Abstract
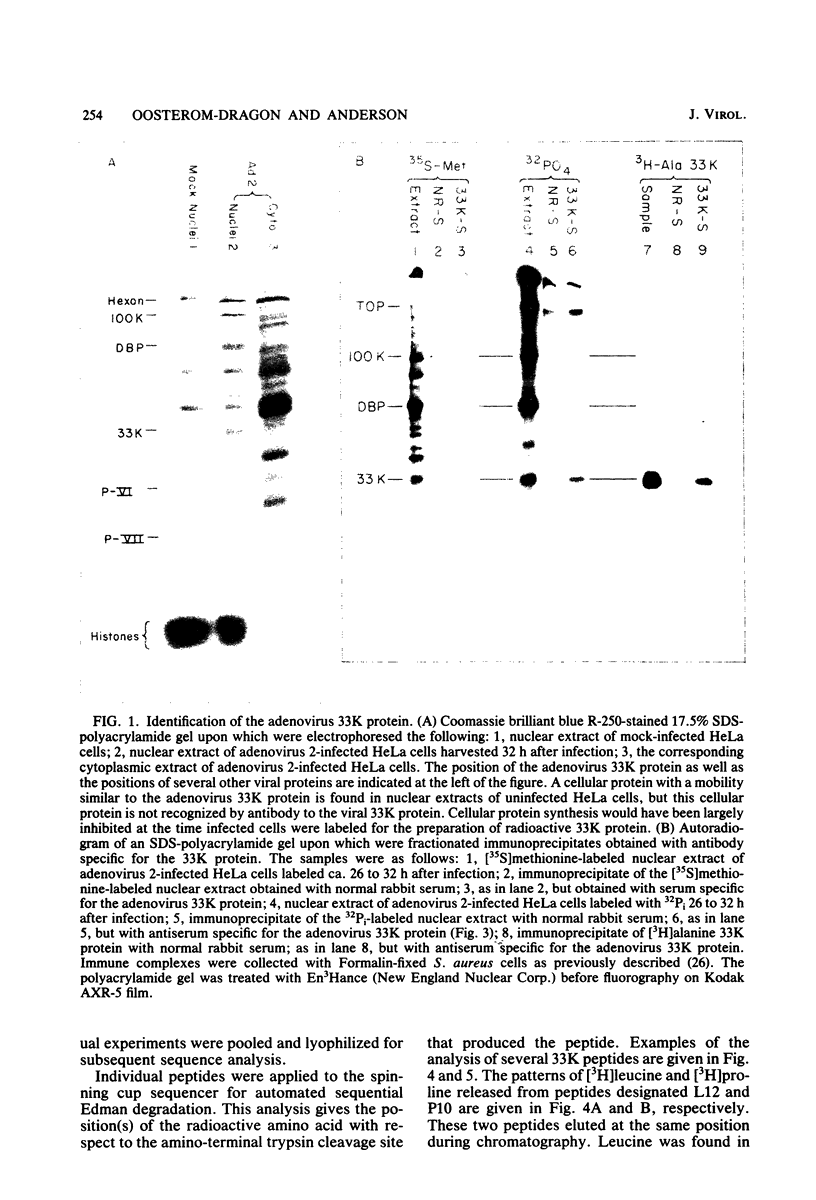
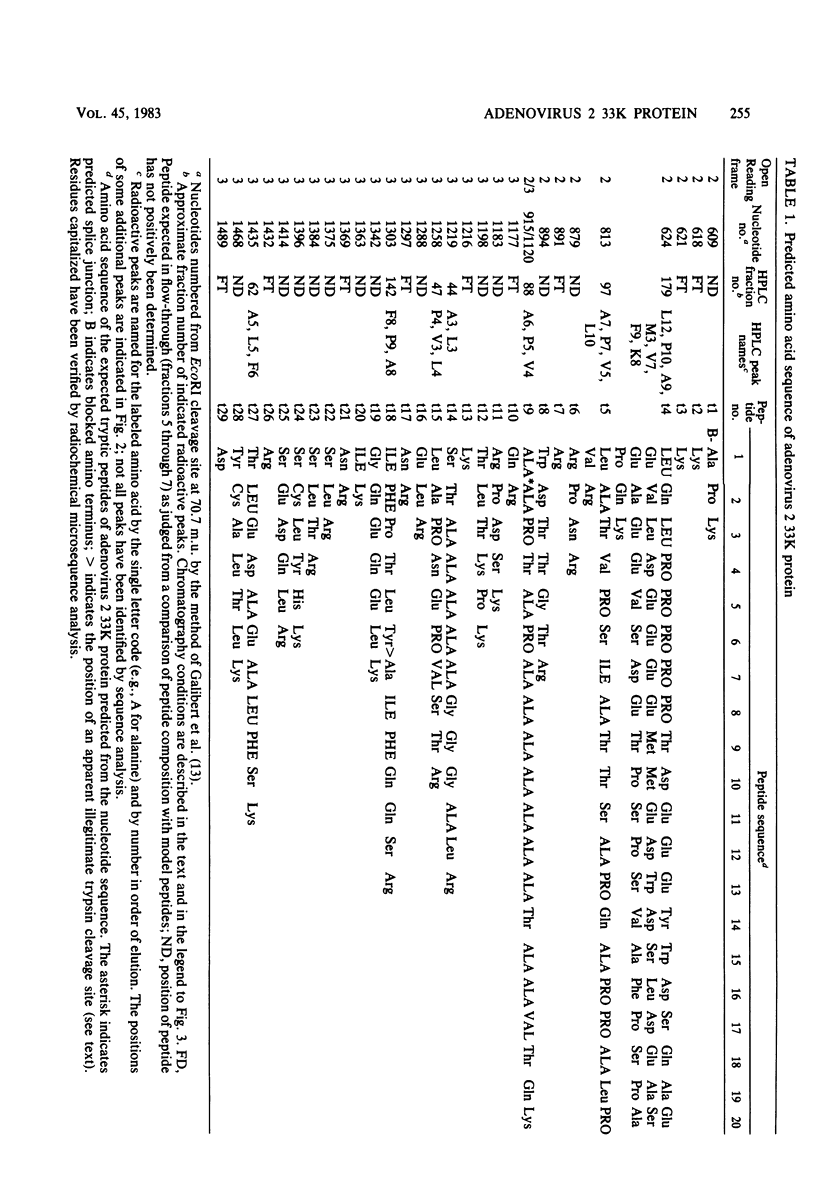
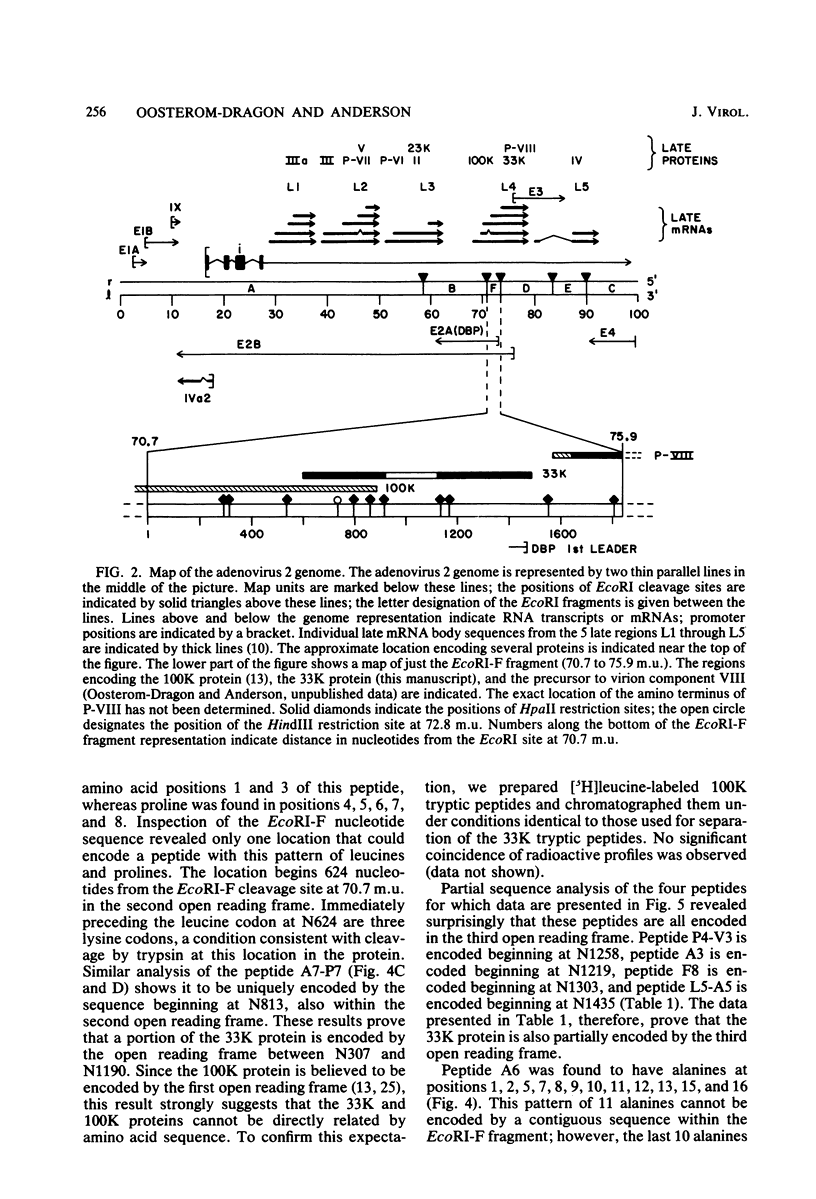
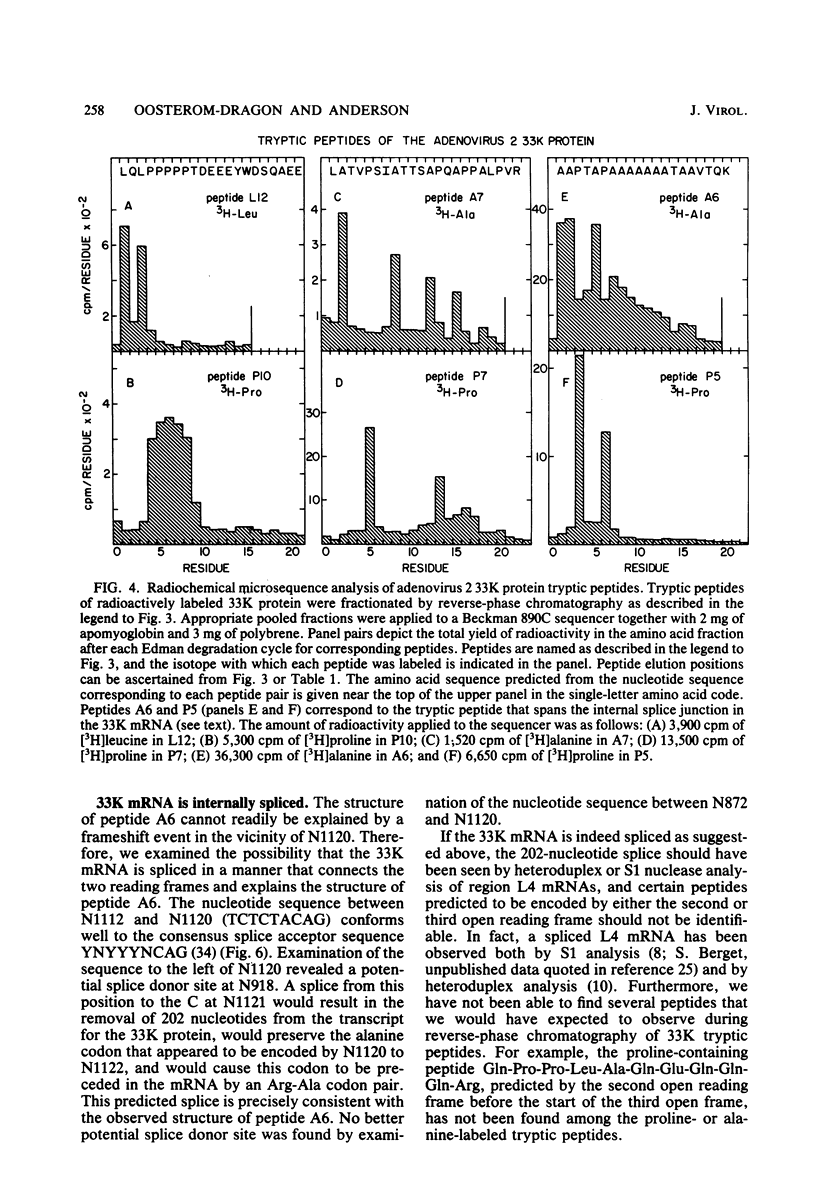
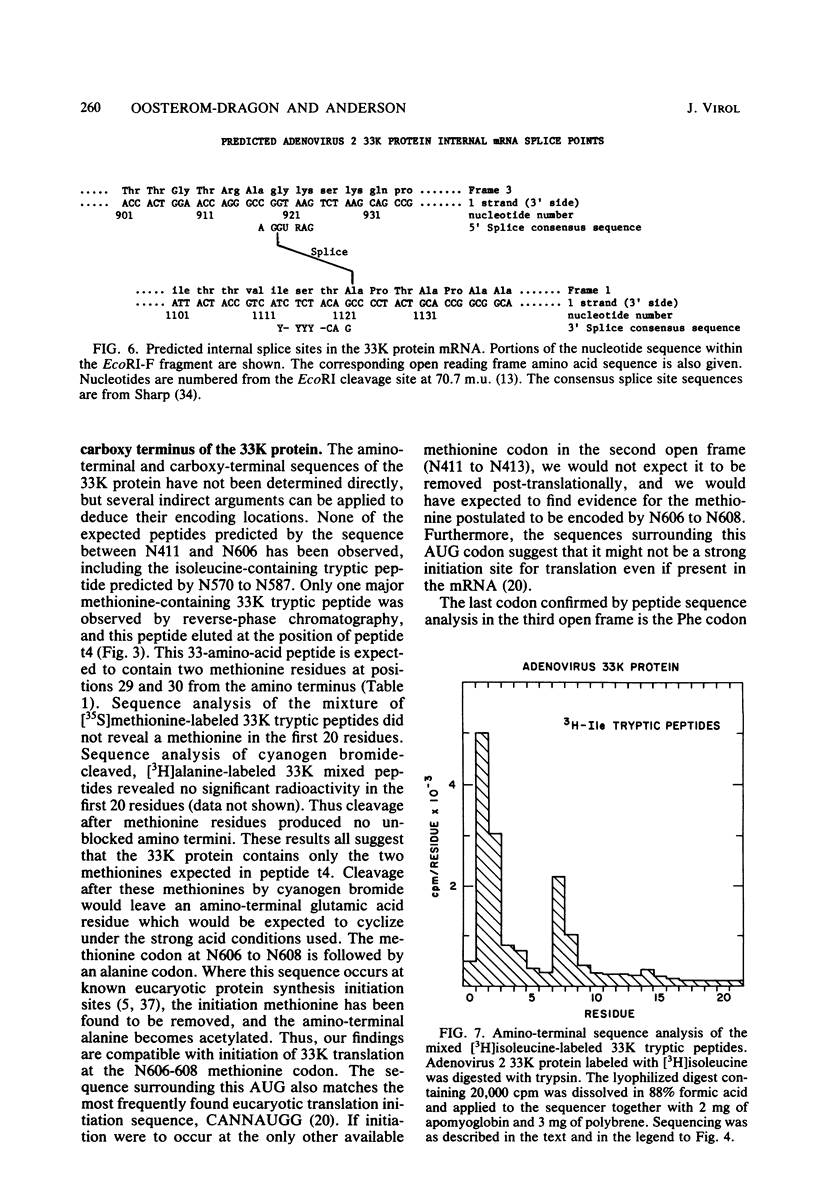
Radiochemical microsequence analysis of selected tryptic peptides of the adenovirus type 2 33K nonstructural protein has revealed the precise region of the genomic nucleotide sequence that encodes this protein. The initiation codon for the 33K protein lies 606 nucleotides to the right of the EcoRI restriction site at 70.7 map units and 281 nucleotides to the left of the postulated carboxyterminal codon of the adenovirus 100K protein. The coding regions for these two proteins thus overlap; however, the 33K protein is derived from the +1 frame with respect to the postulated 100K reading frame. Our results contradict an earlier published report suggesting that these two proteins share extensive amino acid sequence homology (N. Axelrod, Virology 87:366-383, 1978). The published nucleotide sequence of the Ad2 EcoRI-F fragment (70.7 to 75.9 map units) cannot accommodate in a single reading frame the peptide sequences of the 33K protein that we have determined. Sequence analysis of DNA fragments derived from virus has confirmed the published nucleotide sequence in all critical regions with respect to the coding region for the 33K protein. Consequently, our data are only consistent with the existence of an mRNA splice within the coding region for 33K. Consensus donor and acceptor splice sequences have been located that would predict the removal of 202 nucleotides from the transcripts for the 33K protein. Removal of these nucleotides would explain the structure of a peptide that cannot otherwise be directly encoded by the EcoRI-F fragment. Identification of the precise splice points by peptide sequencing has permitted a prediction of the complete amino acid sequence for the 33K protein.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akusjärvi G., Zabielski J., Perricaudet M., Pettersson U. The sequence of the 3' non-coding region of the hexon mRNA discloses a novel adenovirus gene. Nucleic Acids Res. 1981 Jan 10;9(1):1–17. doi: 10.1093/nar/9.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson C. W., Baum P. R., Gesteland R. F. Processing of adenovirus 2-induced proteins. J Virol. 1973 Aug;12(2):241–252. doi: 10.1128/jvi.12.2.241-252.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson C. W., Lewis J. B. Amino-terminal sequence of adenovirus type 2 proteins: hexon, fiber, component IX, and early protein 1B-15K. Virology. 1980 Jul 15;104(1):27–41. doi: 10.1016/0042-6822(80)90363-3. [DOI] [PubMed] [Google Scholar]
- Anderson C. W. Spontaneous mutants of the adenovirus-simian virus 40 hybrid, Ad2+ND3, that grow efficiently in monkey cells. Virology. 1981 May;111(1):263–269. doi: 10.1016/0042-6822(81)90670-x. [DOI] [PubMed] [Google Scholar]
- Axelrod N. Phosphoproteins of adenovirus 2. Virology. 1978 Jun 15;87(2):366–383. doi: 10.1016/0042-6822(78)90141-1. [DOI] [PubMed] [Google Scholar]
- Baker C. C., Herisse J., Courtois G., Galibert F., Ziff E. Messenger RNA for the Ad2 DNA binding protein: DNA sequences encoding the first leader and heterogenity at the mRNA 5' end. Cell. 1979 Oct;18(2):569–580. doi: 10.1016/0092-8674(79)90073-4. [DOI] [PubMed] [Google Scholar]
- Berget S. M., Sharp P. A. Structure of late adenovirus 2 heterogeneous nuclear RNA. J Mol Biol. 1979 Apr 25;129(4):547–565. doi: 10.1016/0022-2836(79)90468-6. [DOI] [PubMed] [Google Scholar]
- Bhatti A. R., Weber J. Protease of adenovirus type 2: partial characterization. Virology. 1979 Jul 30;96(2):478–485. doi: 10.1016/0042-6822(79)90105-3. [DOI] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Empirical predictions of protein conformation. Annu Rev Biochem. 1978;47:251–276. doi: 10.1146/annurev.bi.47.070178.001343. [DOI] [PubMed] [Google Scholar]
- Dunn J. J., Studier F. W. Nucleotide sequence from the genetic left end of bacteriophage T7 DNA to the beginning of gene 4. J Mol Biol. 1981 Jun 5;148(4):303–330. doi: 10.1016/0022-2836(81)90178-9. [DOI] [PubMed] [Google Scholar]
- Galibert F., Hérissé J., Courtois G. Nucleotide sequence of the EcoRI-F fragment of adenovirus 2 genome. Gene. 1979 May;6(1):1–22. doi: 10.1016/0378-1119(79)90081-7. [DOI] [PubMed] [Google Scholar]
- Gambke C., Deppert W. Late nonstructural 100,000- and 33,000-dalton proteins of adenovirus type 2. I. Subcellular localization during the course of infection. J Virol. 1981 Nov;40(2):585–593. doi: 10.1128/jvi.40.2.585-593.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gambke C., Deppert W. Late nonstructural 100,000- and 33,000-dalton proteins of adenovirus type 2. II. Immunological and protein chemical analysis. J Virol. 1981 Nov;40(2):594–598. doi: 10.1128/jvi.40.2.594-598.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassell J. A., Weber J. Genetic analysis of adenovirus type 2. VIII. Physical locations of temperature-sensitive mutations. J Virol. 1978 Dec;28(3):671–678. doi: 10.1128/jvi.28.3.671-678.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson L. E., Oroszlan S., Konigsberg W. A micromethod for complete removal of dodecyl sulfate from proteins by ion-pair extraction. Anal Biochem. 1979 Feb;93(1):153–157. [PubMed] [Google Scholar]
- Kessler S. W. Rapid isolation of antigens from cells with a staphylococcal protein A-antibody adsorbent: parameters of the interaction of antibody-antigen complexes with protein A. J Immunol. 1975 Dec;115(6):1617–1624. [PubMed] [Google Scholar]
- Kozak M. Possible role of flanking nucleotides in recognition of the AUG initiator codon by eukaryotic ribosomes. Nucleic Acids Res. 1981 Oct 24;9(20):5233–5252. doi: 10.1093/nar/9.20.5233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruijer W., van Schaik F. M., Sussenbach J. S. Nucleotide sequence analysis of a region of adenovirus 5 DNA encoding a hitherto unidentified gene. Nucleic Acids Res. 1980 Dec 20;8(24):6033–6042. doi: 10.1093/nar/8.24.6033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levinson A. D., Postel E. H., Levine A. J. In vivo and in vitro phosphorylation of the adenovirus type 5 single strand-specific DNA-binding protein. Virology. 1977 Jun 1;79(1):144–159. doi: 10.1016/0042-6822(77)90341-5. [DOI] [PubMed] [Google Scholar]
- Lewis J. B., Atkins J. F., Anderson C. W., Baum P. R., Gesteland R. F. Mapping of late adenovirus genes by cell-free translation of RNA selected by hybridization to specific DNA fragments. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1344–1348. doi: 10.1073/pnas.72.4.1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Miller J. S., Ricciardi R. P., Roberts B. E., Paterson B. M., Mathews M. B. Arrangement of messenger RNAs and protein coding sequences in the major late transcription unit of adenovirus 2. J Mol Biol. 1980 Oct 5;142(4):455–488. doi: 10.1016/0022-2836(80)90258-2. [DOI] [PubMed] [Google Scholar]
- Oosterom-Dragon E. A., Ginsberg H. S. Characterization of two temperature-sensitive mutants of type 5 adenovirus with mutations in the 100,000-dalton protein gene. J Virol. 1981 Nov;40(2):491–500. doi: 10.1128/jvi.40.2.491-500.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oosterom-Dragon E. A., Ginsberg H. S. Purification and preliminary immunological characterization of the type 5 adenovirus, nonstructural 100,000-dalton protein. J Virol. 1980 Mar;33(3):1203–1207. doi: 10.1128/jvi.33.3.1203-1207.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmiter R. D. Prevention of NH2-terminal acetylation of proteins synthesized in cell-free systems. J Biol Chem. 1977 Dec 25;252(24):8781–8783. [PubMed] [Google Scholar]
- Ricciardi R. P., Miller J. S., Roberts B. E. Purification and mapping of specific mRNAs by hybridization-selection and cell-free translation. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4927–4931. doi: 10.1073/pnas.76.10.4927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell W. C., Blair G. E. Polypeptide phosphorylation in adenovirus-infected cells. J Gen Virol. 1977 Jan;34(1):19–35. doi: 10.1099/0022-1317-34-1-19. [DOI] [PubMed] [Google Scholar]
- Schechter N. M., Davies W., Anderson C. W. Adenovirus coded deoxyribonucleic acid binding protein. Isolation, physical properties, and effects of proteolytic digestion. Biochemistry. 1980 Jun 10;19(12):2802–2810. doi: 10.1021/bi00553a041. [DOI] [PubMed] [Google Scholar]
- Semler B. L., Anderson C. W., Kitamura N., Rothberg P. G., Wishart W. L., Wimmer E. Poliovirus replication proteins: RNA sequence encoding P3-1b and the sites of proteolytic processing. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3464–3468. doi: 10.1073/pnas.78.6.3464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp P. A. Speculations on RNA splicing. Cell. 1981 Mar;23(3):643–646. doi: 10.1016/0092-8674(81)90425-6. [DOI] [PubMed] [Google Scholar]
- Smart J. E., Lewis J. B., Mathews M. B., Harter M. L., Anderson C. W. Adenovirus type 2 early proteins: assignment of the early region 1A proteins synthesized in vivo and in vitro to specific mRNAs. Virology. 1981 Jul 30;112(2):703–713. doi: 10.1016/0042-6822(81)90315-9. [DOI] [PubMed] [Google Scholar]
- White D. O., Scharff M. D., Maizel J. V., Jr The polypeptides of adenovirus. 3. Synthesis in infected cells. Virology. 1969 Jul;38(3):395–406. doi: 10.1016/0042-6822(69)90152-4. [DOI] [PubMed] [Google Scholar]
- Wold F. In vivo chemical modification of proteins (post-translational modification). Annu Rev Biochem. 1981;50:783–814. doi: 10.1146/annurev.bi.50.070181.004031. [DOI] [PubMed] [Google Scholar]
- Zain S., Sambrook J., Roberts R. J., Keller W., Fried M., Dunn A. R. Nucleotide sequence analysis of the leader segments in a cloned copy of adenovirus 2 fiber mRNA. Cell. 1979 Apr;16(4):851–861. doi: 10.1016/0092-8674(79)90100-4. [DOI] [PubMed] [Google Scholar]




