). EU showed a large complex mass with a hyperechoic rim, within which were several distinct anechoic areas (arrow), characteristic of contained pus within an abscess cavity (fig 1B). A pyogenic cervical lymph node abscess was later drained in the operating theatre. Submandibular lymph node abscesses are common in Malawi, and occasionally breach platysma, extending into the parapharyngeal and retropharyngeal spaces, where they may compromise the upper airway. These collar stud abscesses can be excluded by EU where urgent CT scanning is not available.
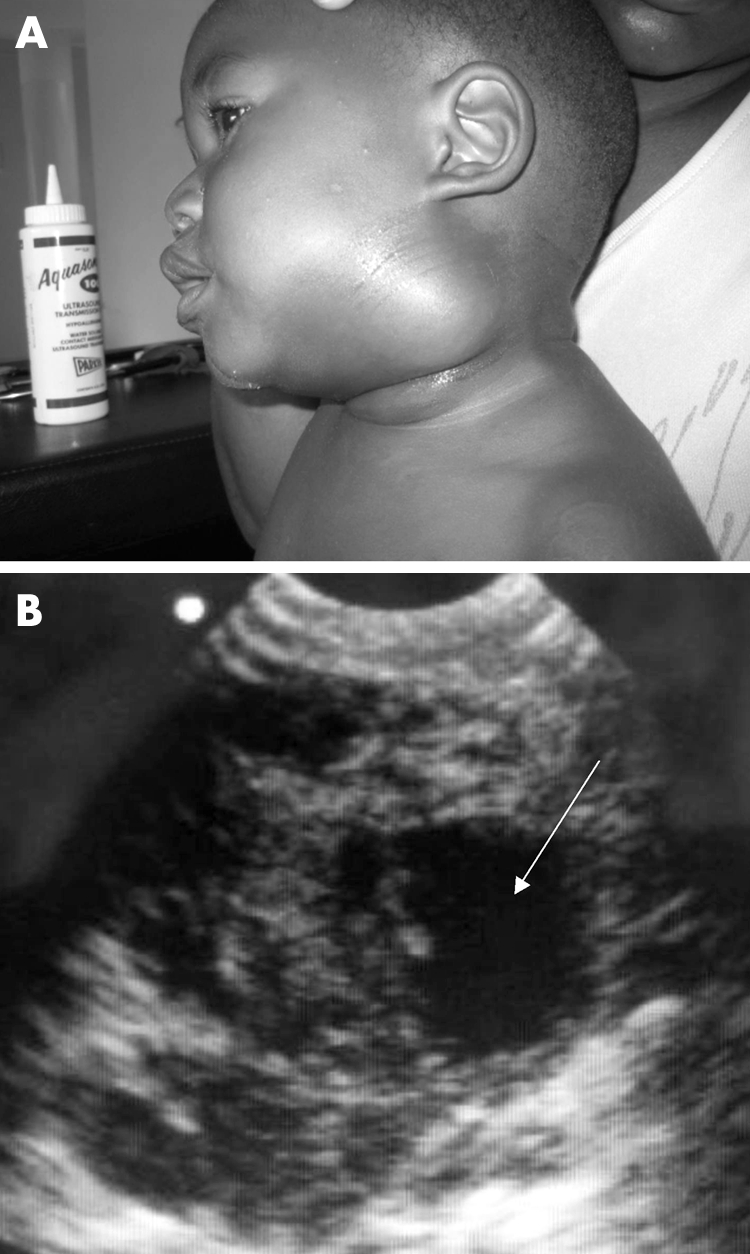
Figure 1 (A) Pyogenic lymph node; (B) EU image of complex mass. Photo reproduced with permission.
A child presented with symptoms of partial upper airway obstruction and superior vena cava syndrome (fig 2A). Note (fig 2B) the presence of two discrete homogenously isoechoic masses (arrows). Real time sonography in this situation was useful in demonstrating that compression of the airway was extrinsic, and maximal patency in both inspiration and expiration could be achieved when the child was positioned upright and slightly forward with the head supported. Following review of the patient and real time EU images by a senior anaesthetist, we were satisfied that management of this potentially difficult airway could be managed conservatively. Later histopathology confirmed the clinical suspicion of Kaposi's sarcoma lymph node disease.
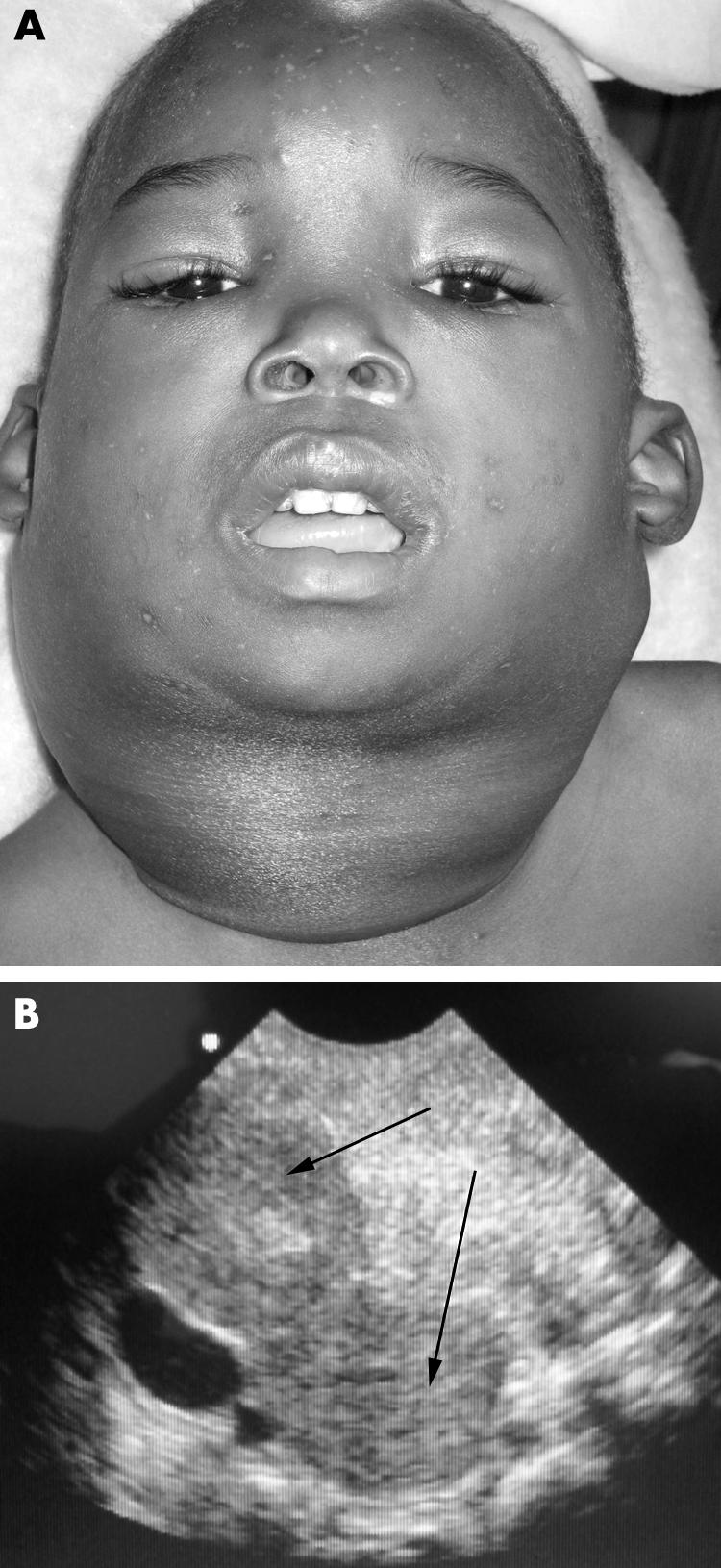
Figure 2 (A) Kaposi's sarcoma lymph node disease; (B) EU image of malignant lymphadenopathy. Photo reproduced with permission.
Breathing: cyanosis
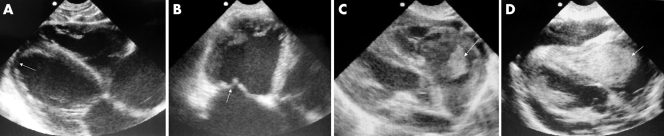
A 3 year old child presented to the ED with a short history of diarrhoea, and at triage was found to be blue, tachycardic, and unresponsive. In the resuscitation room he had a clear airway and increased effort of breathing on oxygen saturation of 40% in air. Air entry was good, lung fields were clear, and blood pressure was normal, but a delayed peripheral capillary refill time was noted. His blood glucose was normal. On closer inspection, he had digital clubbing and Harrison's sulci. No cardiac murmur was detected, although his heart rate was 185 beats/min and oxygen saturation was 40% despite supplementary oxygen. Echocardiography was performed. A large high ventricular septal defect with an over‐riding aorta was noted (fig 3A). A diagnosis of a hypercyanotic episode associated with tetralogy of Fallot was made, oxygen was discontinued, a small dose of morphine administered, and the child's knees brought up to the chest. His condition improved rapidly and he was admitted to the ward (fig 3B). A "tet" spell, as a first presentation of Fallot's, is not unusual in a sub‐Saharan setting where contact with health services can be minimal until a life threatening situation arises. EU in this case was immediately diagnostic and may have prevented possible over‐zealous and potentially harmful resuscitative measures.
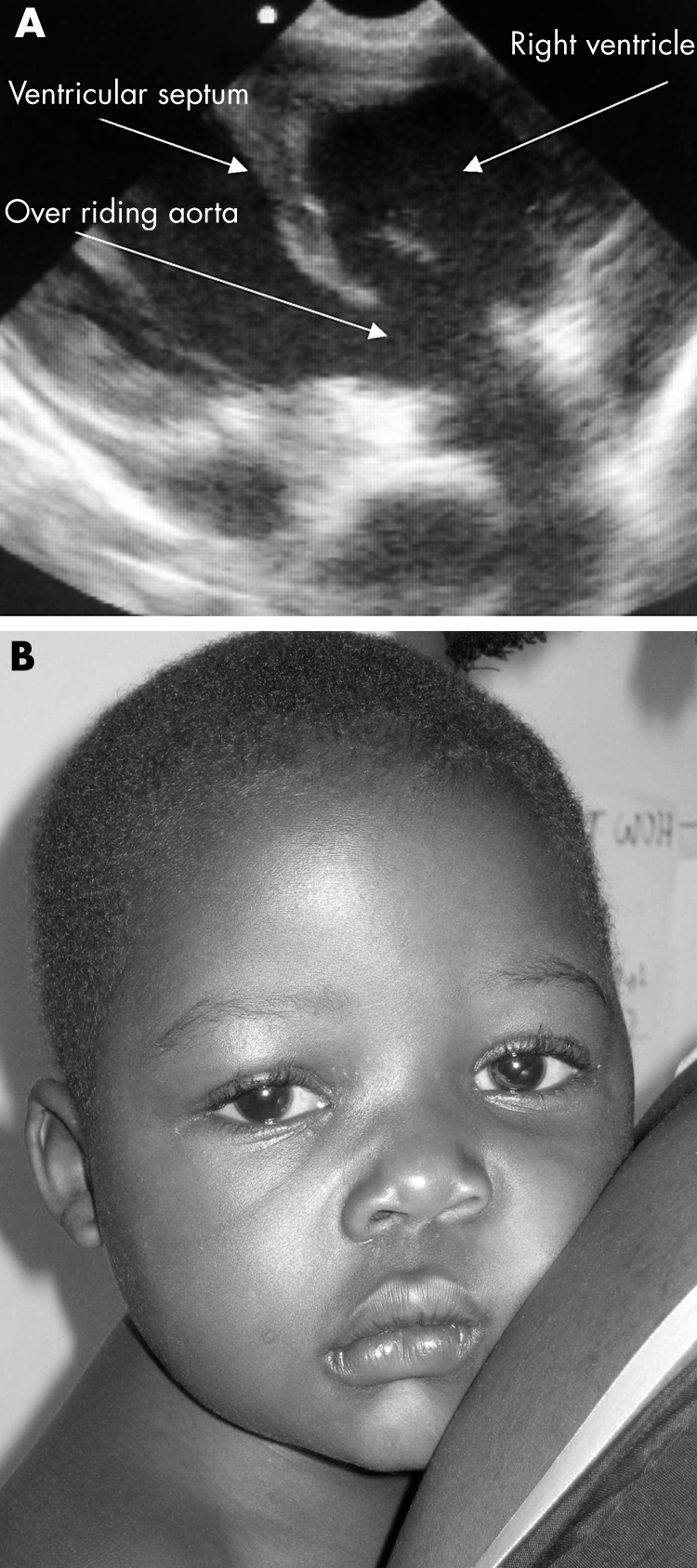
Figure 3 (A) EU image of over‐riding aorta; (B) patient with tetralogy of Fallot with central cyanosis. Photo reproduced with permission.
Breathing: respiratory failure secondary to severe pulmonary oedema
In our practice, mobile radiography is generally unavailable and children are often too unstable to travel to the radiology department for a chest radiograph. EU can facilitate early diagnosis and influence treatment decisions when there is doubt over the primary cause of respiratory failure, such as cardiac or pulmonary failure, where presenting symptoms and signs can overlap. In the older child, in Malawi, new onset heart failure is often the result of myocarditis or dilated cardiomyopathy, although an echocardiogram cannot differentiate between the two. Cases of chronic congestive cardiac failure in the older child are usually due to long standing rheumatic heart disease and these children are often referred for tertiary hospital care when the clinical condition worsens acutely despite maximum medical therapy at a district hospital. EU can provide useful information as to whether decompensation is due to an additional insult, such as bacterial endocarditis, or deteriorating myocardial function. A crude description of overall contractility during real time two dimensional EU can be useful if markedly abnormal, for example, if it is globally hypokinetic or akinetic, suggesting a primary cardiac pathology.
Four of our patients are shown (fig 4A–D). They were all boys aged between 7 and 11 years who presented with features of pneumonia (fever, productive cough, tachypnoea, focal chest signs, and hypoxaemia) in addition to features of predominantly right heart failure (raised jugular venous pressure (JVP), s3 gallop, and generalised oedema).
Figure 4 EU image of (A) dilated cardiomyopathy; (B) rheumatic mitral valve and dilated cardiomyopathy; (C) tricuspid valve vegetation; (D) intracavity mass.
An apical four chamber view of a dilated cardiomyopathy is shown (fig 4A). Note the four dilated chambers with a thinned myocardium (arrow). Real time scanning showed severe global hypokinesis. Long standing thickened and incompetent rheumatic mitral valves (fig 4B, arrow) can produce similar features to a dilated cardiomyopathy and can be a diagnostic pitfall. A large irregular hyperechoic mass attached to the tricuspid valve, which prolapsed in and out of the right ventricle during each cardiac cycle on two dimensional real time scanning, was found in the third child (fig 4C). This child was treated for infective endocarditis on the basis of the EU diagnosis. Subsequently S faecalis was identified on blood culture. The fourth child had a large homogenous right atrial mass (fig 4D), which was compromising right ventricular diastolic filling and right ventricular outflow tract ejection. Note also the associated global pericardial effusion. This child responded to chemotherapy for Burkitts' lymphoma and is one of the few cases of intracardiac Burkitts lymphoma to be reported.1
Breathing: respiratory failure secondary to massive pleural effusion with mediastinal shift
EU can be useful in the rapid identification and precise localisation of pleural effusions. Clinical examination can be inadequately sensitive in young children in differentiating lobar collapse from consolidation and pleural effusion. Radiographs are useful, but may not demonstrate fluid collections of less than 300–400 ml.2 Early diagnosis and EU guided thoracocentesis can be both diagnostic and immediately therapeutic. In Malawi, children often attend for hospital care late in the disease process and it is not unusual at presentation to find large collections of fluid in a hemithorax causing mediastinal shift and life threatening hypoxaemia. These pleural effusions are usually infective (commonly S pneumoniae or Mycobacterium tuberculosis) or malignant in origin. A transverse view taken between the fourth and fifth ribs on the left side in a 6 year old boy with Burkitt's lymphoma is shown (fig 5). Note the large area of anechoic hemithorax, which represents a massive pleural effusion, and the echogenic collapsed "free floating" lung (arrow). This child's immediate condition improved rapidly following insertion of a chest drain.
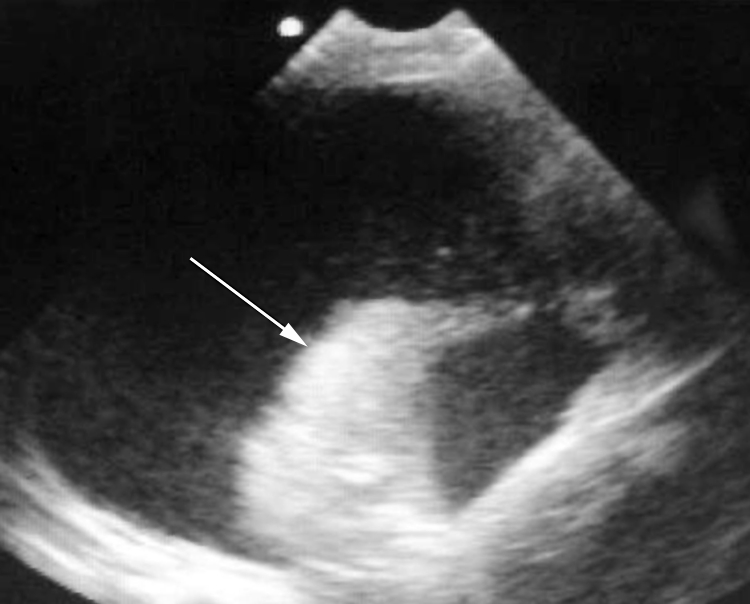
Figure 5 EU image of massive pleural effusion.
Circulation: shock secondary to cardiac tamponade
EU can be helpful when there is shock in the presence of a raised JVP, distant heart sounds, and tachycardia. Cardiac tamponade should be considered. EU can assess the size of a pericardial effusion and any dysfunction of the chambers. Differentiation among types of pericardial fluid (blood, exudate, transudate) cannot be made, but fibrinous strands, tumour masses, and blood clots can often be distinguished. Rapid accumulation of fluid in the pericardial space is poorly tolerated and is usually seen in the context of trauma or bacterial infection. Slower accumulations of fluid may allow for large amounts to collect without producing symptoms until intrapericardial pressures exceed right sided cardiac filling pressures. EU evidence of right atrial invagination (collapse) at the onset of systole and right ventricular collapse in diastole are signs of haemodynamic compromise. These patients are often extremely breathless and drainage can give immediate relief. Right ventricular diastolic collapse (RVDC) is a sensitive sign of tamponade.3
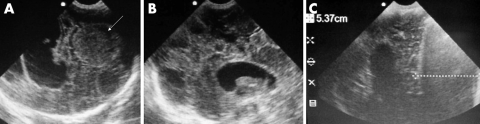
A 10 year girl presented with dyspnoea, elevated neck veins and lethargy (fig 5A). She was tachycardic with a reduced pulse pressure but normal systolic pressure. Note the 60.4 mm anechoic pericardial space with echogenic strands characteristic of a tuberculous effusion. There was no evidence of RVDC. She was treated with anti‐tuberculosis therapy and made a full recovery.
A global pericardial effusion with RVDC is shown (fig 6B; arrow). This 5 year old child presented with a high fever, dyspnoea, and features of tamponade. EU guided pericardiocentesis provided immediate relief. The aspirated fluid was purulent and grew Staphyloccus aureus. EU in both these cases was useful in demonstrating the size and effects of the pericardial effusions. More importantly, EU influenced the decision to intervene medically in the first case and surgically in the second.
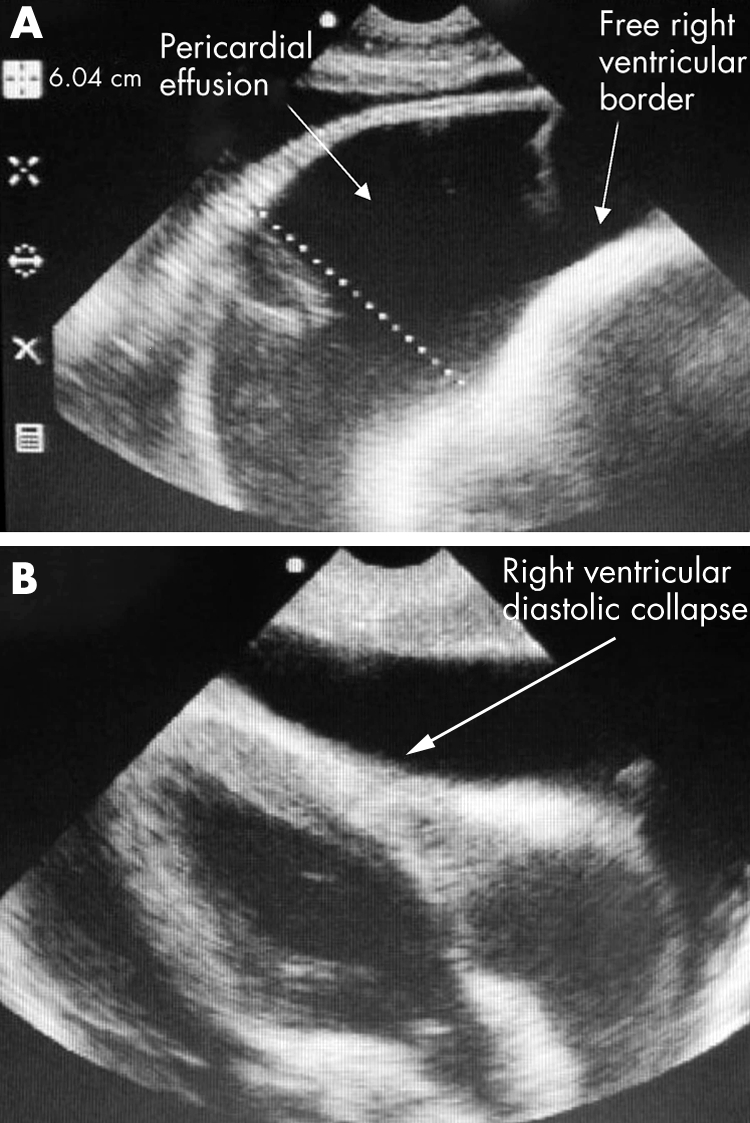
Figure 6 (A) EU of large pericardial effusion; (B) EU image of pericardial tamponade.
Circulation: shock secondary to haemorrhage
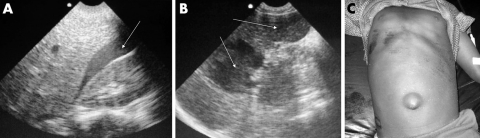
EU in adult blunt thoracoabdominal trauma has been shown to be useful and accurate in detecting haemoperitoneum, haemopericardium, and haemothorax, thus avoiding transfer to CT scan if haemodynamically unstable and justifying surgery.4 In children, the detection of haemoperitoneum alone does not justify surgery as the vast majority of solid organ injuries are successfully managed non‐surgically.5 In sub‐Saharan African children, spleens are often massive due to recurrent infections with malaria, and therefore relatively exposed below the rib cage, increasing their susceptibility to injury. Laparotomy in these children is often hazardous, and splenectomy increases the risk of subsequent infection with non‐encapsulated bacteria. The primary role of thoracoabdominal EU in children following trauma, in our practice, is to guide further management in our high dependency unit or operating theatre (if surgery is required for other reasons) by assessing the actual or potential loss of blood in order to determine the need for transfusion. Using a modified focused assessment with sonography for trauma (FAST) technique, the following areas can be assessed for the presence or absence of free fluid: the pericardium, right pleural space, subphrenic space, Morrison's pouch, right paracolic gutter, left pleural space, splenorenal recess, left paracolic gutter, and pelvis. EU can also be used to assess for and define grades of solid organ injury. However, the technique is highly operator dependent, as gastric air and peritoneal irritation can make the examination difficult. Lower grades of splenic injury do not always lead to haemoperitoneum so the absence of free fluid does not necessarily equate to lack of solid organ injury in children.6 However, the diagnosis of hepatosplenic injury is important in determining the need for hospitalisation, duration of bed rest, resumption of activity, and need for follow up scan. Delayed rupture of subcapsular haematomas can occur and serial EU is therefore of value. As blood becomes organised into haematomas, which are initially hypoechoic, become isoechoic to normal spleen tissue. Resolution can take up to a year, leaving a residual scar as a linear echogenic focus.
An 8 year old child who suffered blunt abdominal trauma was found to have free fluid (fig 7A; arrow) in Morrison's pouch. Two hypoechoic regions consistent with liver haematomas were seen in an 11 year old boy (fig 7B; arrows), 24 hours after injury following a road traffic accident. A child lying supine following thoracoabdominal blunt trauma is shown (fig 7C); note the superficial abrasions and everted umbilicus. EU of the abdomen excluded the presence of free fluid or obvious solid organ disruption in the resuscitation room.
Figure 7 EU image of (A) free fluid in Morrisons' pouch and (B) liver haematoma. (C) Superficial abdominal injuries. Photo reproduced with permission.
Neurological disability secondary to cerebral abscess or subdural empyema
The commonest causes of coma and/or convulsions in our resuscitation room are acute severe brain infections from severe malaria, viral or bacterial meningitis, poisoning, metabolic insults, or injury. Occasionally children are referred for tertiary hospital care with a diagnosis of pyogenic meningitis, but remain comatose or have persistent, focal seizures several days after presentation, which may be refractory to maximum medical therapy. In these children, EU through an open fontanelle can be useful in detecting the presence or absence of a cerebral abscess or subdural empyema. Prior to the extended programme of immunisation in Malawi, Salmonella species and Haemophilus influenzae type b (Hib) were the commonest causes of pyogenic meningitis complicated by parenchymal brain abscess or subdural empyema.7 Nowadays, because of the widespread routine use of a pentavalent vaccine in early infancy (diphtheria/pertussis/tetanus, Hib, and hepatitis B), detection of focal infective complications post‐bacterial meningitis is almost invariably due to Salmonella species. In many resource poor settings, microbiological diagnostic facilities are unavailable and treatment is empirical, based on the most likely causative organism, its epidemiology, and the age of the child. The detection of an abscess or empyema in our practice is therefore useful in influencing appropriate antibiotic choice as well as guiding aspiration.
Views of the brain are shown (fig 8). A large complex mass (fig 8A; arrow) can be seen, with an echogenic rim within which lie areas of heterogeneous echogenicity characteristic of a necrotic cerebral abscess. A right mid sagittal view depicts several small focal complex masses consistent with parenchymal cerebral abscesses in various stages of necrosis (fig 8B). A hypoechoic area 53.7 mm in diameter can be seen (fig 8C), consistent with a large left frontoparietal subdural empyema. Culture of this fluid following an EU guided subdural tap grew Salmonella typhimurium.
Figure 8 (A) Coronal EU image of multiple cerebral abscesses; (B) sagittal EU image of multiple cerebral abscesses; (C) EU image of subdural empyema.
Neurological disability secondary to acute elevated intracranial pressure
In the severely head injured child, aggressive attention to hypoxia and hypotension during the primary survey is important to prevent secondary brain injury. The early detection and management of acute elevated intracranial pressure (ICP) due to intracranial bleeding or oedema associated with injury hours or days after the event then becomes critical to outcome. Early EU measurement of optic nerve sheath diameters (ONSDs) has been proposed as a rapid, non.finvasive method to detect raised ICP in patients in the ED following head injury.[8] It is not yet known how superior this test is to traditional clinical methods for detection of clinically relevant raised ICP. Its attractiveness as a clinically useful investigation in the ED lies in its ability to assess changes in ONSDs. When combined with clinical parameters such as coma score and vital signs, risk stratification may potentially influence decisions regarding the need for and timing of referral for neurointensive care.
In the severely head injured child, aggressive attention to hypoxia and hypotension during the primary survey is important to prevent secondary brain injury. The early detection and management of acute elevated intracranial pressure (ICP) due to intracranial bleeding or oedema associated with injury hours or days after the event then becomes critical to outcome. Early EU measurement of optic nerve sheath diameters (ONSDs) has been proposed as a rapid non‐invasive method to detect raised ICP in patients in the ED following head injury.8 It is not yet known how superior this test is to traditional clinical methods for detection of clinically relevant raised ICP. Its attractiveness as a clinically useful investigation in the ED lies in its ability to assess changes in ONSDs. When combined with clinical parameters such as coma score and vital signs, risk stratification may potentially influence decisions regarding the need for and timing of referral for neurointensive care.
Previous paediatric studies demonstrating the clinical usefulness of measurement of ONSDs have compared it with indirect measures of raised ICP such as findings of cerebral oedema on CT scan, cerebrospinal fluid manometry, or changes on fundoscopy.9,10,11 In neurosurgical patients, a positive linear relationship between ONSDs and the gold standard of invasive ICP monitoring has been demonstrated, with changes in ICP values reflecting changes in ONSDs without a lag period.12,13,14 If measurement of ONSDs is to become routine in the paediatric ED then further studies will need to demonstrate that raised values precede clinical signs of raised ICP in order for the technique to influence decisions regarding proactive clinical care or timing of referral for higher levels of care. It may then become a useful investigation in children who present with non‐traumatic coma/seizures where knowledge of serial ONSDs may enable detection of evolving intracranial hypertension. For example, EU measurement of baseline ONSDs may be indicated in children where there is a risk of cerebral oedema associated with refractory status epilepticus, acute bacterial meningitis, hypo‐osmolar intravenous fluid administration, or diabetic ketoacidosis. Control data suggest that the upper limit of normal for ONSD is 4.5 mm in patients older than 1 year of age and 4.0 mm in those younger than 1 year (measured 3 mm behind the globe).15
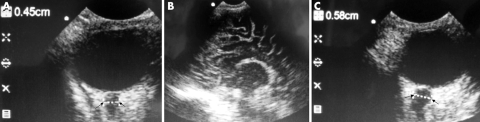
A 36 month old child presented with status epilepticus for 86 minutes prior to arrival to hospital. Note the presenting ONSD of 4.5 mm (fig 9A; arrow), which had reduced to 3.9 mm the next morning. His final diagnosis was cerebral malaria. A cranial ultrasound of a 4 month old child who presented with fever, posturing, and coma shows bright gyri (fig 9B), and presenting ONSD of 5.8 mm (fig 9C; arrow). These features are consistent with acute bacterial meningitis complicated by cerebral oedema and significantly raised intracranial pressure. CSF later grew S pneumoniae.
Figure 9 A) ONSD post convulsive status; (B) bright gyri with bacterial meningitis; (C) ONSD in acute bacterial meningitis
CONCLUSION
EU is extremely useful in a resource poor setting as an adjunct but not substitute to clinical assessment where other diagnostic modalities are not readily available such as emergency radiography or CT scanning. In the resuscitation room, integrating EU into clinical practice can facilitate earlier diagnosis and expedite definitive care whether medical or surgical. The advantages of EU being performed by the paediatric emergency physician are diagnosis, interpretation, and clinically appropriate action can be initiated in real time. In our experience, the major limitation to EU is not device but operator related and for this reason, we emphasise the essential need for training and early confirmation of findings by a trained radiologist. Future roles of EU in the paediatric resuscitation room may lie in its non‐invasive potential for detecting development of intracranial hypertension and measurements of cardiac filling pressures to assess acute changes in vascular filling state in order to guide safe intravenous volume resuscitation.
ACKNOWLEDGEMENTS
We would like to thank the children and their guardians who permitted us to describe their conditions and reproduce their clinical images for publication. We are particularly grateful to Dr J Robson, the congregation of St Clements, Toxteth and All Saints, Childwall Churches, and the Childwall and Eleanor Rathbone Charitable Trust for generously raising the funds for and donating the Sonosite 180 Plus portable ultrasound scanner to our department.
Abbreviations
CT - computerised tomography
ED - emergency department
EP - emergency physician
EU - emergency ultrasonography
ICP - intracranial pressure
JVP - jugular venous pressure
ONSD - optic nerve sheath diameter
RVDC - right ventricular diastolic collapse
Footnotes
Funding: none required
Competing interests: none declared
References
- 1.Ahmad S, Molyneux E. Intra‐cardiac Burkitt's lymphoma. Arch Dis Child 200590236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kohan J, Poe R, Israel R.et al Value of chest ultrasonography versus decubitus roentgenography for thoracocentesis. Am Rev Respir Dis 19861331124–1136. [DOI] [PubMed] [Google Scholar]
- 3.Singh S, Wann L S, Schuchard G H.et al Right ventricular and right atrial collapse in patients with cardiac tamponade – a combined echocardiographic and haemodynamic study. Circulation 198470966–971. [DOI] [PubMed] [Google Scholar]
- 4.Goletti O, Ghiselli G, Lippolis P V.et al The role of ultrasonography in blunt abdominal trauma: results in 250 cases. J Trauma 199436178–181. [DOI] [PubMed] [Google Scholar]
- 5.Rouse T M, Eichelberger M R. Trends in pediatric trauma management. Surg Clin North Am 1992721347–1364. [DOI] [PubMed] [Google Scholar]
- 6.Taylor G A, Sivit C J. Post traumatic peritoneal fluid: is it a reliable indicator of intra‐abdominal injury in children? J Pediatr Surg 1995301644–1648. [DOI] [PubMed] [Google Scholar]
- 7.Molyneux E M, Walsh A L, Forsyth H.et al Dexamethasone treatment in childhood bacterial meningitis in Malawi: a randomised controlled trial. Lancet 2002360211–218. [DOI] [PubMed] [Google Scholar]
- 8.Blaivas M, Theodoro D, Sierzenski P R. Elevated intracranial pressure detected by bedside emergency ultrasonography of the optic nerve sheath. Acad Emerg Med 200310376–381. [DOI] [PubMed] [Google Scholar]
- 9.Tsung J W, Blaivas M, Cooper A.et al A rapid non invasive method of detecting elevated intracranial pressure using bedside ocular ultrasound: application to 3 cases of head trauma in the Pediatric Emergency Department. Pediatric Emerg Care 20052194–98. [DOI] [PubMed] [Google Scholar]
- 10.Newman W D, Hollman A S, Dutton G N.et al Measurement of optic nerve sheath diameter by ultrasound: a means of detecting acute raised intracranial pressure in hydrocephalus. Br J Opthalmol 2002861109–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Malayeri A A, Bavarian S, Mehdizadeh M. Sonographic evaluation of optic nerve diameter in children with raised intracranial pressure. J Ultrasound Med 200524143–147. [DOI] [PubMed] [Google Scholar]
- 12.Cennamo G, Gangemi M, Stella L. The correlation between endocranial pressure and optic nerve diameter: an ultrasonographic study. Opthalmic Echography 19877603–606. [Google Scholar]
- 13.Tamburelli C, Aricle C, Mangiola A.et al CSF dynamic parameters and changes of optic nerve diameters measured by standardised echography. Opthalmic Echography 199313101–109. [Google Scholar]
- 14.Gangemi M, Cennamo G, Maiuri F.et al Echographic measurement of the optic nerve in patients with intracranial hypertension. Neurochirugica 19873053–55. [DOI] [PubMed] [Google Scholar]
- 15.Ballantyne J, Hollman A S, Hamilton R.et al Transorbital optic nerve sheath ultrasonography in normal children. Clin Radiol 199954740–742. [DOI] [PubMed] [Google Scholar]