Abstract
Elastic fibers consist of two morphologically distinct components: elastin and 10-nm fibrillin-containing microfibrils. During development, the microfibrils form bundles that appear to act as a scaffold for the deposition, orientation, and assembly of tropoelastin monomers into an insoluble elastic fiber. Although microfibrils can assemble independent of elastin, tropoelastin monomers do not assemble without the presence of microfibrils. In the present study, immortalized ciliary body pigmented epithelial (PE) cells were investigated for their potential to serve as a cell culture model for elastic fiber assembly. Northern analysis showed that the PE cells express microfibril proteins but do not express tropoelastin. Immunofluorescence staining and electron microscopy confirmed that the microfibril proteins produced by the PE cells assemble into intact microfibrils. When the PE cells were transfected with a mammalian expression vector containing a bovine tropoelastin cDNA, the cells were found to express and secrete tropoelastin. Immunofluorescence and electron microscopic examination of the transfected PE cells showed the presence of elastic fibers in the matrix. Biochemical analysis of this matrix showed the presence of cross-links that are unique to mature insoluble elastin. Together, these results indicate that the PE cells provide a unique, stable in vitro system in which to study elastic fiber assembly.
INTRODUCTION
Elastic fibers are an abundant and integral part of many extracellular matrices, in which they provide resilience and deformability to tissues. These properties are critical for the functioning of tissues such as arterial vessels, lungs, and skin. Elastic fibers consist of two distinct components: elastin, an insoluble polymer of 70-kDa tropoelastin monomers, and microfibrils, 10-nm unbranching fibrillin-containing fibrils. During development, the microfibrils form linear bundles that appear to act as a scaffold for the deposition and/or orientation of tropoelastin monomers. Lysine residues on adjacent monomers are then modified by the enzyme lysyl oxidase to form covalent cross-links, two of which (desmosine and isodesmosine) are unique to elastin and can be distinctive markers of the fully cross-linked insoluble fiber (Mecham and Davis, 1994). Although microfibrils can assemble independent of elastin to form functional structures within the matrix, insoluble elastin has not been observed in the absence of microfibrils. Remarkably, little is known concerning the specific molecular interactions that are involved in, and required for, the formation of elastic fibers.
Unlike many components of the extracellular matrix, elastic fibers are formed only in developing tissues, with little or no synthesis in adults (Davis, 1993a). Elastic fibers formed during early development, therefore, must be of accurate size and orientation to function for a lifetime. In several diseases, elastic fibers are damaged or degraded and can become solubilized during the natural progression of the disease. Examples of such diseases include aortic aneurysms (Campa et al., 1987; Thompson et al., 1995), emphysema (Snider, 1992; Finlay et al., 1996), and Marfan syndrome (Dietz et al., 1991; McKusick, 1991). In other instances, the initial formation of elastic fibers may be affected. This occurs primarily in those diseases that have been linked to the elastin gene, such as supravalvular aortic stenosis (Curran et al., 1993; Ewart et al., 1993, 1994) and cutis laxa (Zhang et al., 1999). Upon destruction of elastic fibers, elastogenic cells are often reactivated and begin to synthesize at least some of the elastic fiber components. In most situations, however, the elastic fibers produced in response to disease are aberrant and may even disrupt or impair normal function of the tissue (Kuhn et al., 1976).
To better understand the changes that occur to elastic fibers in these destructive and genetic diseases, it is becoming increasingly clear that we must first understand the events that lead to the normal formation of elastic fibers. Studies on elastin biology have relied heavily on the use of a limited number of elastogenic cell types isolated and established as primary cultures from fetal and neonatal tissues (Mecham, 1987). In the present study, we have investigated the potential of an immortalized cell line derived from the pigmented epithelium of the ciliary body in the eye to serve as an in vitro model of elastic fiber assembly. Our results demonstrate that these cells not only assemble fibrillin-containing microfibrils in culture but, upon transfection with a tropoelastin cDNA, can assemble the tropoelastin monomers into mature elastic fibers.
MATERIALS AND METHODS
Cells
Fetal bovine chondroblasts (FBCs) were isolated from 150- to 170-d gestation auricular cartilage by collagenase digestion, as described previously (Mecham, 1987). Immortal ciliary body pigmented epithelial (PE) cells derived from human eyes were the generous gift of Dr. Martin Wax (Washington University School of Medicine) (Coca-Prados and Wax, 1986). All cells were grown to confluence in DMEM supplemented with l-glutamine, nonessential amino acids, antibiotics, and 10% fortified FCS (Hyclone Laboratories, Logan, UT). Experiments with FBCs were conducted with first-passage cells.
Antibodies and Probes
Antibodies used for immunofluorescence included BA4, a mAb to tropoelastin; anti-microfibril–associated glycoprotein (MAGP), a rabbit polyclonal antibody raised to an MAGP-GST fusion protein; fibrillin-1 anti-C-terminal, a rabbit polyclonal antibody raised to bacterially expressed exons 64–65 of fibrillin-1; and fibrillin-2 Gly, a rabbit polyclonal antibody raised to bacterially expressed protein from the glycine-rich region of fibrillin-2 (Wrenn et al., 1986; Trask et al., 1999). Secondary antibodies included fluorescein- or rhodamine-conjugated goat anti-rabbit or goat anti-mouse antibodies diluted 1:200 (ICN/Cappel, Costa Mesa, CA). Probes used for Northern analysis included a 2.3-kilobase bovine tropoelastin probe (Parks et al., 1988), a 500-base pair human fibrillin-1 probe and a 221-base pair human fibrillin-2 probe (gifts from Dr. Francesco Ramirez, Mount Sinai School of Medicine, New York, NY), a 537-base pair bovine MAGP probe (Gibson et al., 1991), and a 1.1-kilobase rat GAPDH probe. The fibrillin probes were to the 3′ untranslated regions, and the remaining probes were to small segments of coding sequence.
RNA Isolation and Northern Analysis
FBCs and PE cells were plated at a density of 1 × 106 cells in 100-mm dishes. At 2, 4, and 7 d, total RNA was isolated with Trizol reagent (GIBCO-BRL, Gaithersburg, MD) and 10 μg was separated by electrophoresis through a 1% agarose gel containing 1 M formaldehyde. RNA was transferred overnight to a Hybond-N+ nylon membrane (Amersham, Arlington Heights, IL) and UV cross-linked with a Stratagene (La Jolla, CA) Stratalinker II. cDNA probes were labeled with α-[32P]CTP with the use of Rediprime II random prime labeling (Amersham), and unincorporated nucleotides were removed with QIAquick nucleotide removal kit (Qiagen, Valencia, CA). Membranes were hybridized overnight at 65°C (tropoelastin probe) or 60°C (all other probes) in 250 mM Na2PO4, 7% SDS and then washed two times in 2× SSPE, 0.1% SDS for 15 min at room temperature followed by two washes in 1× SSPE, 0.1% SDS for 15 min at the hybridization temperature. Hybridized complexes were detected by exposure of the membrane to X-OMAT AR film (Kodak, Rochester, NY). For reprobing, membranes were stripped with boiling 0.5% SDS.
Immunofluorescence Staining
Confluent cultures of FBCs, as well as nontransfected and transfected PE cells, plated on four-well LabTek chamber slides (Nunc no. 177437, Fisher Scientific, Pittsburgh, PA) were washed with PBS and fixed with 2% paraformaldehyde in PBS for 30 min. After several washes in PBS, reactive aldehydes were quenched by incubating the cells in 50 mM NH4Cl for 30 min. To better expose antigenic epitopes, the cell layers were treated with guanidine HCl followed by iodoacetamide, as described previously (Gibson et al., 1989). Nonspecific immunoreactivity was blocked with 1% BSA in PBS for 1 h at room temperature. The cell layers were then incubated with primary antibody for 1 h at room temperature, followed by several washes in blocking buffer and a second incubation with fluorescence-conjugated immunoglobulin G. Cell layers were then washed in blocking buffer, rinsed once with PBS, mounted in 80% glycerol in PBS containing 1 mg/ml p-phenylenediamine, and visualized with a Leica (Exton, PA) confocal microscope.
Stable Transfections
A cDNA of a natural splice variant of bovine tropoelastin lacking exons 13–14 (a generous gift of Dr. Len Grosso, St. Louis University, St. Louis, MO) was inserted into a pCi-neo expression vector (Promega, Madison, WI) behind an engineered Kozak consensus sequence (Kozak, 1989). PE cells were plated in six-well tissue culture plates at 1 × 105 cells/well and allowed to grow in DMEM with 10% FCS until visually 50–60% confluent (∼2 d). The tropoelastin cDNA in the pCi-neo expression vector and Lipofectin (GIBCO-BRL) were incubated together in serum-free medium (Opti-Mem I, GIBCO-BRL) according to the manufacturer’s directions. Cells were rinsed with serum-free medium and then incubated with the DNA-Lipofectin complex for 5 h at 37°C. Plates were rinsed with DMEM containing 10% FCS and allowed to grow for 48–72 h. The cells from each well were then trypsinized and divided onto three 100-mm tissue culture plates with medium containing 0.6 mg/ml active Geneticin (GIBCO-BRL). When discrete colonies were formed, the plates were rinsed two times with sterile PBS and cloning rings were placed around the individual colonies. The cells in each ring were trypsinized, transferred onto 24-well plates, and allowed to grow to confluence in selection medium (5–7 d). The medium in each well was then changed to serum-free medium for 12 h and analyzed by Western analysis for colonies that expressed tropoelastin. Positive colonies were expanded, and an aliquot of each positive colony was plated onto 96-well plates at 0.5 cells/well to obtain individual clones. After 3–4 d, wells growing single isolated colonies were identified. After 7 d, the clones were passed onto six-well plates and again screened at confluence for tropoelastin expression. For Northern analysis of tropoelastin message, stably transfected PE cells were plated at a density of 1 × 106 cells on 100-mm dishes. Total RNA was isolated after 4 d and probed as described above.
Metabolic Labeling and Immunoprecipitation
Confluent monolayers of FBCs, nontransfected PE cells, and stably transfected PE cells were metabolically labeled with [4,5-3H]l-leucine (1 mCi/ml) (ICN Pharmaceuticals, Irvine, CA) and immunoprecipitated for tropoelastin, as described previously (Davis and Mecham, 1996). Briefly, cells in 35-mm tissue culture dishes were labeled with 50 μCi/ml [3H]l-leucine for 4 h. The medium and cell lysate were then collected separately and incubated with anti-tropoelastin antibody overnight at 4°C. The next day, 40 μl of Pansorbin suspension (Calbiochem-Novabiochem, San Diego, CA) was added to each tube and incubated for 1 h at 4°C. Immune complexes were pelleted, washed, and resuspended in 35 μl of Laemmli sample buffer containing 100 mM DTT. Samples were electrophoresed on SDS-polyacrylamide gels, fixed, treated with En3Hance (New England Nuclear, Boston, MA) for 1 h, dried, and exposed to X-OMAT AR film (Kodak). All immunoprecipitation experiments were conducted several times to ensure reproducibility of results. For densitometric quantitation, autoradiographic bands were scanned with the use of NIH Image software (http://rsb.info.nih. gov/nih-image/). Each band was scanned four times with different sized areas to ensure accurate and consistent measurements. The same four areas were used for each experimental condition. The average of the four measurements was plotted.
Electron Microscopy
FBCs, nontransfected PE cells, and stably transfected PE cells were plated on four-well LabTek chamber slides and grown until 7 d after confluence. Cell layers were washed three times with PBS and fixed in situ with 3% glutaraldehyde in 0.1 M sodium cacodylate buffer, pH 7.4, for 30 min. After several washes with cacodylate buffer, the cell layers were treated sequentially with 1% osmium tetroxide in buffer, 2% tannic acid in buffer, and 2% uranyl acetate in distilled water. Cell layers were then dehydrated in a graded series of methanol to propylene oxide, infiltrated, and embedded with Epon (SPI Supplies, West Chester, PA). En face thin sections were cut on a Reichert (Leica, Deerfield, IL) ultracut ultramicrotome and counterstained with methanolic uranyl acetate (Franc et al., 1984) followed by lead citrate (Reynolds, 1963). Sections were examined with a Jeol (Peabody, MA) transmission electron microscope at an accelerating voltage of 60 kV.
Desmosine Analysis
Nontransfected and stably transfected PE cells were plated at a density of 1 × 106 cells on 100-mm dishes and cultured for 14 d in DMEM containing 10% FCS. Cells were then washed three times with PBS and pooled by scraping into 2 ml of distilled water. The samples were centrifuged in a microfuge for 2 min, and the supernatants were removed. The pellets were then hydrolyzed overnight at 110°C in constant boiling 6 N HCl. Desmosine levels in the hydrolysates were determined by radioimmunoassay (Starcher and Conrad, 1995) and normalized to the number of cells per plate. Cell number was determined by counting trypsinized cells from parallel plates of both transfected and nontransfected cultures.
RESULTS
PE Cells Assemble a Microfibril Scaffold but Lack Tropoelastin Production
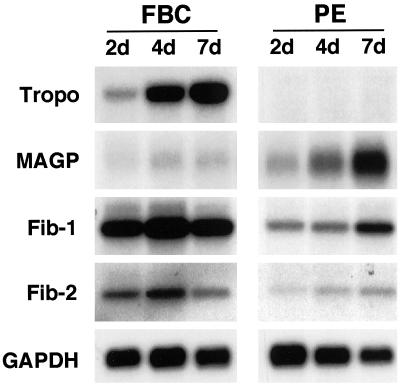
Total RNA isolated from PE cells was probed by Northern analysis to investigate expression levels of tropoelastin and three microfibril proteins: MAGP, fibrillin-1, and fibrillin-2. FBCs grown under similar conditions were used as a control because these cells synthesize and secrete tropoelastin and microfibril proteins in culture and assemble these components into an insoluble elastic fiber matrix. Consistent with existing studies (Mecham et al., 1981; Wachi et al., 1995), FBCs expressed tropoelastin in a cell density–dependent manner (Figure 1). Expression of all three microfibril proteins was detected in the FBC cultures, with levels of MAGP expression being particularly low. In contrast, the PE cells showed no detectable message for tropoelastin. However, preconfluent and postconfluent cultures of PE cells did express both fibrillin-1 and fibrillin-2. Expression of MAGP was also seen in the PE cells, with higher levels than that of FBCs.
Figure 1.
PE cells express microfibril proteins but do not express tropoelastin. Expression of elastic fiber components was determined by Northern analysis for FBCs and PE cells at 2, 4, and 7 d after plating. Both cell types were confluent at ∼4 d. Ten micrograms of total RNA was fractionated on a 1% agarose gel, transferred to a nylon membrane, and probed for tropoelastin (Tropo), three microfibrillar proteins (MAGP, fibrillin-1 [Fib-1], and fibrillin-2 [Fib-2]), and GAPDH.
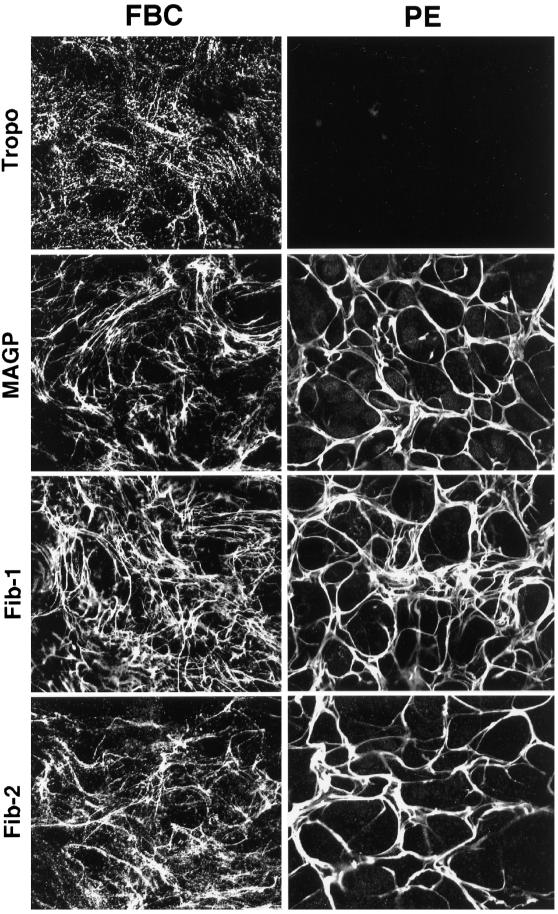
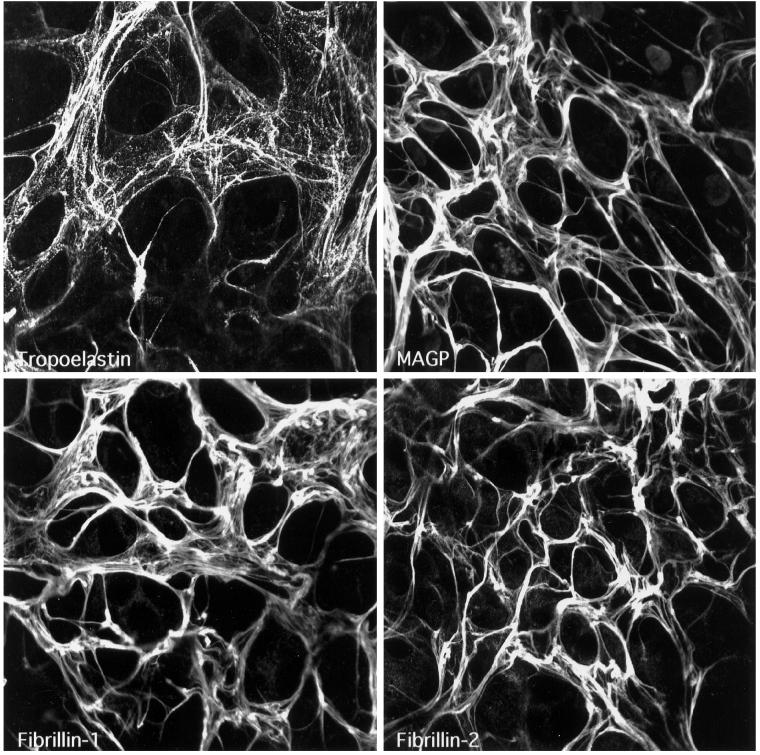
To determine if the microfibril components synthesized by the PE cells could assemble into a microfibrillar scaffold, immunofluorescence staining of the PE cell matrix was performed and compared with that of FBCs. Immunofluorescence labeling of tropoelastin, MAGP, and the two fibrillin proteins in postconfluent FBC cultures showed a filamentous network for all four elastic fiber components (Figure 2). The staining pattern for tropoelastin was slightly different from that seen for the microfibril proteins because it had a “bead-on-a-string” appearance. This observation is typical for FBCs in culture and correlates well with the punctate appearance of elastin deposits as seen by electron microscopy in the extracellular matrix of FBCs (Figure 3A). Consistent with results from the Northern analysis, immunofluorescence staining of PE cultures showed no tropoelastin in the matrix. Staining for MAGP and the fibrillin proteins in the PE cultures, however, showed that the microfibril proteins produced by these cells assemble into an extensive fibrillar network. Although the matrix in PE cell cultures appeared thicker and more compact than that seen in cultures of FBCs, the results indicate that PE cells both express and assemble all of the components necessary to form a microfibrillar matrix with no expression of tropoelastin.
Figure 2.
PE cells assemble microfibril components into a microfibrillar matrix. FBC and PE cultures were grown to confluence and labeled by indirect immunofluorescence for tropoelastin (Tropo), MAGP, fibrillin-1 (Fib-1), and fibrillin-2 (Fib-2). A meshwork of microfibrils can be seen in both cultures, but only FBCs show tropoelastin deposited in the matrix.
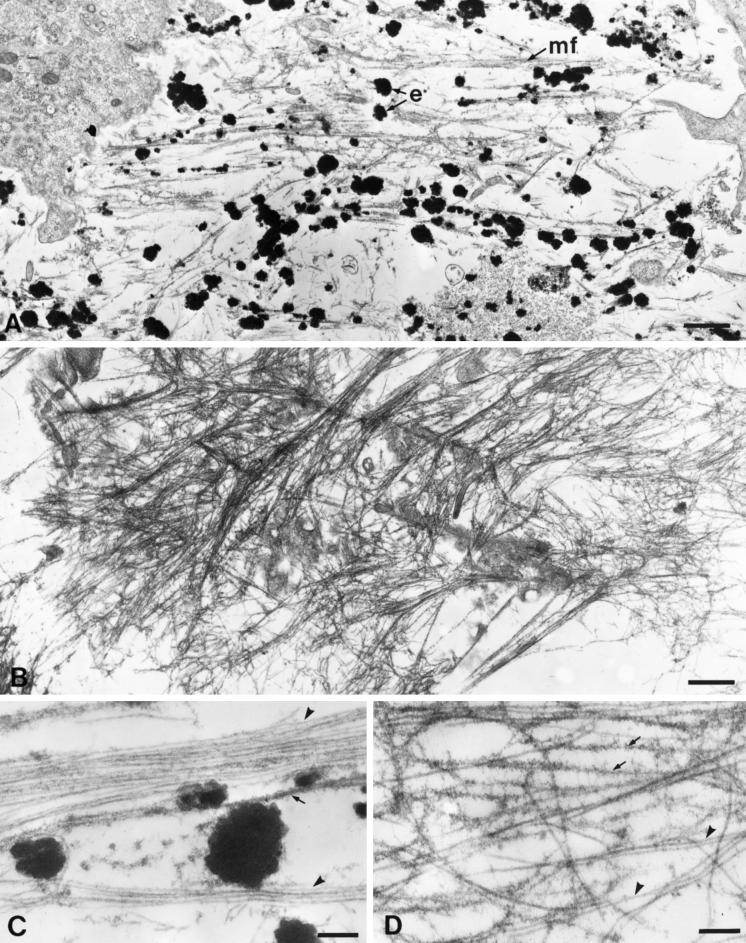
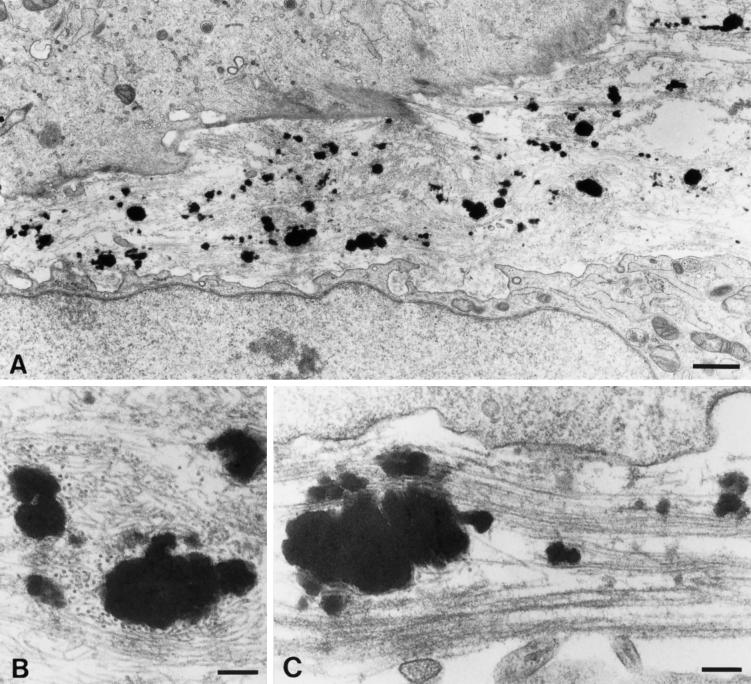
Figure 3.
Electron microscopic examination of the PE cell matrix shows an extensive array of microfibrils. (A) FBCs assemble insoluble elastic fibers in vivo. The matrix of these cells shows bundles of microfibrils (mf) both with and without electron-dense deposits of elastin (e). (B) The PE cell matrix contains extensive microfibril bundles; however, no elastin is present. (C) At higher magnification, individual 10-nm microfibrils can be seen in the FBC culture. These microfibrils appear both uncoated (arrowheads) and coated with fuzzy material (arrow). (D) The microfibrils of the PE cell matrix are identical to those of the FBC culture. Note that as with those in the FBC matrix, the microfibrils in the PE cell matrix are both uncoated (arrowheads) and coated (arrows). Original magnifications: A and B, ×10,600; C and D, ×46,500. Bars: A and B, 1.0 μm; C and D, 0.2 μm.
Electron Microscopic Examination of PE Cell Matrix Reveals Microfibrils Typical of Elastogenic Cells and Tissues
The overall light microscopic appearance of the microfibrillar matrix produced by the PE cells was somewhat different from that made by the FBCs. For this reason, a more detailed investigation of the PE matrix was carried out at the electron microscope level. Figure 3A shows the typical appearance of FBCs in culture. Electron-dense globules of insoluble elastin were seen adjacent to small bundles of microfibrils in the extracellular matrix. At higher magnification, two “types” of microfibrils were observed in the matrix: uncoated or “naked” microfibrils, and those with a fuzzy coated appearance (Figure 3C). The identity of the coating material is unknown; however, microfibrils of similar appearance have been identified in the subendothelial region of the aorta, where they immunolabel strongly for fibronectin (Davis, 1993b, 1994). Low-magnification views of the PE cell matrix showed an extensive array of microfibrils (Figure 3B). The microfibrils were often organized in large bundles, which likely accounted for their appearance as seen by immunofluorescence labeling at the light microscope level. At higher magnification, both coated and uncoated microfibrils were easily recognized in the PE culture (Figure 3D). These observations establish that the PE cells assemble an extracellular matrix that contains 10-nm microfibrils typical of those seen in FBC cultures in vitro and in elastin-rich tissues in vivo.
Tropoelastin Transfected into PE Cells Is Secreted and Assembles into Elastic Fibers
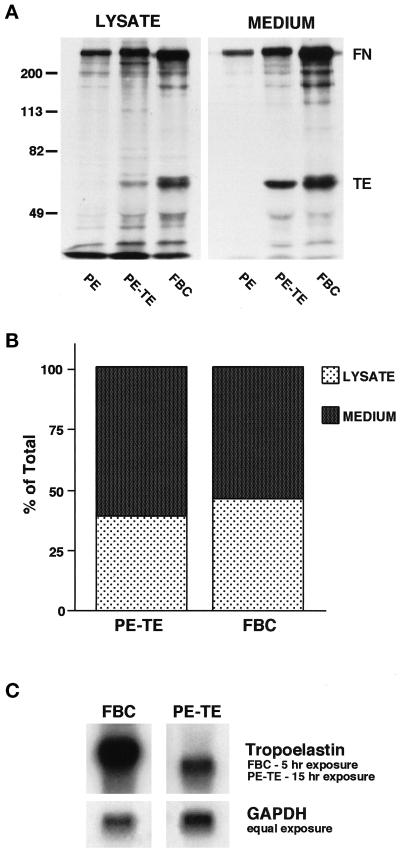
The extensive microfibrillar matrix synthesized by the PE cells suggested that these cells might be able to use this matrix to assemble soluble tropoelastin monomers into an insoluble elastic fiber matrix similar to that seen in the primary cultures of FBCs. To investigate this possibility, a stable PE cell line expressing tropoelastin was prepared. To ensure that the cells were efficiently transporting the tropoelastin monomer through the secretory pathway and secreting the protein into the extracellular matrix, lysates and media from the transfected PE cells were immunoprecipitated for tropoelastin and compared with similar samples obtained from FBC cultures (Figure 4A). After 4 h of continuous metabolic labeling with [3H]leucine, both the transfected PE cells and the FBCs showed the presence of tropoelastin in the cell lysate and medium. Although the level of protein produced by the FBCs was greater, the proportion of tropoelastin in the cell lysates compared with the media for the two cell types was comparable (Figure 4B). This result indicated that the PE cells transport and secrete the transfected tropoelastin in a manner similar to that of a known elastogenic cell type. Consistent with the protein levels observed, Northern analysis of tropoelastin message in the transfected PE cells was found to be considerably lower than that seen in FBCs (Figure 4C).
Figure 4.
Bovine tropoelastin stably expressed in PE cells is transported and secreted in proportions similar to those of FBCs. (A) Nontransfected PE cells (PE), PE cells stably expressing tropoelastin (PE-TE), and FBCs were metabolically labeled for 4 h with [3H]leucine. Tropoelastin (TE) was immunoprecipitated from the cell lysates and media with the use of heat-killed Staphylococcus aureus (Pansorbin cells) to collect the immune complexes. Samples were run on a 7.5% SDS-polyacrylamide gel, fixed, treated with En3Hance, and exposed to x-ray film. Note that fibronectin (FN) binds directly to S. aureus in the absence of primary antibody and is therefore present on the autoradiograph. (B) Densitometric scanning of the tropoelastin bands in A demonstrates efficient secretion of tropoelastin by the transfected PE cells. (C) Northern analysis of tropoelastin expression in PE cells stably expressing tropoelastin (PE-TE) versus FBCs demonstrates abundant but reduced message levels in the transfected cells compared with a typical elastogenic cell.
Immunofluorescence analysis of the stably transfected PE cells showed an extensive network of tropoelastin (Figure 5). A similar network of staining was observed for all three of the microfibril proteins, indicating that the expression of tropoelastin in the PE cells did not alter the assembly of the microfibril scaffold. Similar to that of the FBCs, the tropoelastin staining appeared punctate in nature. This was particularly evident in the more diffuse areas of the microfibrillar network.
Figure 5.
PE cells stably transfected with tropoelastin assemble tropoelastin onto a microfibril scaffold to form elastic fibers in the extracellular matrix. Immunofluorescence labeling of confluent cultures of stably transfected PE cells shows an extensive network of elastic fibers that stain positively for tropoelastin and the three microfibril components MAGP, fibrillin-1, and fibrillin-2. Consistent with that seen in the FBC cultures (see Figure 2), the tropoelastin assembled in the matrix has a beaded appearance.
Electron Microscopic Examination of Stably Transfected PE Cells Shows Assembly of Elastin in the Extracellular Matrix
Cultures of stably transfected PE cells were prepared for electron microscopy to investigate the assembly of elastin in more detail. Similar to that observed in the FBC cultures, the elastin deposited in the extracellular matrix of the PE cells appeared as collections of various sized globular units surrounded by an extensive network of microfibrils (Figure 6A). At higher magnification, the elastin was seen to be in close association with the adjacent microfibrils, although it did not infiltrate the microfibrillar bundle (Figure 6, B and C). Overall, the appearance of the elastin deposits in the PE cultures was identical to that observed in cultures of FBCs.
Figure 6.
PE cells stably transfected with tropoelastin assemble elastin in the extracellular matrix in a manner similar to that of FBCs. (A) Electron microscopic examination of stably transfected PE cells reveals black amorphous deposits of elastin in the extracellular matrix. (B and C) At higher magnification, the deposits are seen to be similar to those observed in FBC cultures (see Figure 3C). In all cases, the elastin is intimately associated with microfibrils. Original magnifications: A, ×10,600; B and C, ×46,500. Bars: A, 1.0 μm; B and C, 0.2 μm.
Biochemical Analysis of the PE Matrix Shows Cross-Linked Elastin in the Transfected PE Cell Matrix
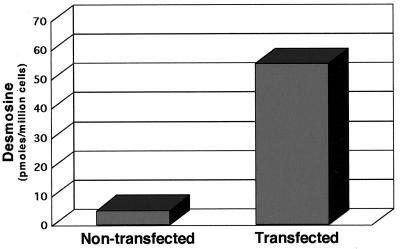
The assembly of elastic fibers in vivo not only involves the deposition of tropoelastin monomers along a microfibril scaffold but also subsequent cross-linking of the monomers to form a functional insoluble polymer. To determine whether the elastic fibers formed in the transfected PE cultures were cross-linked, biochemical analyses of the extracellular matrices of PE cells and PE cells stably transfected with tropoelastin were performed. As expected, nontransfected PE cultures showed no desmosine in the hydrolysate of the extracellular matrix (Figure 7). When transfected with tropoelastin, however, high levels of desmosine were detected, indicating that the secreted tropoelastin was assembled and cross-linked into elastic fibers.
Figure 7.
Quantitation of desmosine in cultures of PE cells stably transfected with tropoelastin indicates that elastin deposited into the extracellular matrix is cross-linked. Desmosine levels in the cell layer of nontransfected PE cells and PE cells stably expressing tropoelastin were determined by ELISA. Values are normalized to cell number.
DISCUSSION
The assembly of elastic fibers is critical for both the structural development and the ultimate function of a number of vital tissues, including the aorta and the lungs. Elastic fiber assembly has often been studied with the use of primary cells isolated from fetal or neonatal auricular cartilage, aorta, or lung because of the ability of chondroblasts, smooth muscle cells, and pulmonary fibroblasts to produce and assemble insoluble elastin in vitro (Campagnone et al., 1987; Mecham, 1987; Faris et al., 1992). Although studies with these cells have provided many important insights into the regulation and production of elastic fiber components, relatively few details concerning the molecular interactions required for the formation of elastic fibers have emerged. This difficulty stems, in part, from the extremely unstable nature of the elastogenic phenotype, which is sensitive to both cell density and passage number (Mecham, 1987). As a result, most experiments have been conducted with primary cultures to avoid the rapid decrease in tropoelastin synthesis that occurs with serial subculture. To date, attempts to immortalize or efficiently transfect these primary elastogenic cells for in vitro studies have been unsuccessful.
In the present study, the potential of an immortalized ciliary body epithelial cell line to serve as a model system for the study of microfibril and elastic fiber assembly was investigated. It was reasoned that the pigmented epithelial cells, from which the immortalized PE cell line was derived (Coca-Prados and Wax, 1986), may produce all of the components necessary to form microfibrils because they are 1) in direct contact with the connective tissue matrix of the ciliary body, which contains microfibril bundles (E.C. Davis, unpublished observation), and 2) in continuity with the pigmented epithelium of the retina, which rests on an elaborate basement membrane (Bruch’s membrane) that contains many matrix proteins, including elastic fiber components (Olson, 1979; Campochiaro et al., 1986). Using Northern analysis and immunostaining, we have confirmed that the PE cells express and assemble an extensive microfibrillar matrix and that this matrix is sufficient to support the assembly of tropoelastin monomers into insoluble, extracellular elastin.
The functional relationship between the microfibril scaffold and the assembly of insoluble elastin in the extracellular matrix is unclear. Like FBCs in culture, the transfected PE cells assemble elastin as globular deposits directly adjacent to bundles of microfibrils. Although this appearance is somewhat different from that seen in normal developing tissues, in which the elastin infiltrates the microfibril bundle (Lee et al., 1994; Mecham and Davis, 1994), the biochemical analyses conducted in this study demonstrate that the transfected PE cells do assemble cross-linked mature elastin.
One reason for the ultrastructural difference between in vivo and in vitro assembly may be that the cultured FBCs and PE cells lack any three-dimensional spatial clues that would be present in intact tissues. Consistent with this idea is the observation that aortic smooth muscle cells form elastic fibers more typical of tissues when maintained in culture for long periods of time without subcultivation (Faris et al., 1992). During this time, the cells form a three-dimensional, multilayered structure that resembles the aortic medium from which they were derived (Stone et al., 1988; Faris et al., 1992). In addition to the spatial orientation of the cells, the organization of the microfibril bundle itself may also be different. We have noted two types of microfibrils, “naked” and “coated,” in both FBC and PE cultures. Similar microfibrils have also been reported in cultures of rat vascular smooth muscle cells (Aggeler, 1988). In this and another report (Lee et al., 1994), we have found that the elastin associates most often with the coated microfibrils. One explanation is that the coating consists of tropoelastin monomers. However, using limited enzymatic digestion, Aggeler (1988) was able to strip the coating entirely off the microfibrils and, based on the enzymes used, determine that the coating consisted of glycoprotein and/or proteoglycan. Because tropoelastin is not glycosylated, these results suggest that the tropoelastin monomers may either preferentially associate with a specific subpopulation of microfibrils in the extracellular matrix or associate with a certain microfibril-associated glycoprotein. Although purely speculation at this time, it is possible that in tissues, the “coated” microfibrils or the coating glycoprotein is incorporated into the microfibril bundle, thus allowing the tropoelastin monomers to assemble within the bundle. Further analysis of microfibril-associated glycoproteins and the ability of these proteins to bind tropoelastin may provide some insight into this step of elastic fiber formation.
Perhaps the most important use of the PE model system will be to determine the domains of tropoelastin that are critical for both intracellular transport and extracellular assembly of the monomers. One domain that is of particular interest is the C terminus of tropoelastin, which contains the only two cysteine residues in the protein. This region has been suggested to play an important role in elastic fiber assembly because the cysteines form an intramolecular disulfide bond that stabilizes a highly conserved, positively charged C-terminal domain (Brown et al., 1992). Furthermore, it has also been reported that a truncated tropoelastin found in the ductus arteriosus remains soluble in the matrix because it lacks the C terminus and is therefore unable to associate and align on the microfibril scaffold (Hinek and Rabinovitch, 1993).
In addition to the use of PE cells to investigate domains in tropoelastin important for its association with microfibrils, the model system can also be used to specifically explore the effects of particular mutations that have been identified in the elastin gene. Supravalvular aortic stenosis (SVAS) is an autosomal dominant trait that may occur sporadically or be inherited as a familial condition. Patients with this disease show a congenital narrowing of the ascending aorta, which often leads to heart failure (Eisenberg et al., 1964). SVAS has been genetically linked to the elastin gene (Ewart et al., 1993), and point mutations, deletions, and translocations in the elastin gene of different SVAS patients have all been found to cause the disease (Curran et al., 1993; Ewart et al., 1994; Olson et al., 1995; Li et al., 1997; Tassabehji et al., 1997). Recently, frameshift mutations in exon 30 of the elastin gene were identified in two families as the underlying cause of another elastin-related disease, cutis laxa (Zhang et al., 1999). In this disease, dermal elastic fibers are fragmented and severely reduced in number, leading to loose skin with decreased resilience and elasticity. Although cutis laxa is a heterogeneous group of disorders that can be caused by several factors, such as low lysyl oxidase activity and reduced elastase inhibitory capacity (Fornieri et al., 1994), the identification of mutations in the elastin gene suggests that initial events of elastic fiber assembly may also be affected in some individuals. The ability to generate stably transfected PE cell lines that express these various mutations will now enable us to better study and understand the pathogenesis of these debilitating diseases.
In summary, we have demonstrated that a cell line derived from the ciliary body pigmented epithelium can synthesize and assemble microfibrils in culture and, upon transfection with tropoelastin, can form insoluble deposits of elastin in the extracellular matrix. To date, these cells represent the first in vitro model system that uses immortalized cells for the study of elastic fiber assembly. Experimental manipulation of the PE cells will allow for the characterization of individual protein roles and protein-protein interactions involved in the formation of elastic fibers.
ACKNOWLEDGMENTS
The authors thank David Schettler and Clarina Tisdale for cell culture assistance, Charles Patterson for Northern analysis of the stably transfected PE cells, and Dr. Robert Mercer for help with the assembly of the tropoelastin construct. We also thank Dr. Barry Starcher at the University of Texas Health Science Center in Tyler, Texas, for performing the desmosine ELISAs. This work was supported by a National Marfan Foundation research grant (E.C.D. and R.P.M.), by National Institutes of Health grants HL53325 (R.P.M.), HL61006 (R.P.M.), and HL60394 (E.C.D.), and by Beginning Grant-in-Aid 98BG056 from the American Heart Association, Texas Affiliate (E.C.D.).
REFERENCES
- Aggeler J. Three-dimensional organization of the extracellular matrix secreted by cultured rat smooth muscle cells. In Vitro Cell Dev Biol. 1988;24:633–638. doi: 10.1007/BF02623600. [DOI] [PubMed] [Google Scholar]
- Brown PL, Mecham L, Tisdale C, Mecham RP. The cysteine residues in the carboxy-terminal domain of tropoelastin form an intrachain disulfide bond that stabilizes a loop structure and positively charged pocket. Biochem Biophys Res Commun. 1992;186:549–555. doi: 10.1016/s0006-291x(05)80843-5. [DOI] [PubMed] [Google Scholar]
- Campa JS, Greenhalgh RM, Powell JT. Elastin degradation in abdominal aortic aneurysms. Atherosclerosis. 1987;65:13–21. doi: 10.1016/0021-9150(87)90003-7. [DOI] [PubMed] [Google Scholar]
- Campagnone R, Regan J, Rich CB, Miller M, Keene DR, Sakai L, Foster JA. Pulmonary fibroblasts: a model system for studying elastin synthesis. Lab Invest. 1987;56:224–230. [PubMed] [Google Scholar]
- Campochiaro PA, Jerdan JA, Glaser BM. The extracellular matrix of human retinal pigment epithelial cells in vivo and its synthesis in vitro. Invest Ophthalmol Vis Sci. 1986;27:1615–1621. [PubMed] [Google Scholar]
- Coca-Prados M, Wax MB. Transformation of human ciliary epithelial cells by SV40: induction of cell proliferation and retention of β2-adrenergic receptors. Proc Natl Acad Sci USA. 1986;83:8754–8758. doi: 10.1073/pnas.83.22.8754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran ME, Atkinson DL, Ewart AK, Morris CA, Leppert MF, Keating MT. The elastin gene is disrupted by a translocation associated with supravalvular aortic stenosis. Cell. 1993;73:159–168. doi: 10.1016/0092-8674(93)90168-p. [DOI] [PubMed] [Google Scholar]
- Davis EC. Stability of elastin in the developing mouse aorta: a quantitative radioautographic study. Histochemistry. 1993a;100:17–26. doi: 10.1007/BF00268874. [DOI] [PubMed] [Google Scholar]
- Davis EC. Endothelial cell connecting filaments anchor endothelial cells to the subjacent elastic lamina in the developing aortic intima of the mouse. Cell Tissue Res. 1993b;272:211–219. doi: 10.1007/BF00302726. [DOI] [PubMed] [Google Scholar]
- Davis EC. Immunolocalization of microfibril and microfibril-associated proteins in the subendothelial matrix of the developing mouse aorta. J Cell Sci. 1994;107:727–736. doi: 10.1242/jcs.107.3.727. [DOI] [PubMed] [Google Scholar]
- Davis EC, Mecham RP. Selective degradation of accumulated secretory proteins in the endoplasmic reticulum: a possible clearance pathway for abnormal tropoelastin. J Biol Chem. 1996;271:3787–3794. [PubMed] [Google Scholar]
- Dietz HC, et al. Marfan syndrome caused by a recurrent de novo missense mutation in the fibrillin gene. Nature. 1991;352:337–339. doi: 10.1038/352337a0. [DOI] [PubMed] [Google Scholar]
- Eisenberg R, Young D, Jacobson B, Boito A. Familial supravalvular aortic stenosis. Am J Dis Child. 1964;108:341–347. doi: 10.1001/archpedi.1964.02090010343002. [DOI] [PubMed] [Google Scholar]
- Ewart AK, Jin W, Atkinson D, Morris CA, Keating MT. Supravalvular aortic stenosis associated with a deletion disrupting the elastin gene. J Clin Invest. 1994;93:1071–1077. doi: 10.1172/JCI117057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewart AK, Morris CA, Ensing GJ, Loer J, Moore C, Leppert M, Keating M. A human vascular disorder, supravalvular aortic stenosis, maps to chromosome 7. Proc Natl Acad Sci USA. 1993;90:3226–3230. doi: 10.1073/pnas.90.8.3226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faris B, Tan OT, Toselli P, Franzblau C. Long-term neonatal rat aortic smooth muscle cell cultures: a model for the tunica media of a blood vessel. Matrix. 1992;12:185–188. doi: 10.1016/s0934-8832(11)80060-0. [DOI] [PubMed] [Google Scholar]
- Finlay GA, O’Donnell MD, O’Connor CM, Hayes JP, Fitzgerald MX. Elastin and collagen remodeling in emphysema: a scanning electron microscopy study. Am J Pathol. 1996;149:1405–1415. [PMC free article] [PubMed] [Google Scholar]
- Fornieri C, Quaglino D, Lungarella G, Cavarra E, Tiozzo R, Giro MG, Canciani M, Davidson JM, Pasquali Ronchetti I. Elastin production and degradation in cutis laxa acquisita. J Invest Dermatol. 1994;103:583–588. doi: 10.1111/1523-1747.ep12396893. [DOI] [PubMed] [Google Scholar]
- Franc S, Garrone R, Bosch A, Franc J-M. A routine method for contrasting elastin at the ultrastructural level. J Histochem Cytochem. 1984;32:251–258. doi: 10.1177/32.2.6198356. [DOI] [PubMed] [Google Scholar]
- Gibson MA, Kumaratilake JS, Cleary EG. The protein components of the 12-nm microfibrils of elastic and nonelastic tissues. J Biol Chem. 1989;264:4590–4598. [PubMed] [Google Scholar]
- Gibson MA, Sandberg LB, Grosso LE, Cleary EG. Complementary DNA cloning establishes microfibril-associated glycoprotein (MAGP) to be a discrete component of the elastin-associated microfibrils. J Biol Chem. 1991;266:7596–7601. [PubMed] [Google Scholar]
- Hinek A, Rabinovitch M. The ductus arteriosus migratory smooth muscle phenotype processes tropoelastin to a 52-kDa product associated with impaired assembly of elastic laminae. J Biol Chem. 1993;268:1405–1413. [PubMed] [Google Scholar]
- Kozak M. The scanning model for translation: an update. J Cell Biol. 1989;108:229–241. doi: 10.1083/jcb.108.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn CI, Yu SY, Chraplyvy M, Linder HE, Senior RM. The induction of emphysema with elastase. II. Changes in connective tissue. Lab Invest. 1976;34:372–380. [PubMed] [Google Scholar]
- Lee KA, Pierce RA, Davis EC, Mecham RP, Parks WC. Conversion to an elastogenic phenotype by fetal hyaline chondrocytes is accompanied by altered expression of elastin-related macromolecules. Dev Biol. 1994;163:241–252. doi: 10.1006/dbio.1994.1140. [DOI] [PubMed] [Google Scholar]
- Li DY, Toland AE, Boak BB, Atkinson DL, Ensing GJ, Morris CA, Keating MT. Elastin point mutations cause an obstructive vascular disease, supravalvular aortic stenosis. Hum Mol Genet. 1997;6:1021–1028. doi: 10.1093/hmg/6.7.1021. [DOI] [PubMed] [Google Scholar]
- McKusick VA. The defect in Marfan syndrome. Nature. 1991;352:279–281. doi: 10.1038/352279a0. [DOI] [PubMed] [Google Scholar]
- Mecham RP. Modulation of elastin synthesis: in vitro models. Methods Enzymol. 1987;144:232–246. doi: 10.1016/0076-6879(87)44181-5. [DOI] [PubMed] [Google Scholar]
- Mecham RP, Davis EC. Elastic fiber structure and assembly. In: Yurchenko PD, Birk DE, Mecham RP, editors. Extracellular Matrix Assembly and Structure. San Diego, CA: Academic Press; 1994. pp. 281–314. [Google Scholar]
- Mecham RP, Lange G, Madaras J, Starcher B. Elastin synthesis by ligamentum nuchae fibroblasts: effects of culture conditions and extracellular matrix on elastin production. J Cell Biol. 1981;90:332–338. doi: 10.1083/jcb.90.2.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson MD. Development of Bruch’s membrane in the chick: an electron microscopic study. Invest Ophthalmol Vis Sci. 1979;18:329–338. [PubMed] [Google Scholar]
- Olson TM, Michels VV, Urban Z, Csiszar K, Christiano AM, Driscoll DJ, Feldt RH, Boyd CD, Thibodeau SN. A 30kb deletion within the elastin gene results in familial SVAS. Hum Mol Genet. 1995;4:1677–1679. doi: 10.1093/hmg/4.9.1677. [DOI] [PubMed] [Google Scholar]
- Parks WC, Secrist H, Wu LC, Mecham RP. Developmental regulation of tropoelastin isoforms. J Biol Chem. 1988;263:4416–4423. [PubMed] [Google Scholar]
- Reynolds ES. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963;17:208–215. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snider GL. Emphysema: the first two centuries—and beyond. Part two. Am Rev Respir Dis. 1992;146:1615–1622. doi: 10.1164/ajrccm/146.6.1615. [DOI] [PubMed] [Google Scholar]
- Starcher B, Conrad M. A role for neutrophil elastase in the progression of solar elastosis. Connect Tissue Res. 1995;31:133–140. doi: 10.3109/03008209509028401. [DOI] [PubMed] [Google Scholar]
- Stone PJ, Morris SM, Martin BM, McMahon MP, Faris B, Franzblau C. Repair of protease-damaged elastin in neonatal rat aortic smooth muscle cell cultures. J Clin Invest. 1988;82:1644–1654. doi: 10.1172/JCI113776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tassabehji M, Metcalfe K, Donnai D, Hurst J, Reardon W, Burch M, Read AP. Elastin: genomic structure and point mutations in patients with supravalvular aortic stenosis. Hum Mol Genet. 1997;6:1029–1036. doi: 10.1093/hmg/6.7.1029. [DOI] [PubMed] [Google Scholar]
- Thompson RW, Holmes DR, Mertens RA, Liao S, Botney MD, Mecham RP, Welgus HG, Parks WC. Production and localization of 92-kilodalton gelatinase in abdominal aortic aneurysms. J Clin Invest. 1995;96:318–326. doi: 10.1172/JCI118037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trask TM, Ritty TM, Broekelmann T, Tisdale C, Mecham RP. N-terminal domains of fibrillin-1 and fibrillin-2 direct the formation of homodimers: a possible first step in microfibril assembly. Biochem J. 1999;340:693–701. [PMC free article] [PubMed] [Google Scholar]
- Wachi H, Seyama Y, Yamashita S, Tajima S. Cell cycle-dependent regulation of elastin gene in cultured chick vascular smooth-muscle cells. Biochem J. 1995;309:575–579. doi: 10.1042/bj3090575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrenn DS, Griffin GL, Senior RM, Mecham RP. Characterization of biologically active domains on elastin: identification of a monoclonal antibody to a cell recognition site. Biochemistry. 1986;25:5172–5176. doi: 10.1021/bi00366a028. [DOI] [PubMed] [Google Scholar]
- Zhang M-C, He L, Giro M, Young SL, Tiller GE, Davidson JM. Cutis laxa arising from frameshift mutations in exon 30 of the elastin gene (ELN) J Biol Chem. 1999;274:981–986. doi: 10.1074/jbc.274.2.981. [DOI] [PubMed] [Google Scholar]