Abstract
OBJECTIVE
To assess the hormonal effects of Fem7® (Merck, KGaA, Darmstadt, Germany) 100 μg transdermal oestrogen patches on men undergoing first-line androgen-deprivation therapy for prostate cancer.
PATIENTS AND METHODS
PATCH is a multicentre, randomized, phase II trial for men with locally advanced or metastatic prostate cancer, comparing luteinizing hormone-releasing hormone agonist therapy with oestrogen patches. To assess the dosing schedule for the patches, as this was the first time that this brand of patch had been used in men, and to reassure patients and participating clinicians, the Independent Data Monitoring Committee agreed to early release of hormonal data from this study.
RESULTS
Oestradiol, testosterone and prostate-specific antigen (PSA) levels are presented for the first group of 14 patients who received the patches (with 1 withdrawal) and for whom there were ≥12 weeks of follow-up by March 2007. After 12 weeks, testosterone levels (nmol/L) in eight of the 13 patients were <1.7, two were 1.7–2 and three were >2. The median (range) serum oestradiol levels was 442 (52.1–1542) pmol/L and all patients had a PSA response, with eight having a PSA level of <4 ng/mL.
CONCLUSION
These results confirm that oestrogen patches produce castrate levels of testosterone and concomitant PSA responses. They also highlighted the potential differences between different brands of oestrogen patches, and the need to monitor hormonal response, toxicity and efficacy until more experience with oestrogen patches for this clinical indication is obtained. The number of patches recommended in the PATCH study has now been increased.
Keywords: transdermal oestrogen patches, hormone therapy, prostate cancer, randomized controlled trial
INTRODUCTION
LHRH analogues are standard therapy for metastatic prostate cancer; they are also used as adjuvant and neoadjuvant therapy in men undergoing radiotherapy for localized tumours of the prostate, as primary treatment in some men with locally advanced disease, and for those with an increasing PSA level after radical therapy [1]. These extended indications and prolonged duration of administration have increased concerns about the long-term toxicity, particularly osteoporosis. For example, a recent longitudinal study showed that 35% (1592/4494) of men who received LHRH therapy for prostate cancer will experience at least one skeletal fracture in the first 7 years and 19% will be diagnosed with osteoporosis/osteopaenia. Furthermore, the risk of fracture appears to be related to the duration of LHRH therapy [2].
Oral oestrogens were used previously as a method of androgen-deprivation therapy, and although effective from the perspective of prostate cancer they are no longer used routinely as first-line therapy because of increased cardiovascular toxicity [3]. This has been attributed to first-pass hepatic metabolism, affecting lipids and coagulation proteins [4]. Administering oestrogen through a patch avoids the entero-hepatic circulation and therefore it should not be associated with the same level of cardiovascular toxicity. A recent single-centre, 20-patient, non-randomized study investigated the use of oestrogen patches in men with prostate cancer who had not received androgen-deprivation therapy previously. The patches (Progynova TS forte, (7.8 mg, Schering Aktiengesellschaft, Berlin, Germany) were tolerated well, all patients achieved castrate levels of testosterone, and apart from one case of oedema there was no cardiovascular toxicity. Furthermore, bone mineral densities of the lumbar spine and the hip were maintained or significantly increased, such that four patients had an improved WHO classification of osteoporotic risk [5,6]. These interesting results led to a randomized phase II study (PATCH trial, MRC PR09) which is comparing LHRH analogues and transdermal oestrogen patches in men with locally advanced or metastatic prostate cancer who have not previously received hormone therapy.
The oral oestrogen diethylstilbestrol (DES) is used in the UK to treat men with prostate cancer whose disease has progressed during first-line hormonal therapy, usually with LHRH analogues. The observation that DES produces and maintains a clinical response in patients with prostate cancer who are thought to already have castrate levels of testosterone is unexpected, and not fully explained. In 2003 DES became temporarily unavailable due to lack of production and some UK clinicians prescribed oestrogen patches instead. Kandola et al. [7] recently published a report documenting their centre's concerns with transdermal oestrogen therapy as a second-line hormonal intervention in prostate cancer.
The purpose of the present report is to document some early data from the PATCH trial. The PATCH Independent Data Monitoring Committee (IDMC), the only group to review the accumulating data while the trial is ongoing, agreed to the release of early hormonal data from the trial for two reasons. First, to allow the trial team to review the dosing schedule, as this was the first time that this brand of patch had been used in men, and second, to reassure participating patients and clinicians that oestrogen patches produce castrate levels of testosterone when used as first-line hormonal therapy.
PATIENTS AND METHODS
PATCH is a multicentre randomized study, comparing oestrogen patches with LHRH analogues in men with locally advanced or metastatic prostate cancer who had not previously received hormone therapy except in the adjuvant or neoadjuvant setting. Eligible patients are randomized in a 2:1 ratio (patches to LHRH) to maximize experience with the patches. The aim is to recruit 200 patients, and the primary outcome measure is cardiovascular morbidity and mortality. The secondary outcome measures are efficacy and toxicity of the patches. If the patches are well tolerated and there is evidence of activity, it is the intention to formally compare efficacy in a much larger study.
The protocol requires hormone levels to be measured at baseline and after randomization. Three Fem7® 100 μg oestradiol patches (Merck KGaA, Darmstadt, Germany) are applied and changed twice weekly. After 4 weeks, if the serum testosterone level is ≤1.7 nmol/L then a maintenance regimen of two patches changed twice weekly is used. If the testosterone level is >1.7 nmol/L at this time point, the three patches are continued and testosterone levels re-assessed 2 weeks later. A level of 50 ng/dL of testosterone was used to define castrate levels previously [8], equivalent to 1.7 nmol/L, and this level was chosen as the minimum target level for the PATCH study.
RESULTS
Between March 2006 and July 2007, 50 patients were recruited from 11 UK centres to the PATCH trial. In March 2007, the IDMC agreed to release hormonal data (testosterone, oestradiol and PSA levels) relating to the first 14 patients receiving the patches, as they had at least 12 weeks of follow-up from randomization. They declined the request to release comparable data for patients who had received LHRH therapy. The baseline characteristics of these patients are given in Table 1. The median (range) age of patients at randomization was 73 (57–79) years; 13 patients were WHO status 0 at randomization and six had metastatic disease.
TABLE 1.
The baseline characteristics of patients entering the PATCH study, with the TNM staging at baseline, and the PSA, oestradiol and testosterone levels at baseline, 4 and 12 weeks. Patient 2 was entered under the category of multiple bone metastases and had a PSA level of ≥50 ng/mL
| Patient no. |
At randomization |
Gleason sum at diagnosis |
PSA, ng/mL |
Testosterone, nmol/L |
Oestradiol, pmol/L 12 weeks |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Age, years | WHO grade | TNM | Base | 4 | 12 | Base | 4 | 12 | |||
| 1 | 69 | 2 | T3N+M0 | 9 | 134 | 14.2 | 4.5 | 10.3 | 0.8 | 1.3 | 314 |
| 2 | 70 | 0 | TXNXM+ | * | 795.7 | 185.9 | 24 | 7.4 | 1 | 0.7 | 289 |
| 3 | 79 | 0 | T3NXM+ | 7 | 18.8 | 3.7 | 0.6 | 10.9 | 0.9 | 1.2 | 382 |
| 4 | 70 | 0 | TXN+M0 | 7 | 10.8 | 7 | 2.8 | 16.9 | 10 | 4.9 | 567 |
| 5 | 69 | 0 | T3NXM0 | 7 | 20.6 | 17.7 | 2 | 11.1 | 7.8 | 0.3 | 708 |
| 6 | 78 | 0 | T3NXM0 | 7 | 28 | 12 | 10 | 21.2 | 1.2 | 3.5 | 52.1 |
| 7 | 64 | 0 | T3NXM+ | 7 | 624.2 | 200.3 | 15.1 | 13.2 | 0.8 | 0.4 | 596 |
| 8 | 57 | 0 | T3NXM0 | 9 | 152.9 | W | W | W | W | W | W |
| 9 | 79 | 0 | T2N+M0 | 7 | 65.8 | 32.8 | 1.7 | 18.2 | 1.1 | 0.3 | 442 |
| 10 | 77 | 0 | T3NXM+ | 9 | 729 | 7.2 | 1.34 | 29.2 | 1.9 | 1.3 | 788 |
| 11 | 76 | 0 | T2N0M0 | 5 | 100.8 | 67.5 | 24.8 | 15.9 | 4.3 | 4 | 313 |
| 12 | 74 | 0 | T2NXM+ | 6 | 22 | 9 | 1.5 | 10.9 | 1.8 | 1.9 | 462 |
| 13 | 78 | 0 | T3N0M0 | 8 | 6.3 | 1 | 0.3 | 12 | 3 | 0.9 | 1542 |
| 14 | 72 | 0 | T0NXM+ | 7 | 20 | 6.3 | 1.5 | 12.2 | 1.5 | 1.9 | 312 |
W, patient withdrew from the study.
No biopsy.
Table 1 shows the oestradiol, testosterone and PSA levels of the patients treated with patches, and changes over time. The median oestradiol level achieved while on the patches was 442 pmol/L; this was lower than expected. The aim of the study was to produce serum oestradiol levels of 1000–1500 pmol/L, a target based on data from Scandinavian studies using polyestradiol phosphate, a long-acting intramuscular oestrogen preparation used to treat prostate cancer, which indicate that oestradiol levels in this range are required to maintain testosterone at castrate levels [9].
In the present protocol the timing of the oestradiol blood test was not specified in relation to the application and changing of the patches, and this might account for some of the variation in the levels obtained. Available information suggests that for preparations delivering 100 μg/day of oestradiol transdermally (including the Progynova patches used in the original pilot study [5]), the Fem7 patches used in the present study and the Evorel-100 patches (Janssen-Cilag Ltd, High Wycombe, Bucks, UK) used in the study of Kandola et al. [7], the average hourly area-under-the-curve for oestradiol might be only 204–250 pmol/L, but the maximum concentration achieved can be 353–621 pmol/L [10-12]. This is illustrated in Fig. 1 for two 100 μg/day patches, Fem7 and Estradot (Novartis Pharmaceuticals UK Ltd., Frimley, Surrey, UK), for which published oestradiol concentration profile information is available [11,13]. Accordingly, different patches might deliver similar overall concentrations but the day-to-day oestradiol concentrations achieved might differ substantially, especially during the early period of patch application, when maximum concentrations are reached. These values were obtained in women and oestradiol pharmacokinetics could vary between men and women.
FIG. 1.
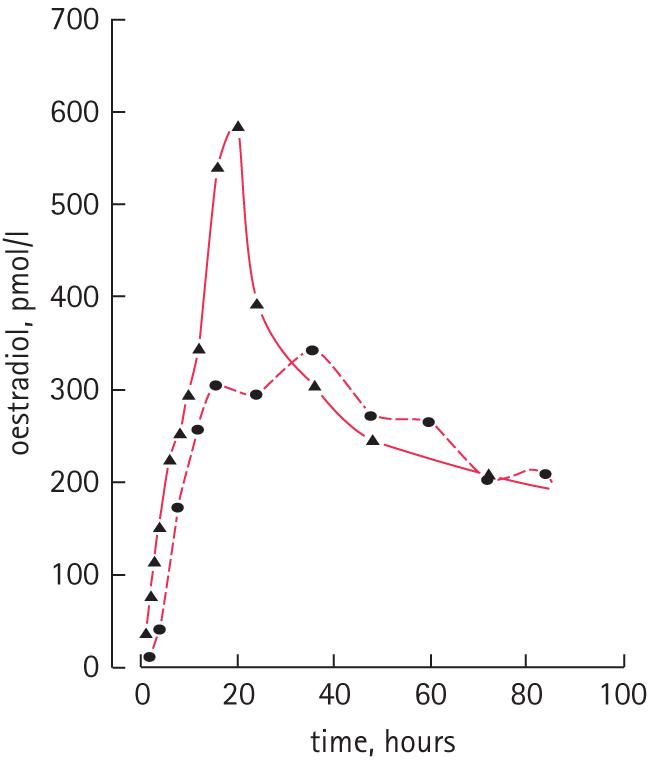
Oestradiol concentration profiles over 3 days (82 h) for patches delivering 100 μg/day oestradiol. The continuous line denotes Fem7 [11], and the dashed line Estradot [13].
The target testosterone level of ≤1.7 nmol/L at 12 weeks was not reached in five of the 13 patients, although two had levels of <2 nmol/L. Of the three other patients, one (no. 6) had a testosterone level of 3.5 nmol/L and an oestradiol level of 52.1 pmol/L, which might indicate a compliance issue, as the normal oestradiol range for men is 30–206 pmol/L, and similar to results we have obtained from patients receiving LHRH analogues.
The median (range) PSA level at randomization was 46.9 (6.3–795) ng/mL. All the present patients had a decrease in PSA level (>70% by 12 weeks, except for patient no. 6 with the possible compliance issues) and nine of 13 had a PSA level of <4 ng/mL. Of the two other patients not considered castrate, the PSA decreased in one from 10.8 to 2.8 ng/mL, and in the other from 100.8 to 24.8 ng/mL.
After comparing the present data with those published previously on the levels of testosterone achieved with orchidectomy and LHRH [8,14], and data from the original single-centre pilot study [15], the PATCH Trial Management Group decided to recommend increasing the dose of the Fem7 patches to an initial dose of four patches changed twice weekly for 4 weeks, followed by a maintenance regimen of three patches changed twice weekly indefinitely, providing that the serum testosterone level is ≤1.7 nmol/L. For patients who are already on the patches and have achieved testosterone levels of ≤1.7 nmol/L, they should remain on two patches. For patients whose testosterone level is >1.7 nmol/L, they should increase from two to three patches changed twice weekly. It is also recommended that serum oestradiol levels be measured the day before the patches are changed, so that a more accurate nadir can be recorded.
DISCUSSION
The present data confirm that oestrogen patches can be used to produce castrate levels of testosterone in men with prostate cancer, and that this is associated with a concomitant reduction in PSA level. They also highlight the differences in expected serum oestradiol profiles between different brands of oestrogen patches, and the importance of measuring serum oestradiol levels, not only to confirm compliance but also to assess dosing schedules until further experience is gained using oestradiol patches for this clinical indication.
One of the reasons for releasing these data was to respond to an article which reported on the substitution of DES with transdermal oestrogen patches in men with prostate cancer who had progressed during first-line hormonal manipulation [7]. In that study, two Evorel-100 patches were administered twice weekly. No data were given on the oestradiol levels obtained or the effect on serum testosterone level. However, two interesting questions are raised by these second-line data. First, what is the mechanism by which DES produces clinical responses in testosterone-suppressed men who have developed androgen-independent prostate cancer; and second, would patches that release oestradiol be expected to act in the same way as DES? Of the several small published studies documenting the use of DES as second- or third-line therapy, PSA responses appear to occur in 30–40% of patients and last for ≈6 months, with a cardiovascular complication rate of 5–28% [16].
DES is thought to act primarily by producing negative feedback on the hypothalamus and anterior pituitary, resulting in down-regulation of LH and a decrease in testosterone to castrate levels. Although castrate levels of testosterone have been defined as <1.7 nmol/L, recent data from Oefelein et al. [8] using current assays show that median testosterone levels after orchidectomy (in 35 men) were 0.5 nmol/L and that for most patients on LHRH therapy (33/38) testosterone levels were <0.7 nmol/L (20 ng/dL) [14]. Thus it is possible that DES could be providing additional down-regulation of LH in patients whose testosterone levels have not reached these lower levels. However, data from the original studies [3] of orchidectomy plus DES (5 mg daily) compared to orchidectomy plus placebo resulted in fewer deaths from prostate cancer in patients with Stage III and IV disease, at 107/473 (23%) vs 132/469 (28%), respectively, suggesting that DES might have additional mechanisms of action.
Additional mechanisms of action that have been postulated for DES include direct cytotoxicity, androgen inactivation, and direct suppression of Leydig cell function in the testis [17]. There are also some in vitro data that question whether DES and other oestrogens would always be expected to have similar clinical effects. Robertson et al. [18] showed that DES inhibits growth and can induce apoptosis in prostate cancer cell lines at physiological concentrations, regardless of androgen sensitivity or oestrogen-receptor status, while oestradiol promoted growth of one of the cell lines positive for oestrogen receptors. Kalach et al. [19] also reported divergent biological effects of oestradiol and DES on a prostate cancer cell line. Oestradiol inhibits the growth of the MOP cell line whereas DES does not, and they suggested that this is due to oestradiol binding to mutated androgen receptors.
In summary, the data presented here provide confirmatory evidence that transdermal oestrogen patches produce castrate levels of testosterone in men. The dosing schedule in the PATCH study has been increased to produce a comparable hormonal response to LHRH therapy, so that there can subsequently be a fair comparison of efficacy. Further information about this trial can be obtained from patch@ctu.mrc.ac.uk. As this is a new clinical indication, the use of transdermal oestrogen patches for treating prostate cancer, whether they are being used as first- or second-line hormonal therapy, should be carefully documented. Preferably data should be obtained in the context of a clinical trial with supporting hormonal, toxicity and efficacy data to aid interpretation.
ACKNOWLEDGEMENTS
Professor Alan Boobis of Imperial College, London for his advice about pharmacokinetics and dosing relating to the administration of the patches. The PATCH study is funded by Cancer Research UK and sponsored by Imperial College London. IFG is supported by the Heart Disease and Diabetes Research Trust.
Abbreviations
- DES
diethylstilbestrol
- IDMC
Independent Data Monitoring Committee
Footnotes
Study Type – Therapy (RCT)
Level of Evidence 1a
CONFLICT OF INTEREST
Paul Abel, Ian Godsland and Stuart Rosen are employees of the sponsor.
REFERENCES
- 1.Carroll PR, Lee K, Fuks ZY, Kantoff PW. Cancer of the prostate. In: DeVita VT, Hellman S, Rosenberg SA, editors. Cancer: Principles and Practice of Oncology. 6th edn. Philadelphia: Lippincott, Williams and Wilkins; 2001. pp. 1435–64. [Google Scholar]
- 2.Krupski TL, Smith MR, Chan Lee W, et al. Natural history of bone complications in men with prostate carcinoma initiating androgen deprivation therapy. Cancer. 2004;101:541–9. doi: 10.1002/cncr.20388. [DOI] [PubMed] [Google Scholar]
- 3.Byar DP. Proceedings: the Veterans Administration Cooperative Urological Research Group's studies of cancer of the prostate. Cancer. 1973;32:1126–30. doi: 10.1002/1097-0142(197311)32:5<1126::aid-cncr2820320518>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 4.Von Schoultz B, Carlstrom K, Collste L, et al. Estrogen therapy and liver function-metabolic effects of oral and parenteral administration. Prostate. 1989;14:389–95. doi: 10.1002/pros.2990140410. [DOI] [PubMed] [Google Scholar]
- 5.Ockrim JL, Lalani EN, Laniado ME, Carter SS, Abel PD. Transdermal estradiol therapy for advanced prostate cancer – forward to the past? J Urol. 2003;169:1735–7. doi: 10.1097/01.ju.0000061024.75334.40. [DOI] [PubMed] [Google Scholar]
- 6.Ockrim JL, Lalani EN, Banks LM, et al. Transdermal estradiol improves bone density when used as single agent therapy for prostate cancer. J Urol. 2004;172:2203–7. doi: 10.1097/01.ju.0000145511.56476.00. [DOI] [PubMed] [Google Scholar]
- 7.Kandola S, Anyamene N, Payne H, Harland S. Transdermal oestrogen therapy as a second-line hormonal intervention in prostate cancer: a bad experience. BJU Int. 2007;99:53–5. doi: 10.1111/j.1464-410X.2007.06543.x. [DOI] [PubMed] [Google Scholar]
- 8.Oefelein MG, Feng A, Scolieri MJ, Ricchiutti D, Resnick MI. Reassessment of the definition of castrate levels of testosterone: implications for clinical decision making. Urology. 2000;56:1021–4. doi: 10.1016/s0090-4295(00)00793-7. [DOI] [PubMed] [Google Scholar]
- 9.Stege R, Carlstrom K, Collste L, Eriksson A, Henriksson P, Posette A. Single drug polyestradiol phosphate therapy in prostate cancer. Am J Clin Oncol. 1988;11:S101–3. [PubMed] [Google Scholar]
- 10.eMC Electronic Medicines Compendium. [Accessed June 2007]. SPC data sheets (Progynova TS 100 and Evorel 100). Available at: http://emc.medicines.org.uk/.
- 11.Geyer D, Gerrits MG, Renoux A, Uhl W. Pharmacokinetics of Fem7, a once-weekly, transdermal oestrogen replacement system in healthy, postmenopausal women. Gynecol Obstet Invest. 1999;48:1–6. doi: 10.1159/000010124. [DOI] [PubMed] [Google Scholar]
- 12.Reginster JY, Albert A, Deroisy R, et al. Plasma estradiol concentrations and pharmacokinetics following transdermal application of Menorest 50 or Systen (Evorel) 50. Maturitas. 1997;27:179–86. doi: 10.1016/s0378-5122(97)00027-3. [DOI] [PubMed] [Google Scholar]
- 13.Hossain M, Quebe-Fehling E, Sergejew T, et al. Dose proportionality study of four doses of an estradiol transdermal system, Estradot. Maturitas. 2003;46:173–85. doi: 10.1016/s0378-5122(03)00189-0. [DOI] [PubMed] [Google Scholar]
- 14.Oefelein MG, Cornum R. Failure to achieve castrate levels of testosterone during luteinizing hormone releasing hormone agonist therapy: the case for monitoring serum testosterone and a treatment decision algorithm. J Urol. 2000;164:726–9. doi: 10.1097/00005392-200009010-00025. [DOI] [PubMed] [Google Scholar]
- 15.Ockrim J. University of London; 2003. Transdermal oestradiol therapy for the treatment of advanced prostate cancer. MD Thesis. [Google Scholar]
- 16.Malkowicz SB. The role of diethylstilboestrol in the treatment of prostate cancer. Urology. 2001;58(Suppl. 2A):108–13. doi: 10.1016/s0090-4295(01)01252-3. [DOI] [PubMed] [Google Scholar]
- 17.Scherr DS, Pitts WR., Jr The nonsteroidal effects of diethylstilbestrol: the rationale for androgen deprivation therapy without estrogen deprivation in the treatment of prostate cancer. J Urol. 2003;170:1703–8. doi: 10.1097/01.ju.0000077558.48257.3d. [DOI] [PubMed] [Google Scholar]
- 18.Robertson CN, Roberson KM, Padilla GM, et al. Induction of apoptosis by diethylstilbestrol in hormone-insensitive prostate cancer cells. JNCI. 1996;88:908–17. doi: 10.1093/jnci/88.13.908. [DOI] [PubMed] [Google Scholar]
- 19.Kalach J, Joly-Pharaboz M, Chantepie J, et al. Divergent biological effects of estradiol and diethylstilbestrol in prostate cancer cell line MOP. J Steroid Biochem Mol Biol. 2005;96:119–29. doi: 10.1016/j.jsbmb.2005.02.012. [DOI] [PubMed] [Google Scholar]


