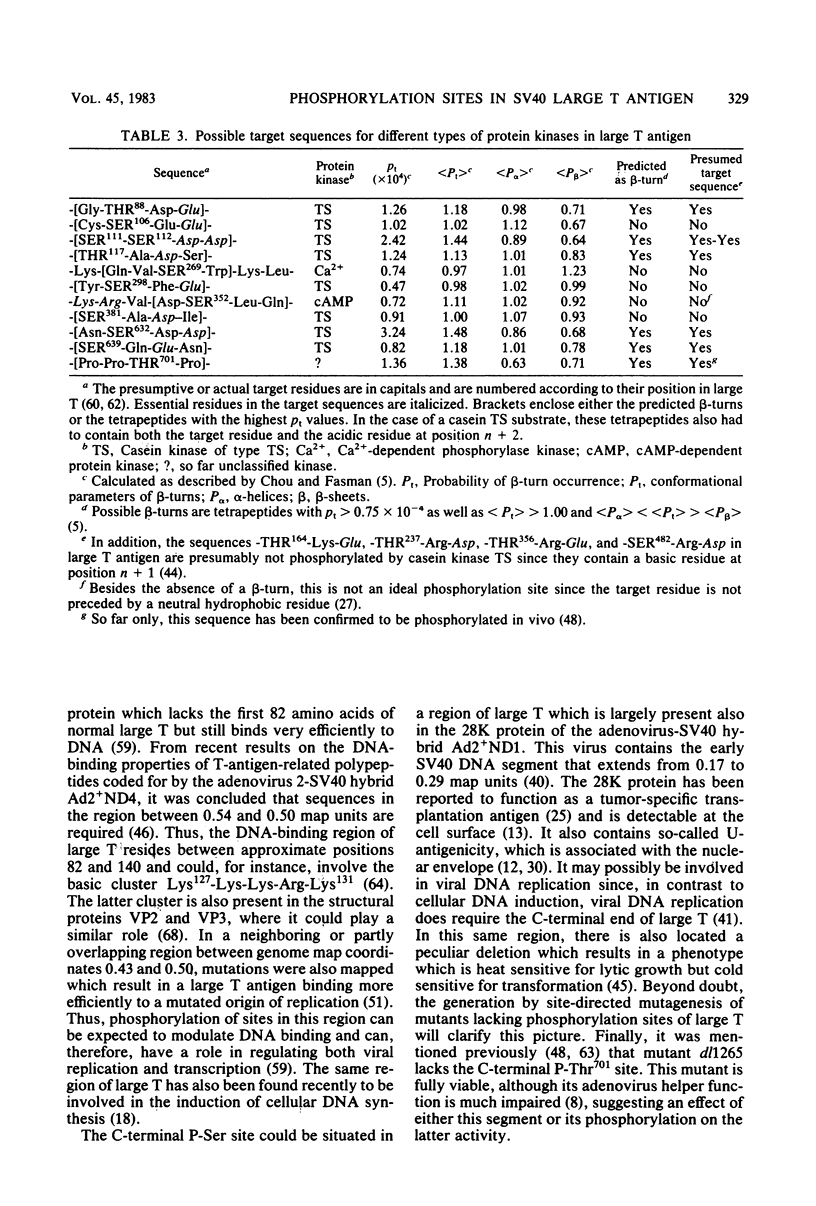
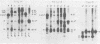Abstract
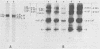
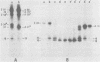
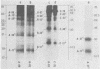
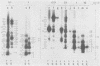
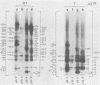
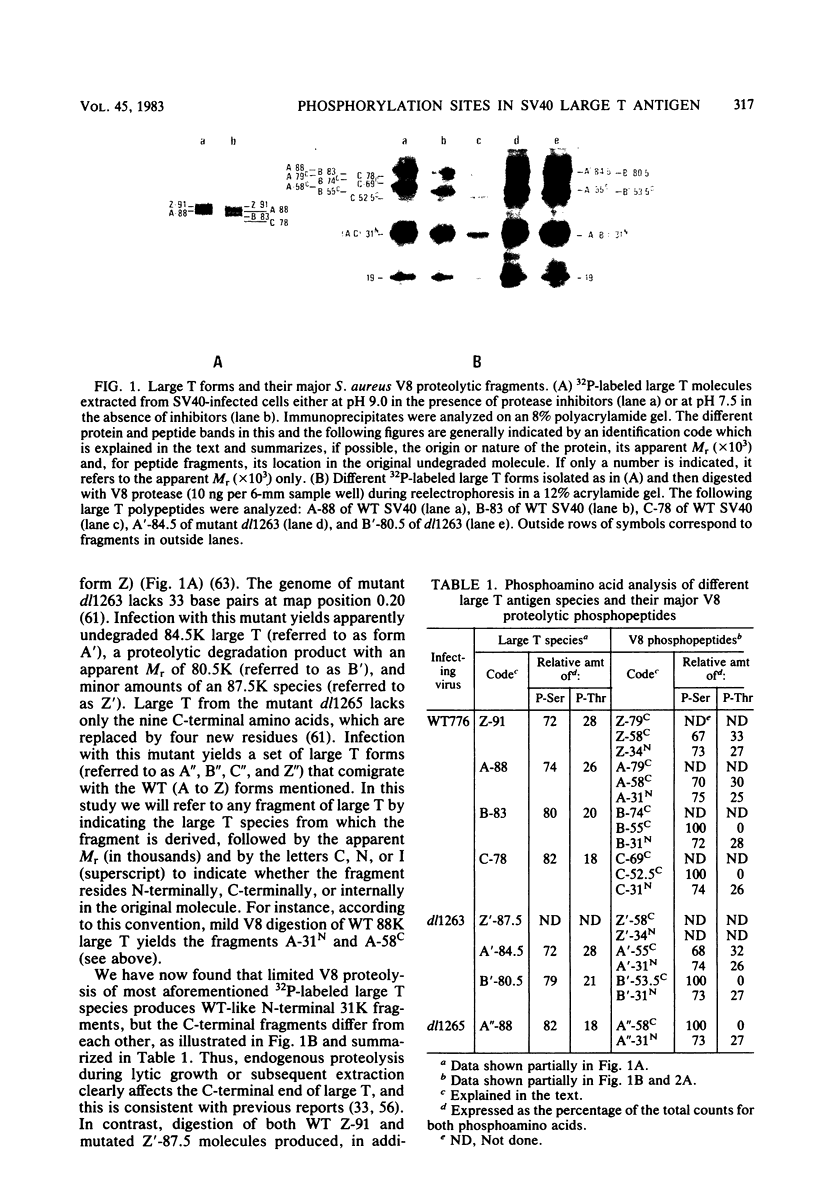
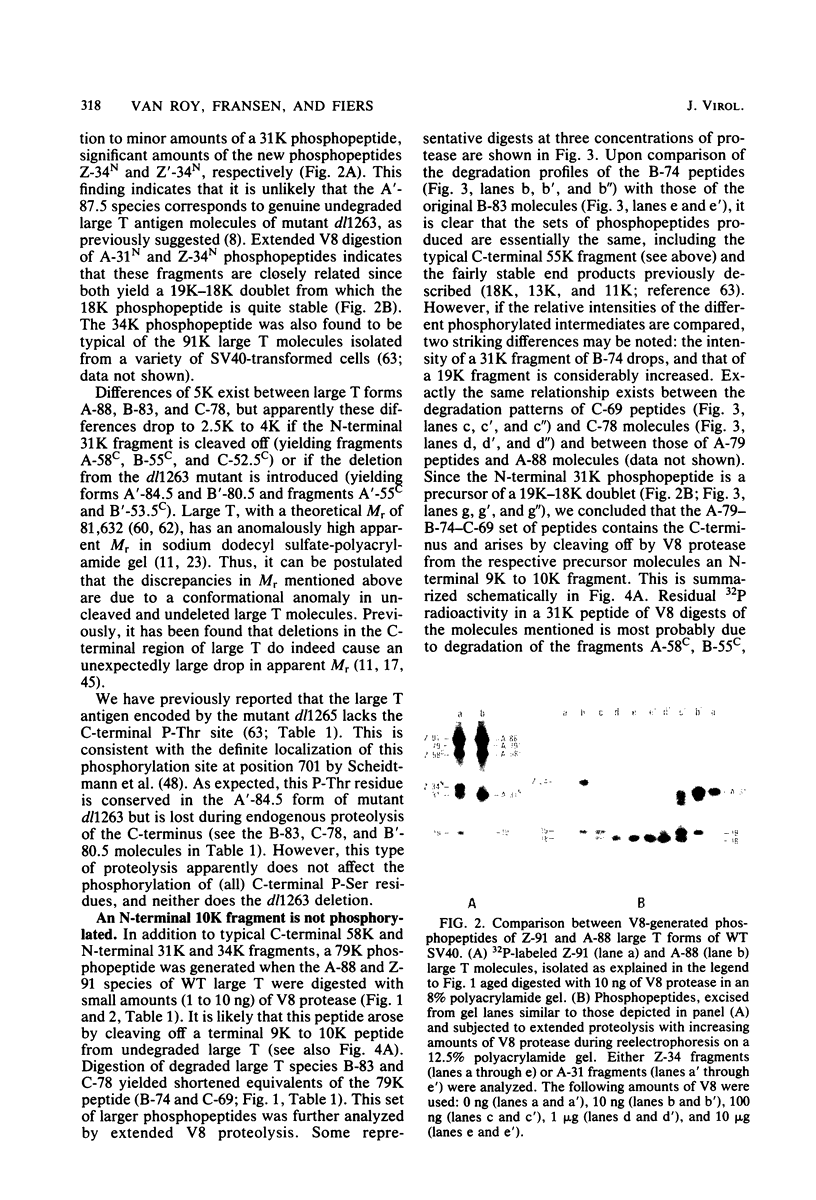
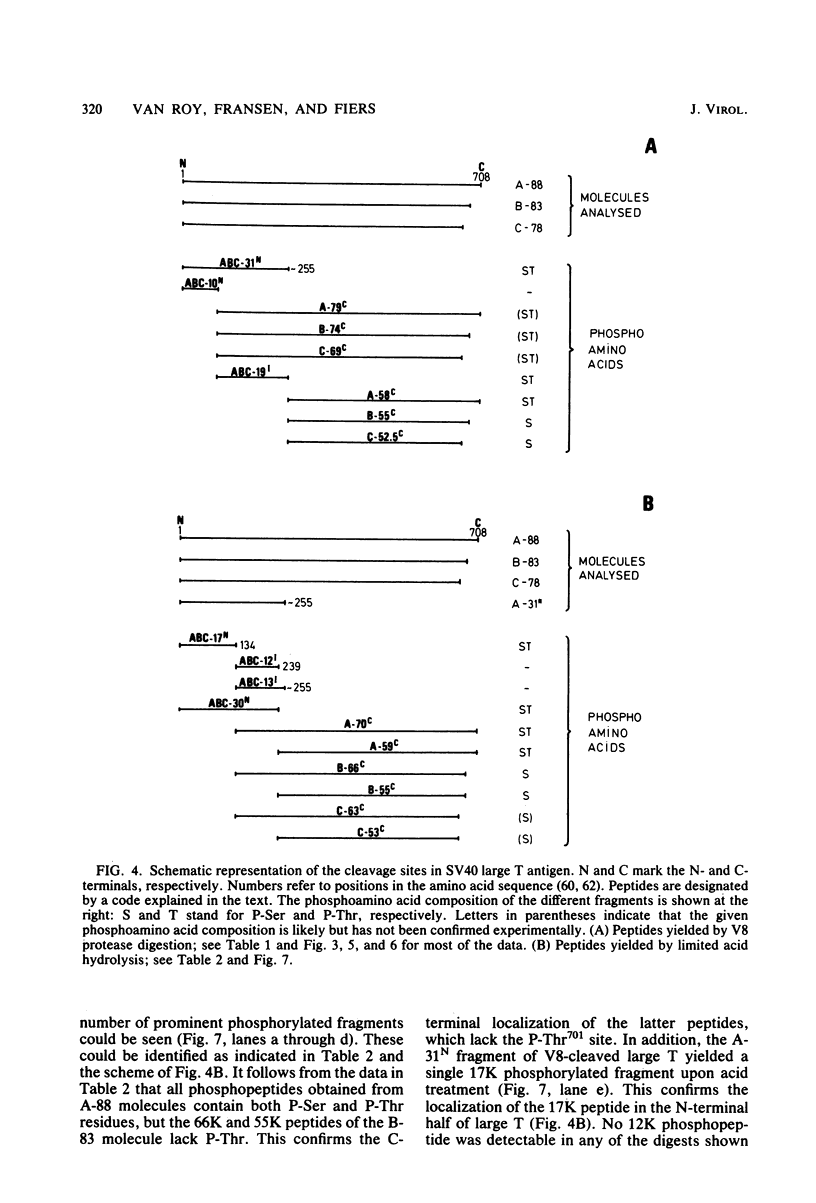
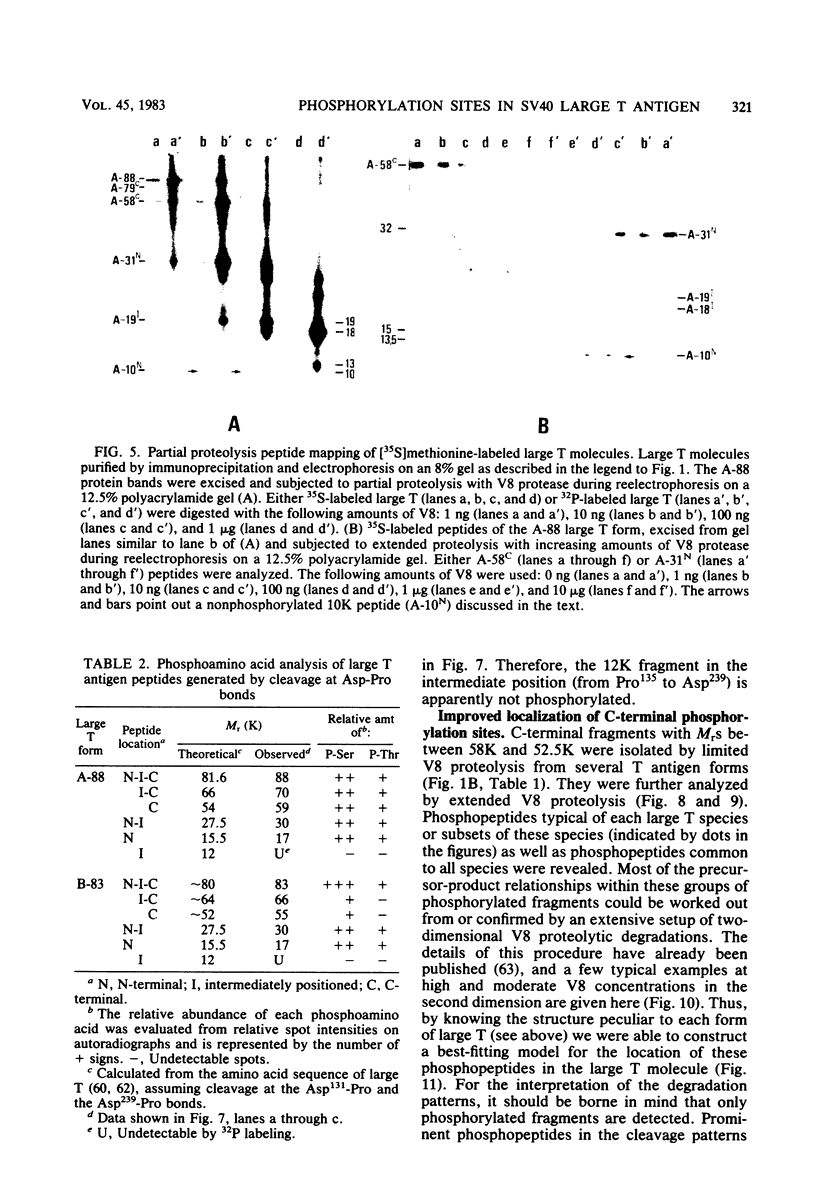
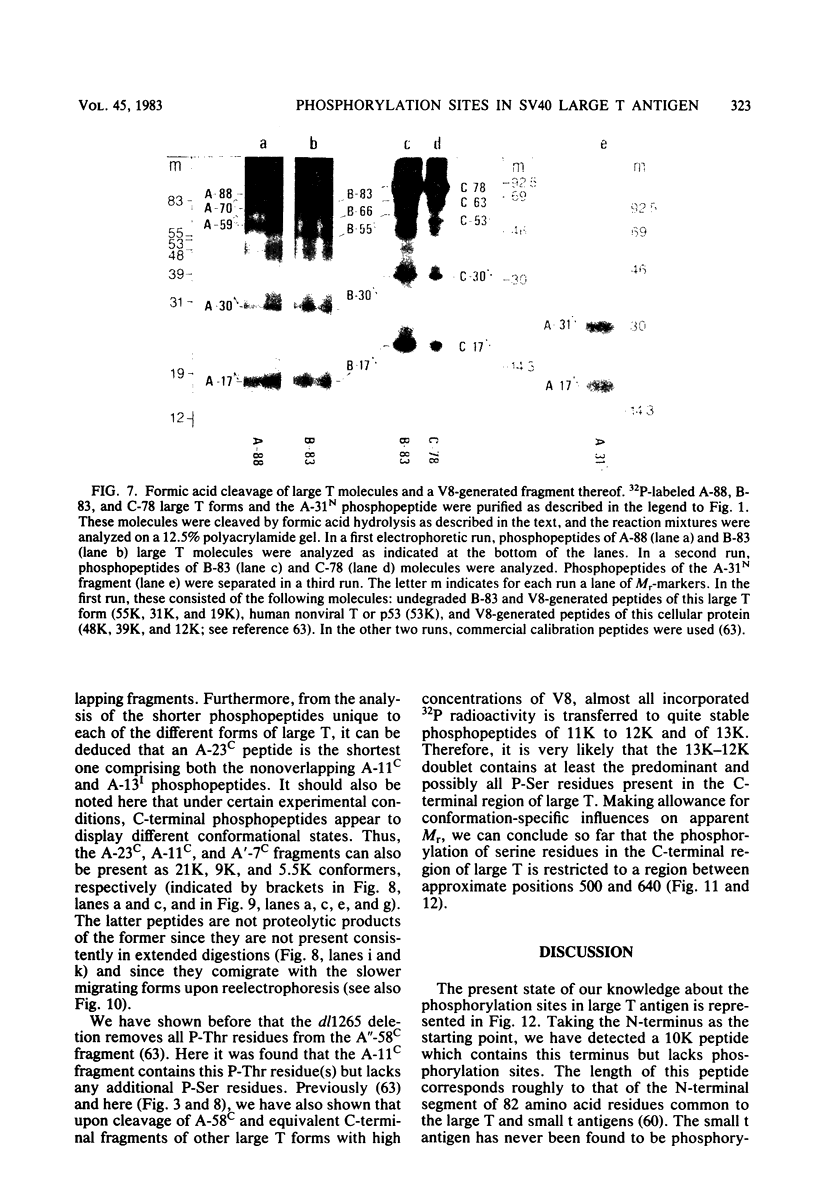
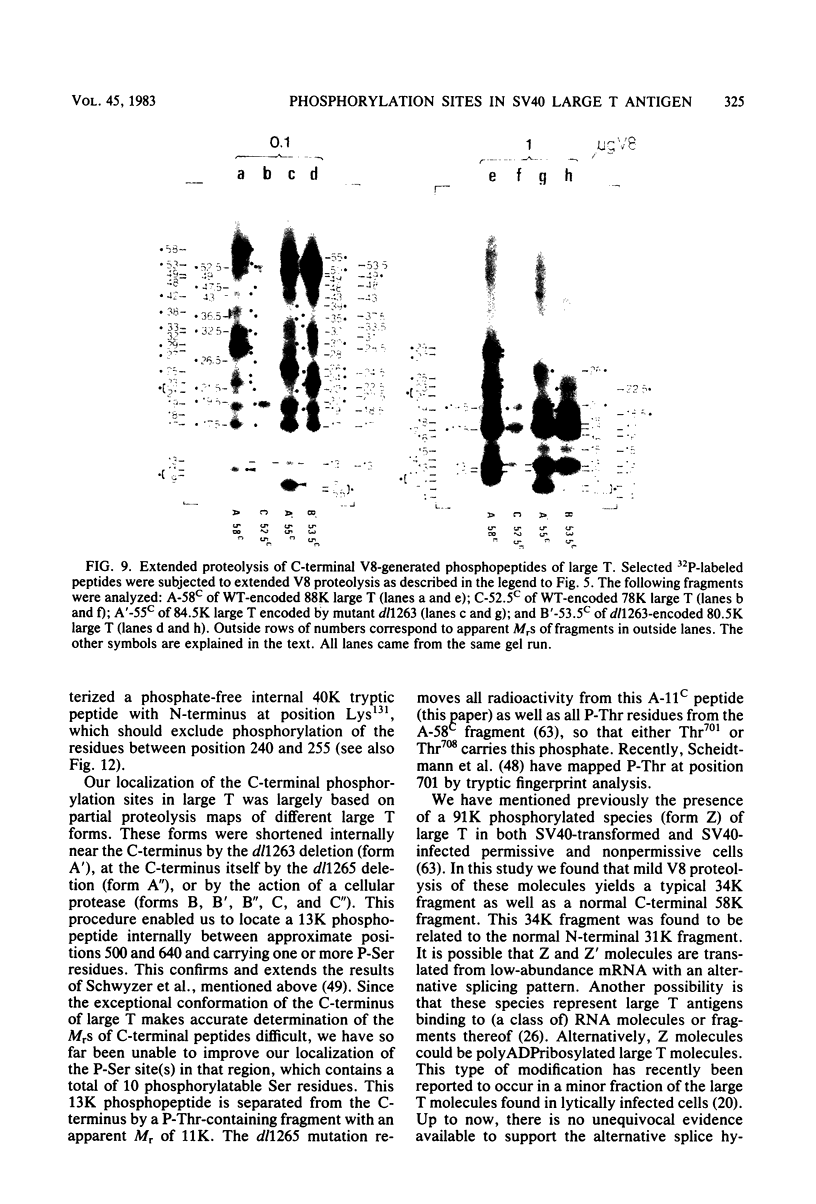
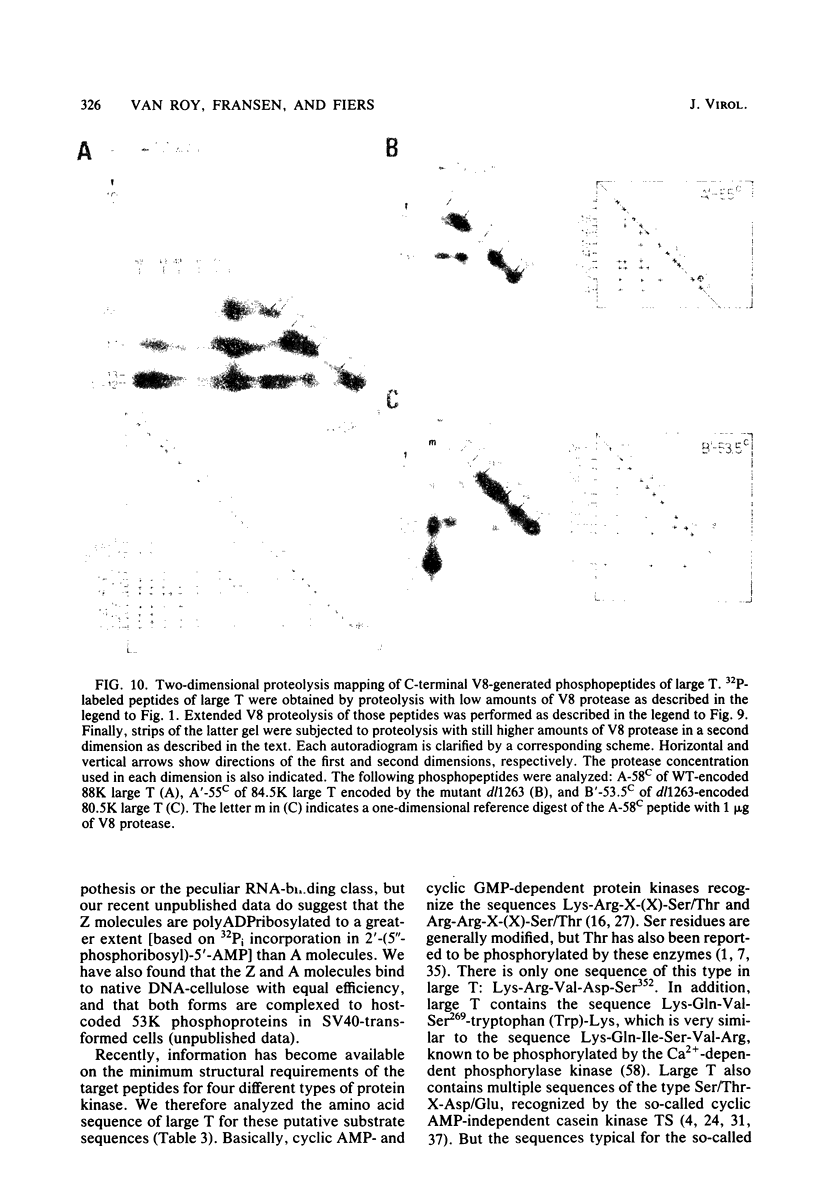
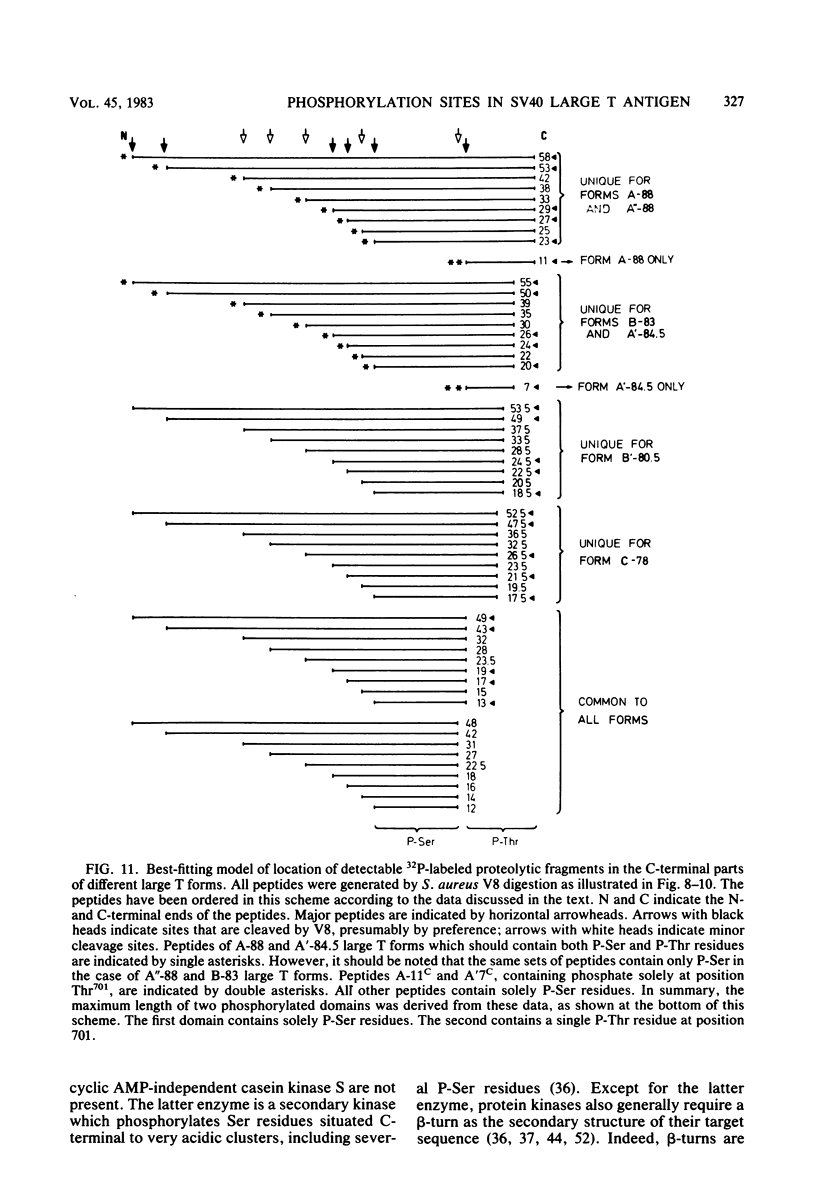
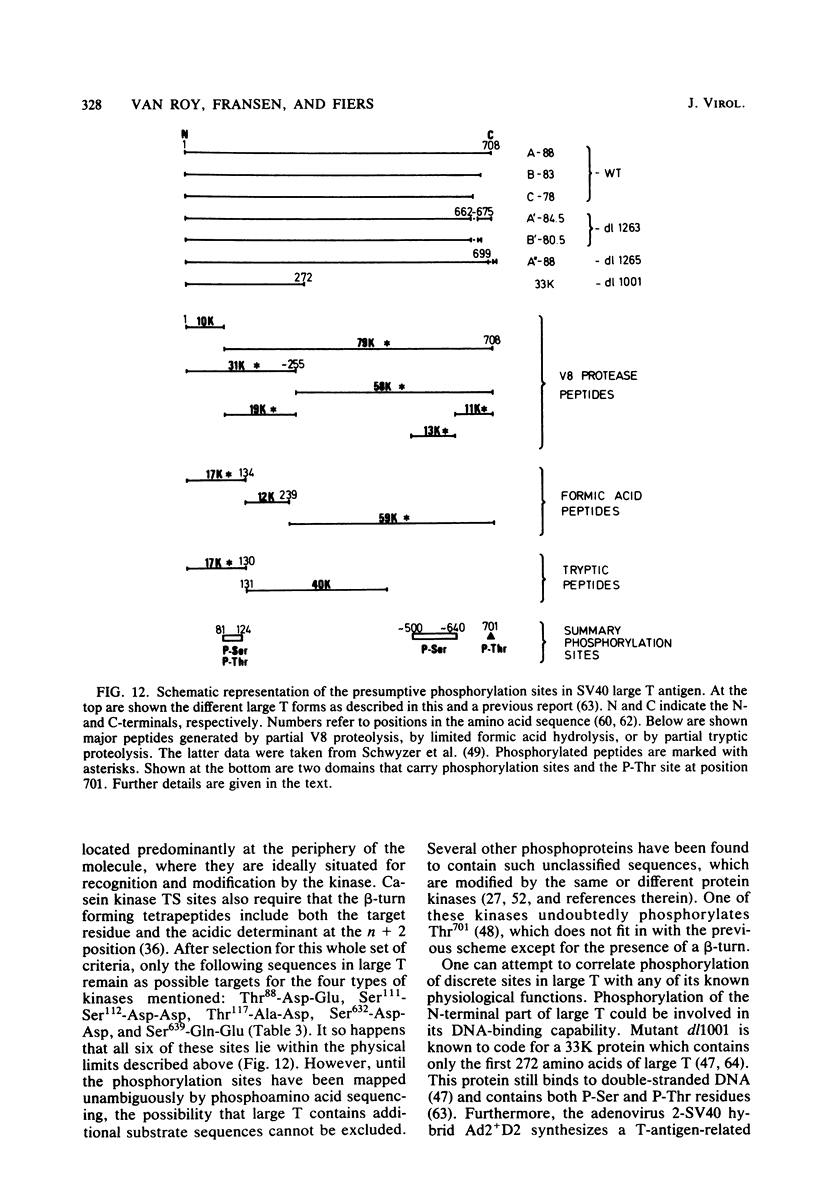
The location of phosphorylation sites in the large T antigen of simian virus 40 has been studied both by partial chemical cleavage and by partial proteolysis of various forms of large T. These included the full-size wild-type molecule with an apparent molecular weight of 88,000, deleted molecules coded for by the mutants dl1265 and dl1263, and several shortened derivatives generated by the action of a cellular protease. These molecules differed from each other by variations in the carboxy-terminal end. In contrast, a ubiquitous but minor large T form with a molecular weight of 91,000 was found to be modified in the amino-terminal half of the molecule. In addition to the phosphorylation of threonine at position 701 (K.-H. Scheidtmann et al., J. Virol. 38:59-69, 1981), two other discrete domains of phosphorylation were recognized, one at either side of the molecule. The amino-terminal region was located between positions 81 and 124 and contained both phosphothreonine and phosphoserine residues. The carboxy-terminal region was located between approximate positions 500 and 640 and contained at least one phosphoserine residue but no phosphothreonine. The presence in the phosphorylated domains of large T of known recognition sequences for different types of protein kinases is discussed, together with possible functions of large T associated with these domains.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aitken A., Bilham T., Cohen P., Aswad D., Greengard P. A specific substrate from rabbit cerebellum for guanosine-3':5'-monophosphate-dependent protein kinase. III. Amino acid sequences at the two phosphorylation sites. J Biol Chem. 1981 Apr 10;256(7):3501–3506. [PubMed] [Google Scholar]
- Amons R., Schrier P. I. Removal of sodium dodecyl sulfate from proteins and peptides by gel filtration. Anal Biochem. 1981 Sep 15;116(2):439–443. doi: 10.1016/0003-2697(81)90385-7. [DOI] [PubMed] [Google Scholar]
- Bradley M. K., Griffin J. D., Livingston D. M. Relationship of oligomerization to enzymatic and DNA-binding properties of the SV40 large T antigen. Cell. 1982 Jan;28(1):125–134. doi: 10.1016/0092-8674(82)90382-8. [DOI] [PubMed] [Google Scholar]
- Brignon G., Ribadeau Dumas B., Mercier J. C., Pelissier J. P., Das B. C. Complete amino acid sequence of bovine alphaS2-casein. FEBS Lett. 1977 Apr 15;76(2):274–279. doi: 10.1016/0014-5793(77)80167-1. [DOI] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Empirical predictions of protein conformation. Annu Rev Biochem. 1978;47:251–276. doi: 10.1146/annurev.bi.47.070178.001343. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Cohen P., Rylatt D. B., Nimmo G. A. The hormonal control of glycogen metabolism: the amino acid sequence at the phosphorylation site of protein phosphatase inhibitor-1. FEBS Lett. 1977 Apr 15;76(2):182–186. doi: 10.1016/0014-5793(77)80147-6. [DOI] [PubMed] [Google Scholar]
- Cole C. N., Crawford L. V., Berg P. Simian virus 40 mutants with deletions at the 3' end of the early region are defective in adenovirus helper function. J Virol. 1979 Jun;30(3):683–691. doi: 10.1128/jvi.30.3.683-691.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole C. N., Landers T., Goff S. P., Manteuil-Brutlag S., Berg P. Physical and genetic characterization of deletion mutants of simian virus 40 constructed in vitro. J Virol. 1977 Oct;24(1):277–294. doi: 10.1128/jvi.24.1.277-294.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford L. V., O'Farrell P. Z. Effect of alkylation on the physical properties of simian virus 40 T-antigen species. J Virol. 1979 Feb;29(2):587–596. doi: 10.1128/jvi.29.2.587-596.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denhardt D. T., Crawford L. V. Simian virus 40 T-antigen: identification of tryptic peptides in the C-terminal region and definition of the reading frame. J Virol. 1980 May;34(2):315–329. doi: 10.1128/jvi.34.2.315-329.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deppert W., Pates R. Cell surface location of simian virus 40-specific proteins on HeLa cells infected with adenovirus type 2-simian virus 40 hybrid viruses Ad2+ND1 and Ad2+ND2. J Virol. 1979 Aug;31(2):522–536. doi: 10.1128/jvi.31.2.522-536.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deppert W. Simian virus 40 T- and U-antigens: immunological characterization and localization in different nuclear subfractions of simian virus 40-transformed cells. J Virol. 1979 Feb;29(2):576–586. doi: 10.1128/jvi.29.2.576-586.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards C. A., Khoury G., Martin R. G. Phosphorylation of T-antigen and control T-antigen expression in cells transformed by wild-type and tsA mutants of simian virus 40. J Virol. 1979 Feb;29(2):753–762. doi: 10.1128/jvi.29.2.753-762.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanning E., Nowak B., Burger C. Detection and characterization of multiple forms of simian virus 40 large T antigen. J Virol. 1981 Jan;37(1):92–102. doi: 10.1128/jvi.37.1.92-102.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feramisco J. R., Glass D. B., Krebs E. G. Optimal spatial requirements for the location of basic residues in peptide substrates for the cyclic AMP-dependent protein kinase. J Biol Chem. 1980 May 10;255(9):4240–4245. [PubMed] [Google Scholar]
- Feunteun J., Carmichael G., Nicolas J. C., Kress M. Mutant carrying deletions in the two simian virus 40 early genes. J Virol. 1981 Dec;40(3):625–634. doi: 10.1128/jvi.40.3.625-634.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galanti N., Jonak G. J., Soprano K. J., Floros J., Kaczmarek L., Weissman S., Reddy V. B., Tilghman S. M., Baserga R. Characterization and biological activity of cloned simian virus 40 DNA fragments. J Biol Chem. 1981 Jun 25;256(12):6469–6474. [PubMed] [Google Scholar]
- Gidoni D., Scheller A., Barnet B., Hantzopoulos P., Oren M., Prives C. Different forms of simian virus 40 large tumor antigen varying in their affinities for DNA. J Virol. 1982 May;42(2):456–466. doi: 10.1128/jvi.42.2.456-466.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman N., Brown M., Khoury G. Modification of SV40 T antigen by poly ADP-ribosylation. Cell. 1981 May;24(2):567–572. doi: 10.1016/0092-8674(81)90347-0. [DOI] [PubMed] [Google Scholar]
- Greenspan D. S., Carroll R. B. Complex of simian virus 40 large tumor antigen and 48,000-dalton host tumor antigen. Proc Natl Acad Sci U S A. 1981 Jan;78(1):105–109. doi: 10.1073/pnas.78.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenspan D. S., Carroll R. B. Simian virus 40 large T antigen isoelectric focuses as multiple species with varying phosphate content. Virology. 1979 Dec;99(2):413–416. doi: 10.1016/0042-6822(79)90020-5. [DOI] [PubMed] [Google Scholar]
- Griffin J. D., Light S., Livingston D. M. Measurements of the molecular size of the simian virus 40 large T antigen. J Virol. 1978 Jul;27(1):218–226. doi: 10.1128/jvi.27.1.218-226.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson J. Y., Moir A. J., Fothergill L. A., Fothergill J. E. Sequences of sixteen phosphoserine peptides from ovalbumins of eight species. Eur J Biochem. 1981 Feb;114(2):439–450. doi: 10.1111/j.1432-1033.1981.tb05165.x. [DOI] [PubMed] [Google Scholar]
- Jay G., Jay F. T., Chang C., Friedman R. M., Levine A. S. Tumor-specific transplantation antigen: use of the Ad2+ND1 hybrid virus to identify the protein responsible for simian virus 40 tumor rejection and its genetic origin. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3055–3059. doi: 10.1073/pnas.75.7.3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khandjian E. W., Loche M., Darlix J. L., Cramer R., Türler H., Weil R. Simian virus 40 large tumor antigen: a "RNA binding protein"? Proc Natl Acad Sci U S A. 1982 Feb;79(4):1139–1143. doi: 10.1073/pnas.79.4.1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs E. G., Beavo J. A. Phosphorylation-dephosphorylation of enzymes. Annu Rev Biochem. 1979;48:923–959. doi: 10.1146/annurev.bi.48.070179.004423. [DOI] [PubMed] [Google Scholar]
- Lam K. S., Kasper C. B. Sequence homology analysis of a heterogenous protein population by chemical and enzymic digestion using a two-dimensional sodium dodecyl sulfate-polyacrylamide gel system. Anal Biochem. 1980 Nov 1;108(2):220–226. doi: 10.1016/0003-2697(80)90572-2. [DOI] [PubMed] [Google Scholar]
- Landon Cleavage at aspartyl-prolyl bonds. Methods Enzymol. 1977;47:145–149. doi: 10.1016/0076-6879(77)47017-4. [DOI] [PubMed] [Google Scholar]
- Lin P. S., Schmidt-Ullrich R., Wallach D. F. Transformation by simian virus 40 induces virus-specific, related antigens in the surface membrane and nuclear envelope. Proc Natl Acad Sci U S A. 1977 Jun;74(6):2495–2499. doi: 10.1073/pnas.74.6.2495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamrack M. D., Olson M. O., Busch H. Amino acid sequence and sites of phosphorylation in a highly acidic region of nucleolar nonhistone protein C23. Biochemistry. 1979 Jul 24;18(15):3381–3386. doi: 10.1021/bi00582a026. [DOI] [PubMed] [Google Scholar]
- Mann K., Hunter T. Phosphorylation of SV40 large T antigen in SV40 nucleoprotein complexes. Virology. 1980 Dec;107(2):526–532. doi: 10.1016/0042-6822(80)90320-7. [DOI] [PubMed] [Google Scholar]
- McCormick F., Chaudry F., Harvey R., Smith R., Rigby P. W., Paucha E., Smith A. E. T antigens of SV40-transformed cells. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 1):171–178. doi: 10.1101/sqb.1980.044.01.020. [DOI] [PubMed] [Google Scholar]
- McCormick F., Harlow E. Association of a murine 53,000-dalton phosphoprotein with simian virus 40 large-T antigen in transformed cells. J Virol. 1980 Apr;34(1):213–224. doi: 10.1128/jvi.34.1.213-224.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meggio F., Chessa G., Borin G., Pinna L. A., Marchiori F. Synthetic fragments of protamines as model substrates for rat liver cyclic AMP-dependent protein kinase. Biochim Biophys Acta. 1981 Nov 13;662(1):94–101. doi: 10.1016/0005-2744(81)90228-x. [DOI] [PubMed] [Google Scholar]
- Meggio F., Deana A. D., Pinna L. A. A study with model substrates of the structure of the sites phosphorylated by rat liver casein kinase TS. Biochim Biophys Acta. 1981 Nov 13;662(1):1–7. doi: 10.1016/0005-2744(81)90215-1. [DOI] [PubMed] [Google Scholar]
- Meggio F., Donella-Deana A., Pinna L. A. Studies on the structural requirements of a microsomal cAMP-independent protein kinase. FEBS Lett. 1979 Oct 1;106(1):76–80. doi: 10.1016/0014-5793(79)80698-5. [DOI] [PubMed] [Google Scholar]
- Mellor A., Smith A. E. Characterization of the amino-terminal tryptic peptide of simian virus 40 small-t and large-T antigens. J Virol. 1978 Dec;28(3):992–996. doi: 10.1128/jvi.28.3.992-996.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montenarh M., Henning R. Simian virus 40 T-antigen phosphorylation is variable. FEBS Lett. 1980 May 19;114(1):107–110. doi: 10.1016/0014-5793(80)80870-2. [DOI] [PubMed] [Google Scholar]
- Morrow J. F., Berg P., Kelly T. J., Jr, Lewis A. M., Jr Mapping of simian virus 40 early functions on the viral chromosome. J Virol. 1973 Sep;12(3):653–658. doi: 10.1128/jvi.12.3.653-658.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller C., Graessmann A., Graessmann M. Mapping of early SV40-specific functions by microinjection of different early viral DNA fragments. Cell. 1978 Oct;15(2):579–585. doi: 10.1016/0092-8674(78)90026-0. [DOI] [PubMed] [Google Scholar]
- Palme K., Henning R. Charge isomers of simian virus 40 T-antigen. FEBS Lett. 1980 Sep 8;118(2):229–232. doi: 10.1016/0014-5793(80)80225-0. [DOI] [PubMed] [Google Scholar]
- Paucha E., Harvey R., Smith A. E. Cell-free synthesis of simian virus 40 T-antigens. J Virol. 1978 Oct;28(1):154–170. doi: 10.1128/jvi.28.1.154-170.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinna L. A., Donella-Deana A., Meggio F. Structural features determining the site specificity of a rat liver cAMP-independent protein kinase. Biochem Biophys Res Commun. 1979 Mar 15;87(1):114–120. doi: 10.1016/0006-291x(79)91654-1. [DOI] [PubMed] [Google Scholar]
- Pintel D., Bouck N., di Mayorca G. Separation of lytic and transforming functions of the simian virus 40 A region: two mutants which are temperature sensitive for lytic functions have opposite effects on transformation. J Virol. 1981 May;38(2):518–528. doi: 10.1128/jvi.38.2.518-528.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prives C., Barnet B., Scheller A., Khoury G., Jay G. Discrete regions of simian virus 40 large T antigen are required for nonspecific and viral origin-specific DNA binding. J Virol. 1982 Jul;43(1):73–82. doi: 10.1128/jvi.43.1.73-82.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rundell K., Collins J. K., Tegtmeyer P., Ozer H. L., Lai C. J., Nathans D. Identification of simian virus 40 protein A. J Virol. 1977 Feb;21(2):636–646. doi: 10.1128/jvi.21.2.636-646.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheidtmann K. H., Kaiser A., Carbone A., Walter G. Phosphorylation of threonine in the proline-rich carboxy-terminal region of simian virus 40 large T antigen. J Virol. 1981 Apr;38(1):59–69. doi: 10.1128/jvi.38.1.59-69.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwyzer M., Weil R., Frank G., Zuber H. Amino acid sequence analysis of fragments generated by partial proteolysis from large simian virus 40 tumor antigen. J Biol Chem. 1980 Jun 25;255(12):5627–5634. [PubMed] [Google Scholar]
- Shaw S. B., Tegtmeyer P. Binding of dephosphorylated A protein to SV40 DNA. Virology. 1981 Nov;115(1):88–96. doi: 10.1016/0042-6822(81)90091-x. [DOI] [PubMed] [Google Scholar]
- Shortle D. R., Margolskee R. F., Nathans D. Mutational analysis of the simian virus 40 replicon: pseudorevertants of mutants with a defective replication origin. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6128–6131. doi: 10.1073/pnas.76.12.6128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small D., Chou P. Y., Fasman G. D. Occurrence of phosphorylated residues in predicted beta-turns: implications for beta-turn participation in control mechanisms. Biochem Biophys Res Commun. 1977 Nov 7;79(1):341–346. doi: 10.1016/0006-291x(77)90101-2. [DOI] [PubMed] [Google Scholar]
- Smith A. E., Smith R., Paucha E. Characterization of different tumor antigens present in cells transformed by simian virus 40. Cell. 1979 Oct;18(2):335–346. doi: 10.1016/0092-8674(79)90053-9. [DOI] [PubMed] [Google Scholar]
- Smith A. E., Smith R., Paucha E. Extraction and fingerprint analysis of simian virus 40 large and small T-antigens. J Virol. 1978 Oct;28(1):140–153. doi: 10.1128/jvi.28.1.140-153.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spangler G. J., Griffin J. D., Rubin H., Livingston D. M. Identification and initial characterization of a new low-molecular-weight virus-encoded T antigen in a line of simian virus 40-transformed cells. J Virol. 1980 Nov;36(2):488–498. doi: 10.1128/jvi.36.2.488-498.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stitt D. T., Carroll R. B., Melero J. A., Mangel W. F. Analysis of the 84K, 55K, and 48K proteins immunoprecipitable by SV40 T antibody from SV40-infected and -transformed cells by tryptic peptide mapping on cation-exchange columns. Virology. 1981 May;111(1):283–288. doi: 10.1016/0042-6822(81)90673-5. [DOI] [PubMed] [Google Scholar]
- Tegtmeyer P., Rundell K., Collins J. K. Modification of simian virus 40 protein A. J Virol. 1977 Feb;21(2):647–657. doi: 10.1128/jvi.21.2.647-657.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tessmer G. W., Skuster J. R., Tabatabai L. B., Graves D. J. Studies on the specificity of phosphorylase kinase using peptide substrates. J Biol Chem. 1977 Aug 25;252(16):5666–5671. [PubMed] [Google Scholar]
- Tjian R. Regulation of viral transcription and DNA replication by the SV40 large T antigen. Curr Top Microbiol Immunol. 1981;93:5–24. doi: 10.1007/978-3-642-68123-3_2. [DOI] [PubMed] [Google Scholar]
- Van Heuverswyn H., Cole C., Berg P., Fiers W. Nucleotide sequence analysis of two simian virus 40 mutants with deletions in the region coding for the carboxyl terminus of the T antigen. J Virol. 1979 Jun;30(3):936–941. doi: 10.1128/jvi.30.3.936-941.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Heuverswyn H., Van de Voorde A., Van Herreweghe J., Volckaert G., De Winne P., Fiers W. Nucleotide sequence of simian virus 40 DNA: structure of the middle segment of the HindII + III restriction fragment B (sixth part of the T antigen gene) and codon usage. Eur J Biochem. 1980 May;106(1):199–209. doi: 10.1111/j.1432-1033.1980.tb06011.x. [DOI] [PubMed] [Google Scholar]
- Van Roy F., Fransen L., Fiers W. Phosphorylation patterns of tumour antigens in cells lytically infected or transformed by simian virus 40. J Virol. 1981 Oct;40(1):28–44. doi: 10.1128/jvi.40.1.28-44.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volckaert G., Van de Voorde A., Fiers W. Nucleotide sequence of the simian virus 40 HindII + III restriction fragment A (second part of the T antigen gene). Eur J Biochem. 1980 May;106(1):169–177. doi: 10.1111/j.1432-1033.1980.tb06008.x. [DOI] [PubMed] [Google Scholar]
- Walter G., Flory P. J., Jr Phosphorylation of SV40 large T antigen. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 1):165–169. doi: 10.1101/sqb.1980.044.01.019. [DOI] [PubMed] [Google Scholar]
- Walter G., Scheidtmann K. H., Carbone A., Laudano A. P., Doolittle R. F. Antibodies specific for the carboxy- and amino-terminal regions of simian virus 40 large tumor antigen. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5197–5200. doi: 10.1073/pnas.77.9.5197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y. C., Hearing P., Rundell K. Cellular proteins associated with simian virus 40 early gene products in newly infected cells. J Virol. 1979 Oct;32(1):147–154. doi: 10.1128/jvi.32.1.147-154.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ysebaert M., van de Voorde A., Fiers W. Nucleotide sequence of the simian virus 40 HindII + III restriction fragment D and the total amino acid sequence of the late proteins VP2 and VP3. Eur J Biochem. 1978 Nov 15;91(2):431–439. doi: 10.1111/j.1432-1033.1978.tb12695.x. [DOI] [PubMed] [Google Scholar]