Abstract
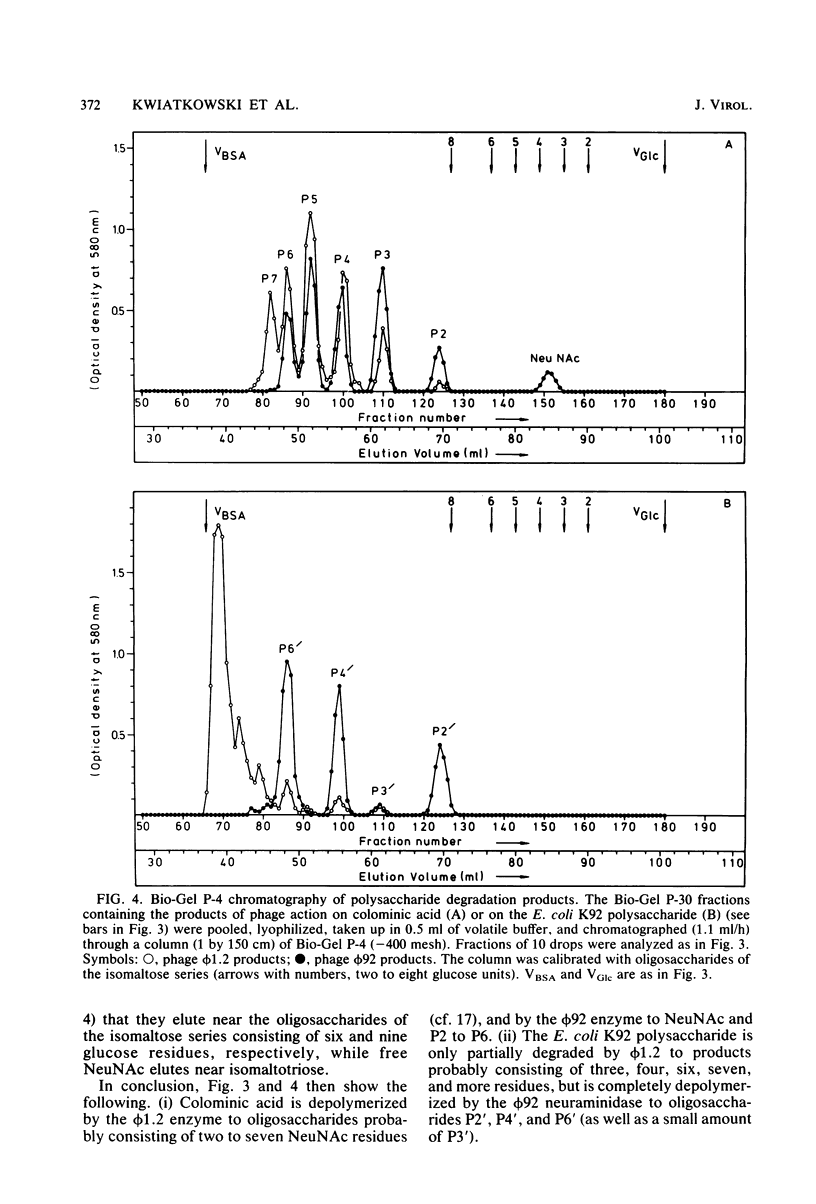
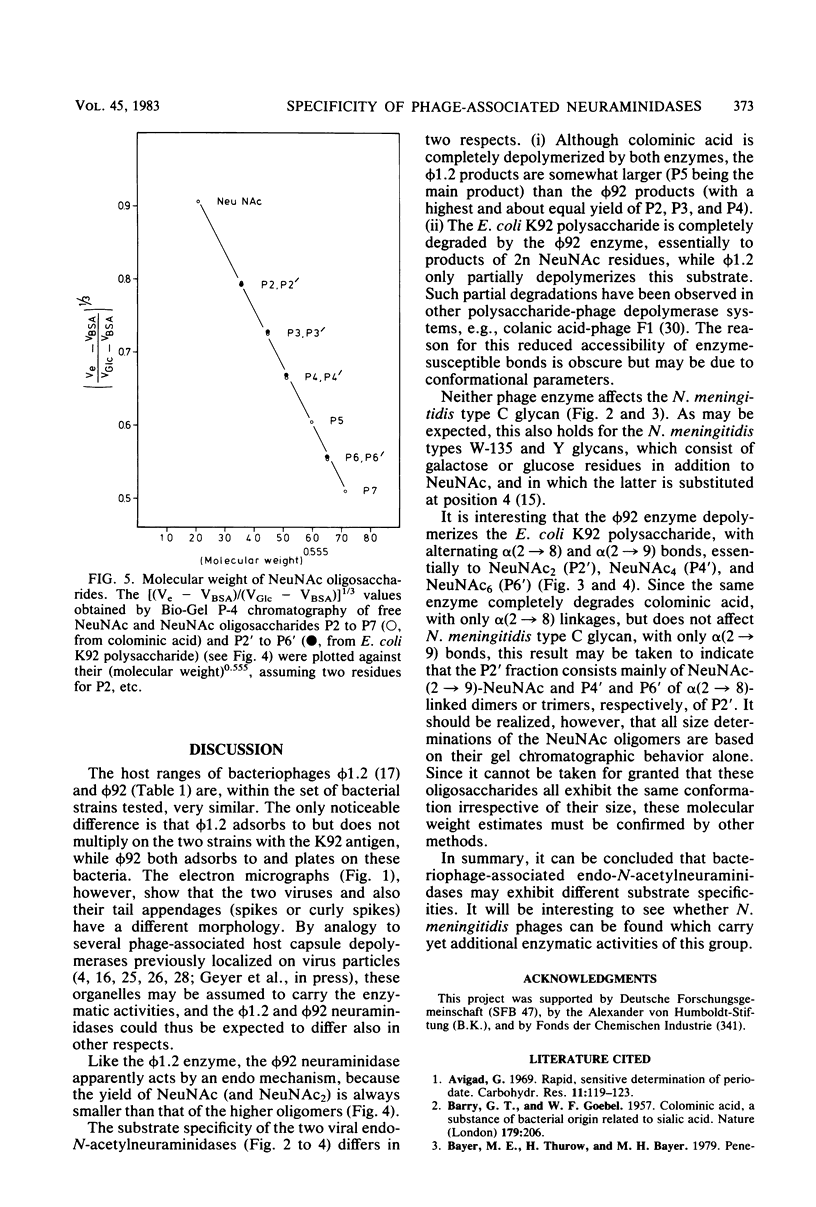
For Escherichia coli Bos12 (O16:K92:H-), a bacteriophage (phi 92) has been isolated which carries a depolymerase active on the K92 capsular polysaccharide. As seen under the electron microscope, phi 92 belongs to Bradley's morphology group A and is different from the phage phi 1.2 previously described (Kwiatkowski et al., J. Virol. 43:697-704, 1982), which grows on E. coli K235 (O1:K1:H-), depolymerizes colominic acid, and belongs to morphology group C. The specificity of the phi 1.2- and phi 92-associated endo-N-acetylneuraminidases has been studied with respect to the following substrates (all alkali treated, and where NeuNAc represents N-acetylneuraminic acid): (i) [-alpha-NeuNAc-(2 leads to 8)-]n (colominic acid), (ii) [-alpha-NeuNAc-(2 leads to 8)-alpha-NeuNAc-(2 leads to 9)-]n (E. coli K92 polysaccharide), and (iii) [-alpha-NeuNAc-(2 leads to 9)-]n (Neisseria meningitidis type C capsular polysaccharide). The increase in periodate consumption of these glycans upon incubation with purified phi 1.2 or phi 92 particles was measured, and the split products obtained from all substrates after exhaustive degradation were analyzed by gel chromatography. It was found that the Neisseria polysaccharide is not appreciably affected by either virus enzyme and that phi 1.2 only depolymerizes a small fraction of the K92 glycan. Colominic acid, however, is completely degraded by both agents, phi 92 yielding smaller fragments (one to six NeuNAc residues) than phi 1.2 (two to seven). Phage phi 92 additionally depolymerizes the K92 glycan, essentially to oligosaccharides of two, four, and six residues. The size distribution of these K92 oligosaccharides indicates that the phi 92 enzyme predominantly cleaves the alpha(2 leads to 8) linkages in this polymer.
Full text
PDF

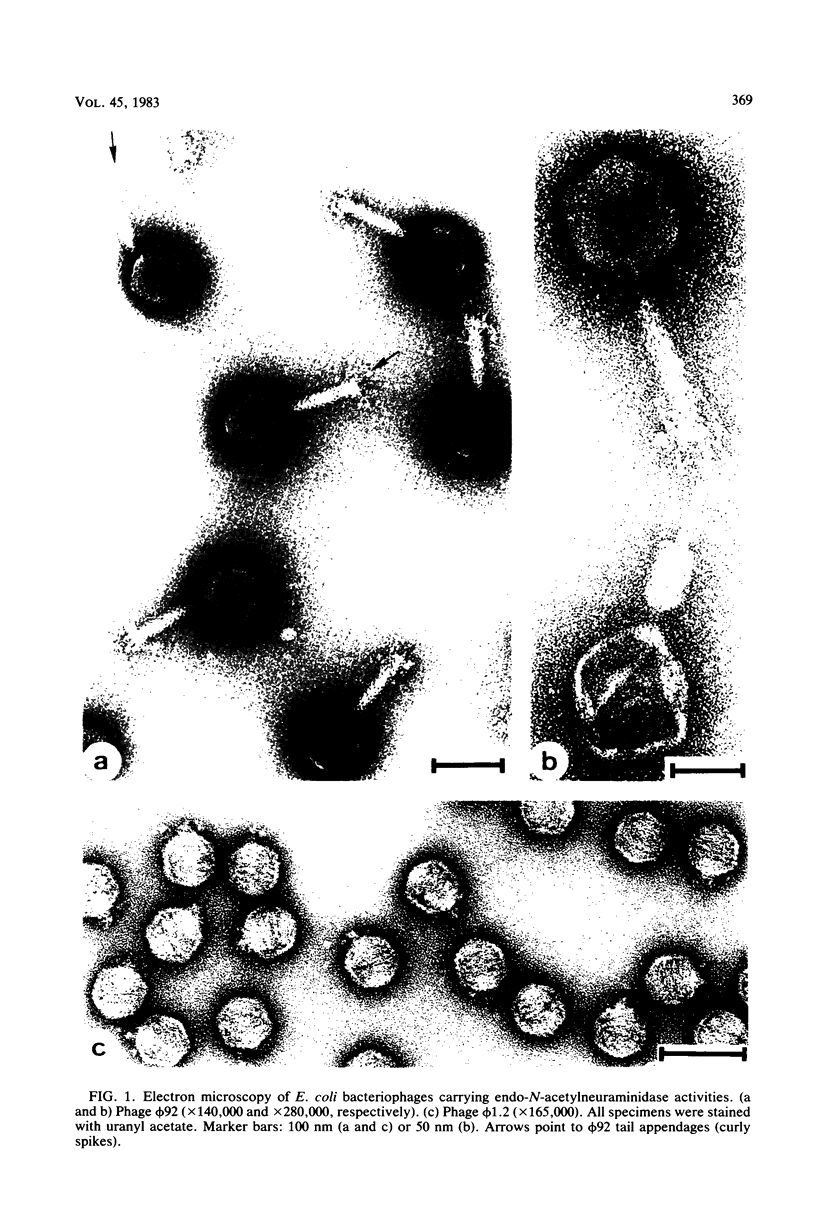
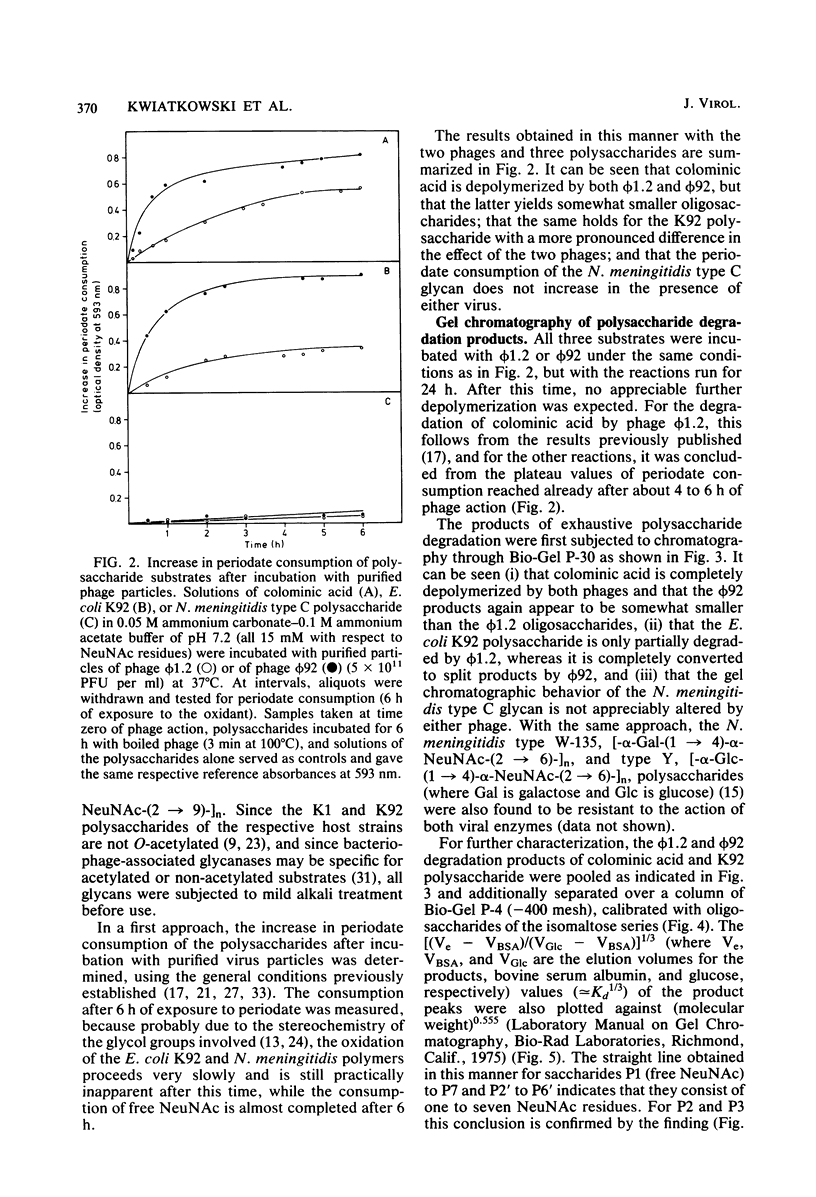
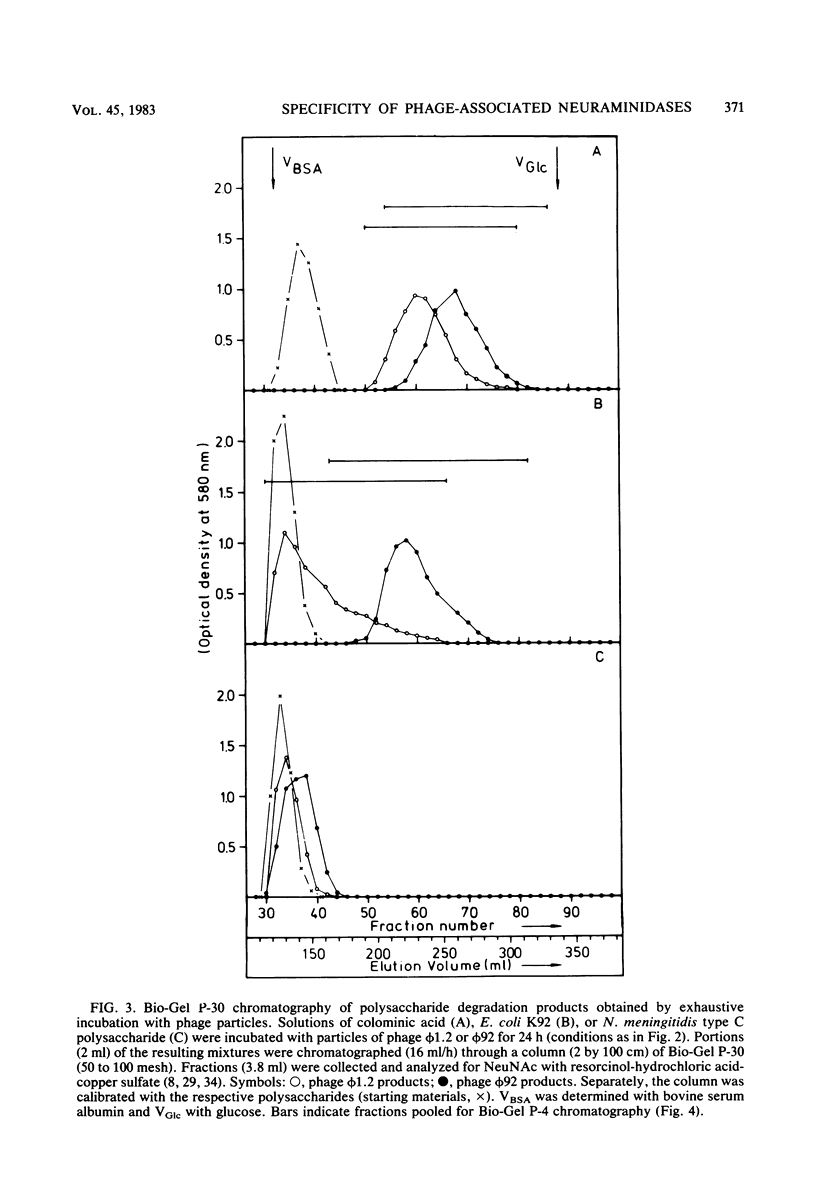

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARRY G. T., GOEBEL W. F. Colominic acid, a substance of bacterial origin related to sialic acid. Nature. 1957 Jan 26;179(4552):206–206. doi: 10.1038/179206a0. [DOI] [PubMed] [Google Scholar]
- Bessler W., Freund-Mölbert E., Knüfermann H., Rduolph C., Thurow H., Stirm S. A bacteriophage-induced depolymerase active on Klebsiella K11 capsular polysaccharide. Virology. 1973 Nov;56(1):134–151. doi: 10.1016/0042-6822(73)90293-6. [DOI] [PubMed] [Google Scholar]
- Bhattacharjee A. K., Jennings H. J., Kenny C. P., Martin A., Smith I. C. Structural determination of the sialic acid polysaccharide antigens of Neisseria meningitidis serogroups B and C with carbon 13 nuclear magnetic resonance. J Biol Chem. 1975 Mar 10;250(5):1926–1932. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Bradley D. E. Ultrastructure of bacteriophage and bacteriocins. Bacteriol Rev. 1967 Dec;31(4):230–314. doi: 10.1128/br.31.4.230-314.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casals-Stenzel J., Buscher H. P., Schauer R. Gas--liquid chromatography of N- and O-acylated neuramic acids. Anal Biochem. 1975 May 12;65(1-2):507–524. doi: 10.1016/0003-2697(75)90536-9. [DOI] [PubMed] [Google Scholar]
- Egan W., Liu T. Y., Dorow D., Cohen J. S., Robbins J. D., Gotschlich E. C., Robbins J. B. Structural studies on the sialic acid polysaccharide antigen of Escherichia coli strain Bos-12. Biochemistry. 1977 Aug 9;16(16):3687–3692. doi: 10.1021/bi00635a028. [DOI] [PubMed] [Google Scholar]
- Gotschlich E. C. Development of polysaccharide vaccines for the prevention of meningococcal diseases. Monogr Allergy. 1975;9:245–258. [PubMed] [Google Scholar]
- Gotschlich E. C., Liu T. Y., Artenstein M. S. Human immunity to the meningococcus. 3. Preparation and immunochemical properties of the group A, group B, and group C meningococcal polysaccharides. J Exp Med. 1969 Jun 1;129(6):1349–1365. doi: 10.1084/jem.129.6.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotschlich E. C., Rey M., Etienne J., Sanborn W. R., Triau R., Cvjetanović B. The immunological responses observed in field studies in Africa with group A meningococcal vaccines. Prog Immunobiol Stand. 1971;5:485–491. [PubMed] [Google Scholar]
- Kwiatkowski B., Beilharz H., Stirm S. Disruption of Vi bacteriophage III and localization of its deacetylase activity. J Gen Virol. 1975 Dec;29(3):267–280. doi: 10.1099/0022-1317-29-3-267. [DOI] [PubMed] [Google Scholar]
- Kwiatkowski B., Boschek B., Thiele H., Stirm S. Endo-N-acetylneuraminidase associated with bacteriophage particles. J Virol. 1982 Aug;43(2):697–704. doi: 10.1128/jvi.43.2.697-704.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCGUIRE E. J., BINKLEY S. B. THE STRUCTURE AND CHEMISTRY OF COLOMINIC ACID. Biochemistry. 1964 Feb;3:247–251. doi: 10.1021/bi00890a017. [DOI] [PubMed] [Google Scholar]
- Orskov F., Orskov I., Jann B., Jann K. Immunoelectrophoretic patterns of extracts from all Escherichia coli O and K antigen test strains: correlation with pathogenicity. Acta Pathol Microbiol Scand B Microbiol Immunol. 1971;79(2):142–152. doi: 10.1111/j.1699-0463.1971.tb02141.x. [DOI] [PubMed] [Google Scholar]
- Orskov F., Orskov I., Sutton A., Schneerson R., Lin W., Egan W., Hoff G. E., Robbins J. B. Form variation in Escherichia coli K1: determined by O-acetylation of the capsular polysaccharide. J Exp Med. 1979 Mar 1;149(3):669–685. doi: 10.1084/jem.149.3.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieger-Hug D., Stirm S. Comparative study of host capsule depolymerases associated with Klebsiella bacteriophages. Virology. 1981 Aug;113(1):363–378. doi: 10.1016/0042-6822(81)90162-8. [DOI] [PubMed] [Google Scholar]
- Rieger D., Freund-Mölbert E., Stirm S. Escherichia coli capsule bacteriophages. III. Fragments of bacteriophage 29. J Virol. 1975 Apr;15(4):964–975. doi: 10.1128/jvi.15.4.964-975.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieger D., Freund-Mölbert E., Stirm S. Escherichia coli capsule bacteriophages. VIII. Fragments of bacteriophage 28-1. J Virol. 1976 Mar;17(3):859–864. doi: 10.1128/jvi.17.3.859-864.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolph C., Freund-Mölbert E., Stirm S. Fragments of Klebsiella bacteriophage no. 11. Virology. 1975 Mar;64(1):236–246. doi: 10.1016/0042-6822(75)90095-1. [DOI] [PubMed] [Google Scholar]
- Schauer R. Characterization of sialic acids. Methods Enzymol. 1978;50:64–89. doi: 10.1016/0076-6879(78)50008-6. [DOI] [PubMed] [Google Scholar]
- Sutherland I. W. Enzymic hydrolysis of colanic acid. Eur J Biochem. 1971 Dec 10;23(3):582–587. doi: 10.1111/j.1432-1033.1971.tb01657.x. [DOI] [PubMed] [Google Scholar]
- Sutherland I. W. Highly specific bacteriophage-associated polysaccharide hydrolases for Klebsiella aerogenes type 8. J Gen Microbiol. 1976 May;94(1):211–216. doi: 10.1099/00221287-94-1-211. [DOI] [PubMed] [Google Scholar]
- Thurow H., Niemann H., Stirm S. Bacteriophage-borne enzymes in carbohydrate chemistry. Part I. On the glycanase activity associated with particles of Klebsiella bacteriophage No. 11. Carbohydr Res. 1975 May;41:257–271. doi: 10.1016/s0008-6215(00)87024-x. [DOI] [PubMed] [Google Scholar]