Abstract
Adiponectin is an insulin sensitizer in muscle and liver and low serum levels characterise obesity and insulin resistance. Eight tagging SNPs in the ADIPOQ gene and promoter were selected and association with serum adiponectin was tested in two independent samples of Caucasian females: the Chingford Study (n=808, mean age 62.8±5.9 years) and Twins UK (n=2718, mean age 47.4±12.6 years). In the Chingford cohort, −11391 G/A, −10066 G/A (rs182052), −7734 C/A (rs16861209), +276 G/T (rs1501299) and +3228 C/T (rs1063537) were significantly associated with fasting serum adiponectin (Ps=1.00 × 10−4 to 1.40 × 10−2). Associations with all except +3228 C/T were replicated in the Twins UK cohort (Ps=3.19 × 10−9 to 6.00 × 10−3). In Chingford subjects, the twelve most common 8-SNP haplotypes (freq. 1.90%) explained 2.85% (p=5.00 × 10−2) and in Twins UK subjects, the four most common 5-SNP haplotypes (freq. >5.00%) explained 1.66% of the variance (p=5.83 × 10−7). To investigate effects of −11391 G/A (rs17300539) and −11377 C/G (rs266729) on promoter activity, 1.2 kb of the ADIPOQ promoter region was cloned in a luciferase reporter plasmid and the four haplotypes were transfected in differentiated 3T3-L1 adipocytes. No significant allelic effects on promoter activity were found.
Keywords: adiponectin, gene transfection, genetic epidemiology, metabolic syndrome, single nucleotide polymorphism
Introduction
Adipose tissue is the major depot for energy storage and an active endocrine organ, secreting a variety of proteins that influence metabolism (Ahima and Flier 2000). Adiponectin, the major adipocyte secretory protein, is one of several adipokines with roles in insulin sensitivity. It is a potent insulin sensitizer in muscle and liver, regulating energy homeostasis and glucose tolerance (Yamauchi et al.2001). Although adiponectin derives exclusively from adipose tissue (Arita et al. 1999), obese subjects have significantly lower plasma adiponectin concentrations than non-obese subjects (Valle et al. 2005). Hypoadiponectinemia is also a general feature of metabolic syndrome traits: insulin resistance and type 2 diabetes (Duncan et al. 2004); cardiovascular disease (Kumada et al. 2003), hypertension (Iwashima et al. 2004) and dyslipidemia (Kazumi et al. 2004).
Adiponectin levels have a strong genetic component, with heritability estimated between 30 and 50% (Comuzzie et al. 2001). Adiponectin is the product of the ADIPOQ gene, which spans approximately 15.8 kb and three exons. It is sited on chromosome 3q27, which has been linked to a susceptibility locus for metabolic syndrome, type 2 diabetes and cardiovascular disease (Vionnet et al. 2000; Francke et al. 2001).
The gene is very polymorphic; associations with adiponectin level and/or the metabolic syndrome have been reported for genetic variants in many populations, but often with conflicting results. Two promoter SNPs −11391 G/A and −11377 C/G have been reported to show the strongest associations with serum adiponectin (Bouatia-Naji et al. 2006; Vasseur et al. 2002; Vasseur et al. 2005; Pollin et al. 2005). The exon 2 synonymous +45 T/G and +276 G/T SNP in intron 2 have been reported to be associated with serum adiponectin, (Bouatia-Naji et al. 2006, Pollin et al. 2005; Menzaghi et al. 2002;Hara et al. 2002;Heid et al. 2006; Qi et al 2005; Menzaghi et al 2004), obesity and insulin sensitivity (Menzaghi et al. 2002), type 2 diabetes (Hara et al (2002) and coronary artery disease (Qi et al (2005). However, some of these associations could not be confirmed in other studies (Filippi et al 2004; Ohashi et al. 2004; Vozarova de Courten et al. 2005).
Most investigations to date have been relatively small case-control studies. Few have been based on healthy individuals from the general population, in which primary genetic effects of ADIPOQ variants preceding the development of disease could be evident. Here we present a systematic investigation involving SNPs tagging a 16.95 kb region including the ADIPOQ gene, in two independent samples of healthy Caucasian females: the Chingford Study (n=808, mean age 62.8±5.9 years) and Twins UK (n=2718, mean age 47.4±12.6 years). We selected a set of 8 tSNPs representing 12 common variants, which include SNPs previously reported to be associated with adiponectin. We tested single SNP and haplotype associations in both cohorts.
Although associations between ADIPOQ promoter SNPs and adiponectin have been reported, (Bouatia-Naji et al. 2006; Vasseur et al. 2002; Vasseur et al. 2004), only one attempt has been made to identify a variant influencing gene expression (Bouatia-Naji et al. 2006), and no potentially functional variants have been investigated in adipocytes. We found the strongest association between −11391 G/A and adiponectin in our two cohorts and proceeded to analyze the effects of four −11391 G/A / −11377 C/G haplotypes on promoter activity in differentiated 3T3-L1 adipocytes.
Materials and Methods
Subjects
The Chingford Longitudinal Study cohort comprises 1,003 women derived from a large general practice in North London, described in detail previously (Hart and Spector 1993). They are similar to the UK population for most demographic variables. Twins UK (St Thomas’ UK Adult Twin Registry) comprises unselected, mostly female volunteers ascertained from the general population through national media campaigns in the UK (Spector and Williams 2006). The study cohort comprised 2718 randomly selected subjects (822 MZ, 1896 DZ). Means and ranges of quantitative phenotypes in Twins UK were similar to an age-matched sample from the general population in the UK (Andrew et al. 2001).
Phenotypes and DNA were collected in the same manner and using the same methods in both cohorts. Informed consent was obtained from participants before they entered the study and approved by the local research ethics committee. The number of individuals in both study cohorts with data on various phenotypic variables is shown in Table 1.
TABLE 1.
General characteristics of subjects
| Variable | Chingford | Twins UK | |||
|---|---|---|---|---|---|
| N* | Mean ± SD | N* | MZ/DZ | Mean ± SD | |
| Age, years | 808 | 62.8, 5.9 | 2718 | 822/1896 | 47.4, 12.6 |
| Post-menopausal, % | 758 | 100 | 2401 | 620/1781 | 47.6 |
| Adiponectin, μg/ml | 800 | 20.35, 12.56 | 1834 | 288/1546 | 8.21, 4.0 |
| Leptin ng/ml | 798 | 20.75, 16.54 | 2718 | 822/1896 | 16.6, 12.0 |
| BMI, kg/m2 | 808 | 26.8, 4.7 | 2702 | 820/1882 | 24.8, 4.4 |
| Weight, kg | 808 | 69.2, 12.7 | 2703 | 820/1883 | 65.4, 11.8 |
| Waist, cm | 807 | 80.4, 10.1 | 2653 | 797/1856 | 78.4, 10.2 |
| Total fat, g | 773 | 29523, 9932 | 2664 | 786/1878 | 23463, 8804 |
| Total fat, % | 773 | 42.5, 7.6 | 2625 | 765/1860 | 35.6, 8.0 |
| Central fat, g | 785 | 2093, 967 | 2643 | 781/1862 | 1333, 727 |
| Central fat, % | 785 | 35.8, 10.6 | 2643 | 781/1862 | 31.2, 11.5 |
Number of subjects with phenotype data on at least one variable and genotype data on at least one tSNP. MZ monozygous twins; DZ Dizygous twins.
Zygosity, body composition and biochemical analyses
Zygosity in Twins UK subjects was determined by standardised questionnaire and confirmed by DNA fingerprinting. Anthropometric measurements were taken as described previously (Jamshidi et al. 2006). Total and central body fat measurements were obtained by DEXA body composition scans (Hologic QDR-2000, Vertec, Waltham, MA). Blood samples for analyses were drawn after a minimum 8-h overnight fast and serum was stored at −45 °C until analyzed. Serum leptin concentration was determined using a radioimmunoassay (Linco Research, St Louis, MO, USA). Fasting serum total adiponectin levels were determined using an ELISA (Chingford) (R&D Systems, Minneapolis, MN, USA) or two-site DELFIA (Twins UK) using antibodies and standards from R&D Systems.
Genotyping for SNP validation and tSNP selection
We undertook a careful literature review and our candidate tSNPs included those previously reported to be significantly associated with serum adiponectin. Eleven validated SNPs with MAF >0.05 on chr3:188,042,000 to 188,058,94 were identified in the NCBI database (http://www.ncbi.nlm.nih.gov/SNP) and one additional SNP, +2109 A/− in the HGV database (http://hgvbase.cgb.ki.SE). These twelve SNPs were genotyped in 94 randomly selected unrelated Twins UK subjects, using PCR and restriction digest. Primers and PCR conditions are given in ESM Table S1. The rs numbers and relative positions of the SNPs are shown in Fig.1. We used the program htSNP2 to identify an optimal subset of tSNPs (Chapman et al. 2003), as described previously (Spencer-Jones et al. 2005). A set of 8 tSNPs that predicted remaining SNPs with a minimum r2 of 0.85 was selected. The relative positions of tSNPs with respect to the first coding base in exon 2 are shown in parentheses: rs16861194 (−11426 A/G), rs17300539 (−11391 G/A), rs182052 (−10066 G/A), rs16861209 (−7734 C/A), rs822395 (−4041 A/C), rs1501299 (+276 G/T), rs3821799 (+639 C/T) and rs1063537 (+3228 C/T).
FIG. 1. Genomic map of ADIPOQ gene with locations of the validated SNPs genotyped in 94 subjects.
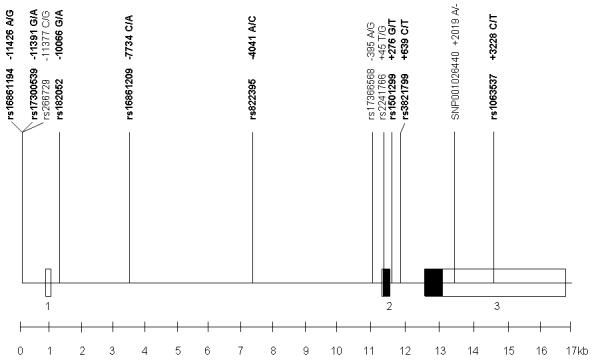
Tagging SNPs in bold. Boxes represent exons, open boxes represent untranslated regions.
Genotyping in cohorts
The 8 tSNPs were genotyped in the complete cohorts by Pyrosequencing (Biotage, Uppsala, Sweden). Genotyping accuracy, as assessed by inclusion of duplicates (pairs of MZ twins) in the arrays, was 98% and negative controls (water blanks) were included on each plate. Genotyping success rates varied between 87% and 96%. Primers and PCR conditions for SNP genotyping in the full cohort by Pyrosequencing, are given in ESM Table S2.
Statistical analysis
Preliminary analyses were performed using STATA 8 (StataCorp, College Station, Texas). Where needed, phenotypic variables were log transformed to obtain better approximations of the normal distribution prior to analysis. Hardy-Weinberg equilibrium was tested by a χ2 test with 1 df in Chingford subjects or in Twins UK, using one twin of each pair chosen at random to prevent inflated significance. For tSNP selection, pairwise linkage disequilibrium (LD) coefficients were calculated using GOLD and reported as r2 (Abecasis and Cookson 2000).
Regular regression analysis was used to test genotype-phenotype association in the Chingford cohort. In the Twins UK cohort Generalized Estimating Equations (GEE) (Trégouët et al. 1997) were used, which allows for the relatedness between twins and yields unbiased standard errors and p-values. For individual SNP association analyses, we first performed a 2-df overall test of genotypic association. In the presence of a significant association, additive, dominant and recessive models (all 1-df) were further tested to find the best mode of inheritance. Age and menopausal status were included as covariates in the models. Associations were also confirmed by sib-TDT based on DZ twin pairs discordant for genotype. We first tested SNP associations with phenotypes in the Chingford cohort and subsequently tested replication of significant associations in the larger Twins UK sample. The Chingford cohort (812 unrelated female subjects) provided 80% power for an alpha = 0.05 to detect a QTL effect as low as 1.2% if the locus is tagged with an r2≥0.85. The Twins UK cohort (with adiponectin and genotype data on 1834 individual twins) provided more than 80% power for an alpha = 0.05 to detect a QTL effect as low as 0.65% if the locus is tagged with an r2≥0.85. Haplotypes were inferred using PHASE software (Stephens et al. 2001). Frequencies of the haplotypes of eight tSNPs were determined in the Chingford cohort and of the five showing positive association with adiponectin in Chingford, were determined in the Twins UK cohort. Association of haplotypes with serum adiponectin was then tested in each cohort.
Cell Culture
3T3-L1 contact inhibited pre-adipocytes were maintained in high-glucose DMEM, 10% fetal bovine serum at 37° C with 5% CO2 and differentiated by treating 3 day postconfluent cells with media containing 0.5 mM 3-isobutyl-1-methyl-xanthine, 0.25 μM dexamethasone and 1 μg/ml insulin for 2 days. On day 2 the media were replaced with DMEM and 10% fetal bovine serum containing insulin (1μg/ml) for a further 48 h before returning the cells to normal cell culture conditions. All reagents were from Sigma-Aldrich, Gillingham, Dorset, UK.
Promoter activity assays
Transient transfection and reporter assays were performed to investigate whether there were differences in promoter activity among the different haplotypes derived from the −11391 G/A (rs17300539) and the adjacent −11377 C/G SNP (rs266729). For each of the four haplotypes, the corresponding ADIPOQ promoter (from position −1169 bp to +15 bp relative to the transcriptional start site) was generated by PCR using genomic DNA as template and then inserted into a plasmid (pGL3-basic vector, Promega, Southampton, UK) containing a firefly luciferase reporter gene. All constructs were verified by DNA sequencing. Differentiated 3T3 cells were transiently transfected with each of the promoter constructs with the use of FuGENE 6 transfection reagent (Roche Diagnostics, Welwyn, UK). A plasmid containing a Renilla luciferase gene under the control of a thymidine kinase promoter (pRL-TK, Promega), was co-transferred into the cells to serve as a reference for transfection efficiency. At 24 hours after transfection cells were lysed and the activities of firefly luciferase and Renilla luciferase in the lysates were measured with the use of a dual-luciferase assay kit (Promega). Promoter activity was determined according to the ratio of firefly luciferase activity to Renilla luciferase activity. Three independent experiments were performed. In each experiment, transfection and luciferase assays were carried out in triplicate for each construct.
Results
All twelve selected SNPs in the ADIPOQ region tested in 94 Twins UK subjects were polymorphic, with MAF >0.05. Fig. 1 shows the positions of these SNPs. All except rs2241766 (+45 T/G, exon 2) were located in non-coding regions and their genotype frequencies were consistent with Hardy-Weinberg proportions. Some SNPs showed strong pairwise LD (linkage disequilibrium), (ESM Table S3) suggesting the feasibility of tSNP selection. 8 tSNPs were selected by htSNP2 (Chapman et al. 2003), which can predict the unmeasured loci with r2≥0.85 (see Methods). The allele frequencies were similar in both cohorts and none of the loci showed deviation from Hardy Weinberg equilibrium.
Table 2 presents analysis of the association of individual tSNPs with fasting serum adiponectin. In the Chingford cohort, the major allele of −10066 G/A and the minor alleles of −11391 G/A, −7734 C/A, +276 G/T and +3228 C/T were significantly associated with elevated adiponectin, explaining between 1.00 and 1.70% of the variance. These five tSNPs were then genotyped in the larger Twins UK cohort. Associations with four tSNPs, −10066 G/A, −7734 C/A, −11391 G/A and +276 G/T were replicated, explaining between 0.93 and 1.88% of the variance. With the exception of −10066 G/A, these were also confirmed by TDT based on DZ twin pairs discordant for genotype (Table 3).
TABLE 2.
Chingford and Twins UK cohorts: Associations of ADIPOQ tSNPs with fasting serum adiponectin
| Chingforda | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| tSNP | Number | Mean ± SD serum adiponectin μg/ml | P | Explained variance | |||||
| 11/12/22 | 11 | 12 | 22 | Codominant | Additive | Dominant | Recessive | ||
| −11423 A/G | 654/111/5 | 20.41, 11.84 | 21.17, 14.75 | 18.55, 12.31 | NS | NS | NS | NS | --- |
| −11391 G/A | 624/129/7 | 19.74, 12.25 | 23.15, 12.31 | 27.03, 13.00 | 6.00 × 10−4 | 1.00 × 10−4 | 1.00 × 10−4 | NS | 1.70% |
| −10066 G/A | 315/317/90 | 21.81, 12.48 | 19.44, 11.47 | 20.07, 14.38 | 1.00 × 10−2 | 6.00 × 10−3 | 3.00 × 10−3 | NS | 1.40% |
| −7734 C/A | 585/140/8 | 19.80, 12.11 | 22.87, 12.28 | 24.29, 14.31 | 4.00 × 10−3 | 1.00 × 10−3 | 1.00 × 10−3 | NS | 1.37% |
| −4041 A/C | 293/341/93 | 20.39, 12.14 | 20.39, 12.69 | 20.07, 10.77 | NS | NS | NS | NS | --- |
| +276 G/T | 391/302/53 | 19.51, 12.70 | 21.61, 11.63 | 20.38, 11.68 | 3.10 × 10−3 | 6.00 × 10−3 | 1.00 × 10−3 | NS | 1.28% |
| +639 C/T | 197/356/140 | 19.22, 12.89 | 20.75, 12.08 | 20.48, 10.79 | NS | NS | NS | NS | --- |
| +3228 C/T | 607/164/10 | 19.90, 12.25 | 21.87, 11.78 | 24.87, 12.10 | 3.60 × 10−2 | 1.00 × 10−2 | 1.40 × 10−2 | NS | 1.00% |
| Twins UKb | |||||||||
| tSNP | Number | Mean ± SD serum adiponectin μg/ml | P | Explained variance | |||||
| 11/12/22 | 11 | 12 | 22 | Codominant | Additive | Dominant | Recessive | ||
| −11391 G/A | 1434/246/5 | 8.00, 3.90 | 9.48, 4.14 | 9.32, 3.48 | 1.23 × 10−8 | 5.66 × 10−9 | 3.19 × 10−9 | NS | 1.88% |
| −10066 G/A | 722/797/187 | 8.67, 4.35 | 8.07, 3.78 | 7.27, 3.28 | 2.50 × 10−3 | 1.00 × 10−3 | 6.00 × 10−3 | 7.00 × 10−3 | 1.03% |
| −7734 C/A | 1363/278/10 | 8.05, 3.89 | 9.11, 4.42 | 9.94, 3.70 | 3.70 × 10−5 | 9.69 × 10−6 | 8.09 × 10−6 | NS | 0.93% |
| +276 G/T | 927/605/94 | 7.89, 3.77 | 8.74, 4.32 | 8.39, 3.48 | 2.00 × 10−4 | 2.80 × 10−4 | 6.18 × 10−5 | NS | 1.04% |
| +3228 C/T | 1244/367/18 | 8.22, 4.00 | 8.40, 4.22 | 8.22, 3.22 | NS | NS | NS | NS | --- |
Linear regression analysis
GEE analysis. NS: Not significant P<0.05.
TABLE 3.
Twins UK cohort: Transmission disequilibrium test ADIPOQ tSNPs with fasting serum adiponectin
| tSNP | Number | Mean ± SD serum adiponectin μg/ml | P | |||
|---|---|---|---|---|---|---|
| 11 | 12 | 22 | Additive | Dominant | ||
| −11391 G/A | 98 | 7.99, 3.69 | 9.98, 4.03 | 9.08, 3.96 | 1.69 × 10−4 | |
| −10066 G/A | 227 | 8.72, 4.76 | 8.00, 4.03 | 7.80, 3.69 | NS | |
| −7734 C/A | 104 | 7.86, 3.71 | 9.70, 4.07 | 9.70, 4.15 | 7.82 × 10−4 | |
| +276 G/T | 184 | 7.62, 3.63 | 8.54, 4.44 | 9.44, 4.31 | 3.62 × 10−2 | |
| +3228 C/T | 114 | 8.50, 4.09 | 8.71, 4.42 | 7.24, 2.33 | NS | |
| 11 | 12/22 | |||||
| −11391 G/A | 95 | 7.99, 3.69 | 9.86, 4.06 | 8.60 × 10−5 | ||
| −10066 G/A | 162 | 8.72, 4.76 | 7.95, 3.84 | NS | ||
| −7734 C/A | 98 | 7.86, 3.71 | 9.60, 4.12 | 3.25 × 10−4 | ||
| +276 G/T | 159 | 7.62, 3.63 | 8.56, 4.53 | 2.53 × 10−2 | ||
| +3228 C/T | 109 | 8.50, 4.09 | 8.70, 4.33 | NS | ||
NS: Not significant P<0.05
We then determined the haplotype frequencies of the eight selected tSNPs in the Chingford cohort and the haplotype frequencies of the five tSNPs showing positive association with adiponectin in Chingford, in the Twins UK cohort. In Chingford, 86 haplotypes were represented (ESM Table S4). We tested association of the twelve most frequent haplotypes with serum adiponectin (Table 4). Two showed significant increases compared to the most common haplotype: numbers 5 and 12. Overall, these twelve haplotypes explained 2.85% of the variance in adiponectin levels (p=5.00 × 10−3). In the Twins UK cohort, eighteen haplotypes were represented (ESM Table S5). We tested association of the four most frequent with serum adiponectin (Table 4). One, number 4, was associated with an increase compared to the most common haplotype and overall, the four haplotypes explained 1.66% of the variance in adiponectin levels (p=5.83 × 10−7).
TABLE 4.
Chingford and Twins UK cohorts: ADIPOQ tSNP haplotype association with serum adiponectin
| Chingford | ||||
|---|---|---|---|---|
| Haplotype* | beta | P | overall P | Explained variance |
| 1. 11211111 | --- | --- | 5.00 × 10−4 | 2.85% |
| 2. 11111221 | 1.522 | NS | --- | --- |
| 3. 11111111 | 1.512 | NS | --- | --- |
| 4. 11112111 | 3.170 | NS | --- | --- |
| 5. 12122221 | 6.719 | 4.00 × 10−3 | --- | --- |
| 6. 11111122 | 4.824 | NS | --- | --- |
| 7. 11212111 | -2.272 | NS | --- | --- |
| 8. 11112121 | -6.734 | NS | --- | --- |
| 9. 21211111 | 2.024 | NS | --- | --- |
| 10. 21211221 | 7.965 | NS | --- | --- |
| 11. 11211122 | -1.493 | NS | --- | --- |
| 12. 12122122 | 12.253 | 6.00 × 10−3 | --- | --- |
| Twins UK | ||||
| Haplotype* | beta | P | overall P | Explained variance |
| 1. 11111 | --- | --- | 5.83 × 10−7 | 1.66% |
| 2. 11121 | 0.42 | NS | --- | --- |
| 3. 11112 | 0.14 | NS | --- | --- |
| 4. 22221 | 3.50 | 1.39 × 10−8 | --- | --- |
tSNP sequence is −11426A/G, −11391G/A, −10066G/A, −7734C/A, −4041A/C, +276G/T, +639C/T, +3228C/T.
NS: Not significant P< 0.05
tSNP sequence is −11391G/A, −10066G/A, −7734C/A, +276G/T, +3228C/T.
NS: Not significant P<0.05.
The influence of −11391 G/A and adjacent SNP −11377 C/G (14 bp 3′) on ADIPOQ promoter activity was tested using a luciferase reporter gene system. A third SNP, rs16861194 (−11423 A/G, also reported as −11426 A/G) was included in the 1.2 kb plasmid insert, with the common allele A present on the four −11391/−11377 haplotypes. We did not test allelic effects of this SNP on promoter activity as we (Table 2) and others found no association between this SNP and adiponectin levels in population studies. The relative activities of promoter constructs carrying the four allelic combinations are shown in Fig. 2. The results are expressed as mean ± SD of the average of the three independent experiments done in triplicate. All four promoter constructs showed increased activity over basic, but there were no significant differences in activity between the four haplotypes.
FIG. 2. Luciferase reporter expression of the rs17300539 (−11391 G/A) and rs266729 (−11377 C/G) ADIPOQ gene promoter polymorphisms in 3T3-L1 cells.
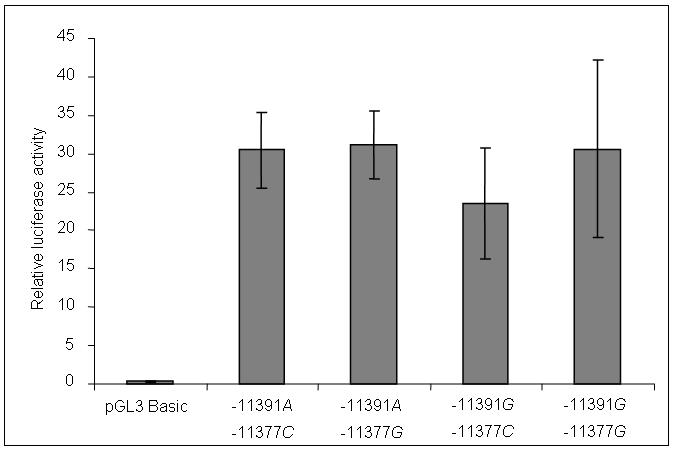
3T3-L1 cells were transfected with the pGL3 basic vector containing 4 allelic combinations of promoter SNPs −11391 G/A and −11377 C/G. Firefly luciferase activity was normalized for transfection efficiency by cotransfection with a Renilla luciferase control plasmid. The results are expressed as mean ± SD of the average of the three independent experiments done in triplicate.
Discussion
We have analysed tagging SNPs covering the full span of ADIPOQ in two large groups of healthy female Caucasians. We observed strong replicated associations of 4 SNPs with adiponectin concentrations: −11391 G/A (promoter), −10066 G/A and −7734 C/A (both intron 1) and +276 G/T (intron 2). We also demonstrated significant single haplotype associations with serum adiponectin in the Chingford and Twin cohorts and overall haplotype effects accounting for up to 2.85% of variance in adiponectin levels. Our transfection experiments demonstrated activity in the proximal promoter of the ADIPOQ gene, but it was not possible to distinguish individual effects of the −11391 G/A and −11377 C/G SNPs on activity.
The main strength of our study lies in use of two large samples of healthy women with serum adiponectin measurements, which permitted replication of significant associations. The Twins UK sample had the advantage of enabling confirmation of associations by sib-transmission disequilibrium tests (sib-TDTs) in DZ twins discordant for genotype, which are insensitive to the effects of population stratification and admixture. We have found the twin subjects to be representative of the UK female population as a whole; the only difference was that MZ twins had a slightly lower weight and a smaller variance for weight than DZ twins and singletons (Andrew et al. 2001). Both samples had adequate statistical power to detect SNPs with small effects and together comprise one of the largest systematic studies of ADIPOQ SNP association with serum adiponectin.
On the current HapMap database (Data Rel#22/phase II Apr 2007), there are currently 25 polymorphic SNPs with MAF >0.05 in the 16.5 kb region spanning our 8 SNPs (including 1.5 kb 5′ and the 3′UTR). According to our analysis of the 90 CEU Trios genotypes using Haploview/Tagger, these are tagged by 15 tSNPs, 6 of which are included in our 8 tSNPs. We have confirmed previously reported associations of elevated adiponectin with −11391 A (Vasseur et al. 2002; Woo et al. 2006); −10066 G (Woo et al. 2006) and +276 T (Vasseur et al. 2002; Menzaghi et al. 2002; Hara et al. 2002; Qi et al. 2005) and found a new association with −7734 A. Most previous investigations have involved relatively small studies comparing patients with type 2 diabetes, hyperglycemia, coronary artery disease or obesity to control subjects. Our study includes the first examination of ADIPOQ variants in over 3000 healthy subjects with measured adiponectin levels. The closest comparable study is that of Heid et al. (2006) based on 1,727 healthy Caucasians. They used the tag SNP program of Stram et al. (2003) to select tSNPs, but interrogated a larger chromosomal region, genotyping 53 SNPs in 81 subjects to select 18 tSNPs.
Vasseur et al. (2002) suggested that the association between +276T and higher serum adiponectin mostly results from LD with −11391A or −11377C. However, according to the current release of HapMap 90 Trios genotypes (HapMap Data Rel#22/phase II Apr 2007) −11377 C/G is not in LD with +276 G/T (also r2=0.10 in our 94 twins, ESM Table S3) and association with +276T resulting from LD with −11391A seems no more likely (r2=0.17 in our 94 twins, ESM Table S3). The association of the +45/+276 T-G haplotype with lower plasma adiponectin level found by Menzaghi et al. (2002), was found to be eliminated in the presence of −11391G (Vasseur et al. 2002) (or failed to appear in the absence of the −11391A (Woo et al. 2006)), so here −11391A seemed to be critical, interacting with the +45/+276 loci to influence adiponectin levels. In a recent twin study, heritability of adiponectin independent of BMI was shown to be partly accounted for by the +45 T/G but not the −11377 C/G SNP (Cesari et al. 2007). All of this evidence seems to favour a role for −11391 G/A in gene expression or as the marker of a functional site. This is in line with our own results, because Chingford, Twins UK and the TDT results all show −11391 G/A to be the most significant locus.
Haplotypes can improve power to detect disease susceptibility regions if they are directly responsible for the observed variation in the trait through the combined effect of multiple variants, or if they are in much higher LD with the functional polymorphism than the individual markers (Bader 2001). In population studies, −11391G (Vasseur et al 2002), −11377G (Hoefle et al. 2007) and the −11391/−11377 G-G haplotype (Vasseur et al 2002; Schwarz et al. 2006; Petrone et al 2006) have been consistently associated with low adiponectin levels. Heid et al. (2006) examined 15-SNP haplotypes in approx. 1500 subjects and those associated with the highest adiponectin levels carried −11391A. We identified two haplotypes in Chingford, nos. 5 and 12 and one in Twins UK no. 4 that were associated with significant elevation of serum adiponectin compared to the most common (reference) haplotypes (Table 4). All haplotypes carried alleles −11391A and −7734A. The situation with regard to the other SNPs is confusing. Chingford no.12 and Twins no. 4 had only −11391A and −7734A in common, yet Chingford no.5, less strongly associated than haplotype 12 with adiponectin, had 4 alleles in common with Twins no. 4 −11391A /−7734A /+276T /+3228C. However, the consistent representation of −11391A in haplotypes associated with elevated adiponectin in both Chingford and Twin samples suggests that this is the strongest candidates for a functional effect and strengthens the evidence from single SNP associations described above, that −11391 G/A might drive adiponectin concentration. With no definitive reports having yet shown this to be the case, we made the first attempt to investigate functionality of −11391 G/A and nearby −11377 C/G in adipocytes.
In the only previously reported functional investigation, Bouatia-Naji et al. (2006) showed that wild type G-C construct had significantly lower ADIPOQ promoter activity than A-C (P=2 × 10−3), compatible with lower serum adiponectin in −11391 allele G carriers. However, this was demonstrated in COS7 cells, which do not naturally express adiponectin. Previous characterization of the human ADIPOQ promoter did not identify any putative binding sites for transcription factors at or around SNPs −11391 and −11377 (Schaffler et al. 1998). We have shown that promoter activity resides in the proximal 1.2 kb region. We have not been able to show that the −11391/−11377 G-G constructs had lower promoter activity than the other haplotypes in differentiated adipocytes. The total length of the ADIPOQ promoter is 2.1 kb, but Kita et al. (2005) demonstrated by deletion analysis that the region from −676 to +41 relative to transcription start site (−11060 to −10343 relative to translation start defining SNPs) is sufficient for basal transcriptional activity in 3T3-L1 adipocytes. LD with an unknown functional site in the promoter region remains the most likely explanation for the consistent associations of the −11391 and −11377 SNPs with serum adiponectin. Alternatively, the SNPs may mark a functional site within the gene which may emerge through sequencing haplotypes associated with elevated adiponectin that we identified in the population studies.
In conclusion, considerable human genetic epidemiological data support an important role for adiponectin in glucose and lipid homeostasis. Studies published to date indicate that polymorphisms at the adiponectin locus are predictors of circulating adiponectin levels, insulin-sensitivity and atherosclerosis, however, no consistent effect on BMI or risk of type 2 diabetes is evident (for review see Menzaghi et al. 2007). The challenge is to find out which SNPs are crucial in affecting adiponectin secretion and activity and to understand the molecular mechanisms involved in maintaining the high levels which protect against the development of metabolic disease.
Supplementary Material
Acknowlegments
This work was funded by the British Heart Foundation Project grant No. PG/04/028 and Wellcome Trust project grant number 073142. The Twin Research and Genetic Epidemiology Unit received support from the Wellcome Trust, Arthritis Research Campaign, the Chronic Disease Research Foundation and and the European Union 5th Framework Programme Genom EU twin no. QLG2-CT-2002-01254 and EuroClot project LSHM-CT-2004-005268.
Abbreviations
- ADIPOQ
Adiponectin gene
- BMI
Body mass index
- CEU
Centre d’Etude du Polymorphisme Humain Utah
- DELFIA
Dissociation-Enhanced Lanthanide Fluorescence Immunoassay
- DEXA
Dual Emission X-ray Absorption
- DMEM
Dulbecco’s Modified Eagle’s Medium
- DZ
dizygous
- ELISA
Enzyme-Linked ImmunoSorbent Assay
- GEE
Generalized Estimating Equations
- HGVbase
Human Genome Variation Database
- LD
Linkage disequilibrium
- MAF
Minor allele frequency
- MZ
Monozygous
- NCBI
National Center for Biotechnology Information
- QTL
Quantitative trait locus
- SNP
Single nucleotide polymorphism
- TDT
Transmission disequilibrium test
- tSNP
tagging SNP
Footnotes
Address all correspondence to Dr Sandra O'Dell, King's College London, Nutritional Sciences Division, Franklin-Wilkins Building, London, SE1 9NH, UK.
References
- Abecasis GR, Cookson WO. GOLD - graphical overview of linkage disequilibrium. Bioinformatics. 2000;16:182–183. doi: 10.1093/bioinformatics/16.2.182. [DOI] [PubMed] [Google Scholar]
- Ahima RS, Flier JS. Adipose tissue as an endocrine organ. Trends Endocrinol Metab. 2000;11:327–332. doi: 10.1016/s1043-2760(00)00301-5. [DOI] [PubMed] [Google Scholar]
- Andrew T, Hart D, Snieder H, de Lange M, Spector TD, MacGregor AJ. Are twins and singletons comparable? A study of disease-related and lifestyle characteristics in adult women. Twin Res. 2001;4:464–477. doi: 10.1375/1369052012803. [DOI] [PubMed] [Google Scholar]
- Arita Y, Kihara S, Ouchi N, Takahashi M, Maeda K, Miyagawa J, Hotta K, Shimomura I, Nakamura T, Miyaoka K, Kuriyama H, Nishida M, Yamashita S, Okubo K, Matsubara K, Muraguchi M, Ohmoto Y, Funahashi T, Matsuzawa Y. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem Biophys Res Commun. 1999;257:79–83. doi: 10.1006/bbrc.1999.0255. DOI 10.1006/bbrc.1999.0255. [DOI] [PubMed] [Google Scholar]
- Bader JS. The relative power of SNPs and haplotype as genetic markers for association tests. Pharmacogenomics. 2001;2:11–24. doi: 10.1517/14622416.2.1.11. DOI 10.1517/14622416.2.1.11. [DOI] [PubMed] [Google Scholar]
- Bouatia-Naji N, Meyre D, Lobbens S, Séron K, Fumeron F, Balkau B, Heude B, Jouret B, Scherer PE, Dina C, Weill J, Froguel P. ACDC/adiponectin polymorphisms are associated with severe childhood and adult obesity. Diabetes. 2006;55:545–550. doi: 10.2337/diabetes.55.02.06.db05-0971. [DOI] [PubMed] [Google Scholar]
- Cesari M, Narkiewicz K, De Toni R, Aldighieri E, Williams CJ, Rossi GP. Heritability of plasma adiponectin levels and body mass index in twins. J Clin Endocrinol Metab. 2007;92:3082–3088. doi: 10.1210/jc.2007-0403. DOI 10.1210/jc.2007-0403. [DOI] [PubMed] [Google Scholar]
- Chapman JM, Cooper JD, Todd JA, Clayton DG. Detecting disease associations due to linkage disequilibrium using haplotype tags: a class of tests and the determinants of statistical power. Hum Hered. 2003;56:18–31. doi: 10.1159/000073729. DOI 10.1159/000073729. [DOI] [PubMed] [Google Scholar]
- Comuzzie AG, Funahashi T, Sonnenberg G, Martin LJ, Jacob HJ, Black AE, Maas D, Takahashi M, Kihara S, Tanaka S, Matsuzawa Y, Blangero J, Cohen D, Kissebah A. The genetic basis of plasma variation in adiponectin, a global endophenotype for obesity and the metabolic syndrome. J Clin Endocrinol Metab. 2001;86:4321–4325. doi: 10.1210/jcem.86.9.7878. [DOI] [PubMed] [Google Scholar]
- Duncan BB, Schmidt MI, Pankow JS, Bang H, Couper D, Ballantyne CM, Hoogeveen RC, Heiss G. Adiponectin and the development of type 2 diabetes: the Atherosclerosis Risk in Communities study. Diabetes. 2004;53:2473–2478. doi: 10.2337/diabetes.53.9.2473. [DOI] [PubMed] [Google Scholar]
- Filippi E, Sentinelli F, Trischitta V, Romeo S, Arca M, Leonetti F, et al. Association of the human adiponectin gene and insulin resistance. Eur J Hum Genet. 2004;12:199–205. doi: 10.1038/sj.ejhg.5201120. DOI 10.1007/s00109-005-0667-z. [DOI] [PubMed] [Google Scholar]
- Francke S, Manraj M, Lacquemant C, Lecoeur C, Leprêtre F, Passa P, Hebe A, Corset L, Yan SL, Lahmidi S, Jankee S, Gunness TK, Ramjuttun US, Balgobin V, Dina C, Froguel P. A genome-wide scan for coronary heart disease suggests in Indo-Mauritians a susceptibility locus on chromosome 16p13 and replicates linkage with the metabolic syndrome on 3q27. Hum Mol Genet. 2001;10:2751–2765. doi: 10.1093/hmg/10.24.2751. [DOI] [PubMed] [Google Scholar]
- Hara K, Boutin P, Mori Y, Tobe K, Dina C, Yasuda K, Yamauchi T, Otabe S, Okada T, Eto K, Kadowaki H, Hagura R, Akanuma Y, Yazaki Y, Nagai R, Taniyama M, Matsubara K, Yoda M, Nakano Y, Tomita M, Kimura S, Ito C, Froguel P, Kadowaki T. Genetic variation in the gene encoding adiponectin is associated with an increased risk of type 2 diabetes in the Japanese population. Diabetes. 2002;51:536–540. doi: 10.2337/diabetes.51.2.536. [DOI] [PubMed] [Google Scholar]
- Hart DJ, Spector TD. The relationship of obesity, fat distribution and osteoarthritis in women in the general population: the Chingford Study. J Rheumatol. 1993;20:331–335. [PubMed] [Google Scholar]
- Heid IM, Wagner SA, Gohlke H, Iglseder B, Mueller JC, Cip P, Ladurner G, Reiter R, Stadlmayr A, Mackevics V, Illig T, Kronenberg F, Paulweber B. Genetic architecture of the APM1 gene and its influence on adiponectin plasma levels and parameters of the metabolic syndrome in 1,727 healthy Caucasians. Diabetes. 2006;55:375–384. doi: 10.2337/diabetes.55.02.06.db05-0747. [DOI] [PubMed] [Google Scholar]
- Hoefle G, Muendlein A, Saely CH, Risch L, Rein P, Koch L, Schmid F, Aczel S, Marte T, Langer P, Drexel H. The −11377 C/G promoter variant of the adiponectin gene, prevalence of coronary atherosclerosis, and incidence of vascular events in men. Thromb Haemost. 2007;97:451–457. DOI 10.1160/TH06-11-0646. [PubMed] [Google Scholar]
- Iwashima Y, Katsuya T, Ishikawa K, Ouchi N, Ohishi M, Sugimoto K, Fu Y, Motone M, Yamamoto K, Matsuo A, Ohashi K, Kihara S, Funahashi T, Rakugi H, Matsuzawa Y, Ogihara T. Hypoadiponectinemia is an independent risk factor for hypertension. Hypertension. 2004;43:1318–1323. doi: 10.1161/01.HYP.0000129281.03801.4b. DOI 10.1161/01.HYP.0000129281.03801.4b. [DOI] [PubMed] [Google Scholar]
- Jamshidi Y, Snieder H, Wang X, Pavitt MJ, Spector TD, Carter ND, O’Dell SD. Phosphatidylinositol 3-kinase p85 alpha regulatory subunit gene PIK3R1 haplotype is associated with body fat and serum leptin in a female twin population. Diabetologia. 2006;49:2659–2667. doi: 10.1007/s00125-006-0388-z. DOI 10.1007/s00125-006-0388-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazumi T, Kawaguchi A, Hirano T, Yoshino G. Serum adiponectin is associated with high-density lipoprotein cholesterol, triglycerides, and low-density lipoprotein particle size in young healthy men. Metabolism. 2004;53:589–593. doi: 10.1016/j.metabol.2003.12.008. DOI 10.1016/j.metabol.2003.12.008. [DOI] [PubMed] [Google Scholar]
- Kita A, Yamasaki H, Kuwahara H, Moriuchi A, Fukushima K, Kobayashi M, Fukushima T, Takahashi R, Abiru N, Uotani S, Kawasaki E, Eguchi K. Identification of the promoter region required for human adiponectin gene transcription: Association with CCAAT/enhancer binding protein-beta and tumor necrosis factor-alpha. Biochem Biophys Res Commun. 2005;331:484–490. doi: 10.1016/j.bbrc.2005.03.205. DOI 10.1016/j.bbrc.2005.03.205. [DOI] [PubMed] [Google Scholar]
- Kumada M, Kihara S, Sumitsuji S, Kawamoto T, Matsumoto S, Ouchi N, Arita Y, Okamoto Y, Shimomura I, Hiraoka H, Nakamura T, Funahashi T, Matsuzawa Y, Osaka CAD Study Group Coronary artery disease 2003 Association of hypoadiponectinemia with coronary artery disease in men. Arterioscler Thromb Vasc Biol. 23:85–89. doi: 10.1161/01.atv.0000048856.22331.50. DOI 10.1161/01.ATV.0000048856.22331.50. [DOI] [PubMed] [Google Scholar]
- Menzaghi C, Ercolino T, Di Paola R, Berg AH, Warram JH, Scherer PE, Trischitta V, Doria A. A haplotype at the adiponectin locus is associated with obesity and other features of the insulin resistance syndrome. Diabetes. 2002;51:2306–2312. doi: 10.2337/diabetes.51.7.2306. [DOI] [PubMed] [Google Scholar]
- Menzaghi C, Ercolino T, Salvemini L, Coco A, Kim SH, Fini G, Doria A, Trischitta V. Multigenic control of serum adiponectin levels: evidence for a role of the ADIPOQ gene and a locus on 14q13. Physiol Genomics. 2004;19:170–174. doi: 10.1152/physiolgenomics.00122.2004. DOI 10.1152/physiolgenomics.00122.2004. [DOI] [PubMed] [Google Scholar]
- Menzaghi C, Trischitta V, Doria A. Genetic influences of adiponectin on insulin resistance, type 2 diabetes, and cardiovascular disease. Diabetes. 2007;56:1198–1209. doi: 10.2337/db06-0506. [DOI] [PubMed] [Google Scholar]
- Ohashi K, Ouchi N, Kihara S, Funahashi T, Nakamura T, Sumitsuji S, et al. Adiponectin I164 T mutation is associated with the metabolic syndrome and coronary artery disease. J Am Coll Cardiol. 2004;43:1195–1200. doi: 10.1016/j.jacc.2003.10.049. DOI 10.1016/j.jacc.2003.10.049. [DOI] [PubMed] [Google Scholar]
- Petrone A, Zavarella S, Caiazzo A, Leto G, Spoletini M, Potenziani S, Osborn J, Vania A, Buzzetti R. The promoter region of the adiponectin gene is a determinant in modulating insulin sensitivity in childhood obesity. Obesity (Silver Spring) 2006;14:1498–1504. doi: 10.1038/oby.2006.172. [DOI] [PubMed] [Google Scholar]
- Pollin TI, Tanner K, O’Connell JR, Ott SH, Damcott CM, Shuldiner AR, McLenithan JC, Mitchell BD. Linkage of plasma adiponectin levels to 3q27 explained by association with variation in the ADIPOQ gene. Diabetes. 2005;54:268–274. doi: 10.2337/diabetes.54.1.268. [DOI] [PubMed] [Google Scholar]
- Qi L, Li T, Rimm E, Zhang C, Rifai N, Hunter D, Doria A, Hu FB. The +276 polymorphism of the ADIPOQ gene, plasma adiponectin concentration, and cardiovascular risk in diabetic men. Diabetes. 2005;54:1607–1610. doi: 10.2337/diabetes.54.5.1607. [DOI] [PubMed] [Google Scholar]
- Schaffler A, Langmann T, Palitzsch KD, Scholmerich J, Schmitz G. Identification and characterization of the human adipocyte apM-1 promoter. Biochim Biophys Acta. 1998;1399:187–197. doi: 10.1016/s0167-4781(98)00106-7. [DOI] [PubMed] [Google Scholar]
- Schwarz PE, Towers GW, Fischer S, Govindarajalu S, Schulze J, Bornstein SR, Hanefeld M, Vasseur F. Hypoadiponectinemia is associated with progression toward type 2 diabetes and genetic variation in the ADIPOQ gene promoter. Diabetes Care. 2006;29:1645–1650. doi: 10.2337/dc05-2123. DOI 10.2337/dc05-2123. [DOI] [PubMed] [Google Scholar]
- Spector TD, Williams FM. The UK Adult Twin Registry (Twins UK) Twin Res Hum Genet. 2006;9:899–906. doi: 10.1375/183242706779462462. [DOI] [PubMed] [Google Scholar]
- Spencer-Jones NJ, Wang X, Snieder H, Miller CS, Spector TD, Carter ND, O'Dell SD. Protein tyrosine phosphatase-1B gene PTPN1: Selection of tagging SNPs and association with body fat, insulin sensitivity and the metabolic syndrome in a normal female population. Diabetes. 2005;54:3296–3304. doi: 10.2337/diabetes.54.11.3296. [DOI] [PubMed] [Google Scholar]
- Stephens M, Smith NJ, Donnelly P. A new statistical method for haplotype reconstruction from population data. Am J Hum Genet. 2001;68:978–989. doi: 10.1086/319501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stram DO, Haiman CA, Hirschhorn JN, Altshuler D, Kolonel LN, Henderson BE, Pike MC. Choosing haplotype-tagging SNPs based on unphased genotype data using a preliminary sample of unrelated subjects with an example from the Multiethnic Cohort Study. Hum Hered. 2003;55:27–36. doi: 10.1159/000071807. DOI 10.1159/000071807. [DOI] [PubMed] [Google Scholar]
- Trégouët D-A, Ducimetère P, Tiret L. Testing association between candidate-gene markers and phenotype in related individuals, by use of estimating equations. Am J Hum Genet. 1997;61:189–199. doi: 10.1086/513895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valle M, Martos R, Gascon F, Canete R, Zafra MA, Morales R. Low-grade systemic inflammation, hypoadiponectinemia and a high concentration of leptin are present in very young obese children, and correlate with metabolic syndrome. Diabetes Metab. 2005;31:55–62. doi: 10.1016/s1262-3636(07)70167-2. DOI 10.1019/200512008. [DOI] [PubMed] [Google Scholar]
- Vasseur F, Helbecque N, Dina C, Lobbens S, Delannoy V, Gaget S, Boutin P, Vaxillaire M, Leprêtre F, Dupont S, Hara K, Clément K, Bihain B, Kadowaki T, Froguel P. Single-nucleotide polymorphism haplotypes in the both proximal promoter and exon 3 of the ADIPOQ gene modulate adipocyte-secreted adiponectin hormone levels and contribute to the genetic risk for type 2 diabetes in French Caucasians. Hum Mol Genet. 2002;11:2607–2614. doi: 10.1093/hmg/11.21.2607. [DOI] [PubMed] [Google Scholar]
- Vasseur F, Helbecque N, Lobbens S, Vasseur-Delannoy V, Dina C, Clément K, Boutin P, Kadowaki T, Scherer PE, Froguel P. Hypoadiponectinaemia and high risk of type 2 diabetes are associated with adiponectin-encoding (ACDC) gene promoter variants in morbid obesity: evidence for a role of ACDC in diabesity. Diabetologia. 2005;48:892–899. doi: 10.1007/s00125-005-1729-z. DOI 10.1007/s00125-005-1729-2. [DOI] [PubMed] [Google Scholar]
- Vionnet N, Hani EH, Dupont S, Gallina S, Francke S, Dotte S, De Matos F, Durand E, Leprêtre F, Lecoeur C, Gallina P, Zekiri L, Dina C, Froguel P. Genomewide search for type 2 diabetes susceptibility genes in French whites: evidence for a novel susceptibility locus for early-onset diabetes on chromosome 3q27-qter and independent replication of a type 2 diabetes locus on chromosome 1q21q24. Am J Hum Genet. 2000;67:1470–1480. doi: 10.1086/316887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vozarova de Courten B, Hanson RL, Funahashi T, Lindsay RS, Matsuzawa Y, Tanaka S, Thameem F, Gruber JD, Froguel P, Wolford JK. Common polymorphisms in the adiponectin gene ACDC are not associated with diabetes in Pima Indians. Diabetes. 2005;54:284–289. doi: 10.2337/diabetes.54.1.284. [DOI] [PubMed] [Google Scholar]
- Woo JG, Dolan LM, Deka R, Kaushal RD, Shen Y, Pal P, Daniels SR, Martin LJ. Interactions between noncontiguous haplotypes in the adiponectin gene ACDC are associated with plasma adiponectin. Diabetes. 2006;55:523–529. doi: 10.2337/diabetes.55.02.06.db05-0446. [DOI] [PubMed] [Google Scholar]
- Yamauchi T, Kamon J, Waki H, Terauchi Y, Kubota N, Hara K, Mori Y, Ide T, Murakami K, Tsuboyama-Kasaoka N, Ezaki O, Akanuma Y, Gavrilova O, Vinson C, Reitman ML, Kagechika H, Shudo K, Yoda M, Nakano Y, Tobe K, Nagai R, Kimura S, Tomita M, Froguel P, Kadowaki T. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat Med. 2001;7:941–946. doi: 10.1038/90984. DOI 10.1038/90984. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


