Abstract
Severe sepsis is a common and commonly fatal disease and is essentially an exaggerated inflammatory response. The epidemiology of severe sepsis and septic shock has been difficult to determine because of an inconsistent approach to definitions and diagnosis. Patients with sepsis account for approximately a third of hospital and intensive care unit bed days in the UK and mortality ranges from 25% to 80%. A number of interventions have recently been shown to improve outcomes. The Surviving Sepsis Campaign recommends a package of evidence based interventions known as the sepsis resuscitation bundles and the sepsis treatment bundles. The aim is to ensure that eligible patients receive all appropriate treatments in a timely fashion, utilising protocol driven prescriptions.
Keywords: emergency department, sepsis, septic shock
The epidemiology of severe sepsis and septic shock has been difficult to determine because of an inconsistent approach to definitions and diagnosis. Generally speaking the disease is not notifiable. Not all patients are admitted to the intensive care unit (ICU), many are elderly, and sepsis may be the final stage in a chronic disease, especially in patients with immunosuppression. More than half of all patients treated in hospital for severe sepsis are managed exclusively in the general ward1 and some elderly, chronically sick patients may be treated at home or in nursing homes. When a patient dies as a result of an infectious disease, sepsis may not appear on the death certificate; associated conditions such as bronchopneumonia, perforated viscus, or malignancy may be recorded instead.
A classification scheme was proposed in 1991 following a consensus conference that included the American College of Chest Physicians and the Society of Critical Care Medicine.2 An international group reiterated the guidelines with minor amendments in 2001.3 The spectrum of the sepsis syndrome represents a continuum of increasing clinical severity and associated mortality (table 1). The end stage is multiple organ dysfunction syndrome, which carries a very poor prognosis.
Table 1 Definitions of systemic inflammatory response syndrome, sepsis, and septic shock.
| SIRS | Two or more of: |
| Temperature >38°C or <36°C | |
| Heart rate >90 | |
| Respiratory rate >20 or PaCO2 <32 mm Hg (4.2 kPa) | |
| White blood count >12×109, <4×109, or 10% | |
| immature (band) forms | |
| Sepsis | SIRS associated with documented infection and at least |
| one of the following: | |
| Altered mental state | |
| Hypoxaemia: PaO2 <72 mm Hg (9.47 kPa) at FiO2 0.21, | |
| not due to primary pulmonary disease | |
| Elevated plasma lactate level | |
| Oliguria (urine output <0.5 ml/kg/h) | |
| Severe | Sepsis associated with organ dysfunction, hypotension, or |
| sepsis | evidence of hypoperfusion, including but not limited to |
| acutely altered mental status, oliguria, or lactic acidosis | |
| Septic | Sepsis induced hypotension (systolic blood pressure |
| shock | <90 mm Hg, MAP <60 or a fall of >40 mm Hg |
| from baseline), not responsive to fluid resuscitation |
MAP, mean arterial pressure; SIRS, systemic inflammatory response syndrome.
Incidence
Using the 1992 guidelines, Angus and Wax published an update on the epidemiology of sepsis in 2001. They reported an increase in the annual incidence from 73.6 to 175.9 per 100 000 of the population in the United States between 1979 and 1989.4 This represents up to 11% of all hospital admissions. The financial costs of care are high, especially in the most critically ill patients and non‐survivors. Angus and colleagues estimated the average cost per case as $22 000. The incidence of the condition is expected to increase by 1.5% per annum to 2010.1
In the UK the annual incidence of severe sepsis is around 200 000. In other words, about 1000 patients are admitted to every general or teaching hospital each year. Fewer than half of these patients survive and hence there will be approximately one to two deaths in every UK hospital every day as a result of the condition. Padkin and colleagues reported on 56 000 patients admitted to ICUs in England, Wales, and Northern Ireland between 1995 and 2000. Just over 27% met the criteria for severe sepsis within 24 h of admission. The annual incidence of severe sepsis in patients admitted to ICUs and meeting severe sepsis criteria at 24 h, was 51 per 10 000 of the population and the mortality rate was 47%. Patients with sepsis accounted for 45% of ICU bed days and 33% of hospital bed days. The ICU length of stay (LOS) was between 4 and 8 days and the median hospital LOS was 18 days.5
Aetiology
Lower respiratory tract infections comprise almost half of all infective cases, abdominal sources account for 20%, and uro‐genital sepsis, skin, bone, and soft tissue infections, and miscellaneous conditions, including meningococcaemia, contribute the remainder.1 Multiple sites are involved in 10–15% of cases and multiple organisms are identified in about 10%. Polymicrobial infections are seen most frequently in patients with neutropenia.
Pathphysiology
Sepsis is essentially an exaggerated inflammatory response. It is best understood in relation to endotoxin, a component of the cell walls of Gram negative bacteria. Events may also be triggered by exotoxin released from Gram positive bacteria, by other microbial products, and by elements of the compliment system. A syndrome identical to severe sepsis may occur in other conditions such as trauma, burns, pancreatitis, and amniotic fluid embolism. Only around half of patients with clinical and pathological evidence of severe systemic inflammatory response syndrome (SIRS) have positive blood cultures.6
Bacterial cell wall products are recognised by the monocytes and macrophages via so‐called Toll‐like receptors within their own cell walls. As a result of this interaction, pro‐inflammatory cytokines such as TNF‐α and the interleukins are released from the activated mononuclear cells. The cytokines are responsible for some of the clinical features and also activate the compliment and coagulation cascades and inhibition of fibrinolysis.
The other important aspect of this immuno‐inflammation is the stimulation of the polymorphs to marginate, rolling along the endothelium and adhering to the endothelial cell wall via specialised intracellular adhesion molecules. The polymorphs now migrate into the interstitial space under the influence of IL‐8 (a chemokine) where they degranulate, releasing lytic substances toxic to the adjacent cells and tissues.
At the same time, a number of vasoactive substances, including the cytokines and bradykinin, induce inappropriate vasodilatation (mediated by nitric oxide) and vasoconstriction. Pathological shunting of blood within the microcirculation occurs and oxygen delivery (DO2) to the tissues is impaired. Tissue oxygenation is further impaired by capillary leak of red cells and intravascular fluid, by disseminated intravascular coagulation, and by extrinsic compression of the capillaries by interstitial oedema. The cells switch to anaerobic respiration, manifest as lactic acidosis.
Despite the high and rising incidence of sepsis, the unacceptable death rate, and high costs of care, trends in mortality have been relatively stable over time. However, research in this field is prolific and a number of interventions have recently been shown to improve outcomes.7
Outcome
The mortality of this condition ranges from 25% to 80%, depending on illness severity and the number and severity of organ failures.6 Other factors influencing outcome include the age and health status of the patient, the nature and source of the infection (Gram positive infections may respond less well to treatment), and polymorphism for genes coding for elements of the inflammatory system.8
Illustrative case report
A 76 year old woman is brought to the emergency department (ED) from a residential home. She has a past history of chronic obstructive pulmonary disease, congestive cardiac failure, type II diabetes, and osteoarthritis. Normally mobile, independent, and self caring, she had been discharged from hospital 3 weeks previously following a lengthy admission with poor mobility. Her general practitioner prescribed antibiotics for cellulitis of the legs 7 days before her ED presentation. She is now confused and disorientated and apparently has difficulty in breathing.
On initial assessment her airway is open and she is breathing supplemental oxygen via a reservoir bag. Respiratory rate is 24 and the SpO2 monitor reads 98%. Heart rate is approximately 120 and irregular, and blood pressure is approximately 88/40. The tympanic temperature is 35.7°C and the Glasgow Coma Scale (GCS) is 12 (E = 3, V = 4, M = 5).
Early interventions include continued oxygen therapy, intravenous (IV) normal saline (given slowly because of possible heart failure), and IV cefuroxime. A chest radiograph and ECG are ordered and bloods are drawn for routine haematology, biochemistry, and blood cultures. Arterial puncture for blood gases yields the following results:
pH 7.19
PaCO2 5.94 kPa
PaO2 13.65 kPa
Bicarbonate 12.40 mmol/l
Lactate 6.98 mmol/l
Questions, part 1
What is the diagnosis?
What are the risk factors for poor outcome?
Discussion, part 1
1. This patient has severe sepsis and sepsis associated hypotension. Hypoperfusion is indicated by altered mental status and lactic acidosis. If there is no response to initial fluid bolus then septic shock has supervened. She will probably require vasopressor therapy to support her circulation.
The differential diagnosis is substantial and includes common conditions such as stroke, myocardial infarction, intra‐abdominal catastrophe, and diabetic keto‐acidosis. The patient is apyrexial and confirmatory tests for infection and inflammation may not be immediately available. A high index of suspicion is required if sepsis is to be diagnosed promptly. Atypical presentations are common, especially in the elderly in whom bacteraemia frequently presents without fever.9,10
2. Shapiro and colleagues identified correlates for in‐hospital death in 3179 ED patients in whom blood cultures were ordered during a 1 year period. Significant risk factors included age >65, nursing home residence, altered mental status, tachypnoea or hypoxia, septic shock, terminal illness, and pneumonia as source.11 Jones found a mortality of 45% in patients with bacteraemia where the skin or soft tissues were the source.6 Finally, one should consider the use of antibiotics during the antecedent illness. Inappropriate choice of and dosing with antimicrobials are common, even in hospital, and such errors increase mortality in sepsis by 10–15%.12,13,14,15
The patient has a poor chronic health evaluation and is now severely ill and acidotic. She has a minimum of two organ failures and at least three SIRS criteria, suggestive of a mortality risk of at least 26% and more likely over 50%. Positive blood cultures, hypoalbuminaemia, and abnormalities in the blood count will add to the risk of death.16
3. The patient should be intubated if her airway is judged to be at risk, if she fails to respond to treatment, becomes exhausted or comatose, or develops ventilatory failure. A central venous line should be inserted, preferably an oximeter attached to a device capable of continuously displaying the central venous oxygen saturation (ScvO2). Large volumes (4–6 l) of fluid may be required and concerns over heart failure should not prevent adequate volume resuscitation. Clinical response and monitored data guide the fluid regime. Colloid and crystalloid are equivalent in terms of efficacy and adverse events.17,18,19
An arterial catheter should be inserted and the mean arterial pressure (MAP) targeted at >65 mm Hg with fluids, noradrenaline (norepinephrine) and, if necessary, dobutamine. MAP at this level is required to maintain adequate splanchnic and renal perfusion. A nasogastric tube and urinary catheter should also be inserted since a urine output of at least 0.5 ml/kg/h is desirable. Atrial fibrillation is common in sepsis and may be contributing to the hypotension in this case. Fluid resuscitation and correction of electrolyte abnormalities takes precedence over antidysrhythmics.
Parenteral, broad spectrum antibiotics should be given early and in appropriate doses.
Case progression
The patient has now been in the ED for 1.5 h. Her general condition, vital signs, and GCS are unchanged. Secondary assessment has revealed marked cellulitis of both legs, complicating chronic venous insufficiency. There are infected leg ulcers, widespread erythaema, and some free pus.
The chest radiograph shows diffuse alveolar shadowing and the ECG confirms atrial fibrillation. Results of other investigations are summarised in table 2.
Table 2 Results of early investigations.
| Haemoglobin | 8.9 g/dl |
| White cell count | 11.5×109/l |
| Platelets | 96×109/l |
| Prothrombin time | 21 s |
| Activated partial thromboplastin time | 58 s |
| Sodium | 125 mmol/l |
| Potassium | 5.6 mmol/l |
| Urea | 27.7 mmol/l |
| Creatinine | 226 μmol/l |
| Glucose | 28.2 mmol/l |
| Amylase | 188 IU/l |
An arterial line has been placed in a radial artery. A urinary catheter and a nasogastric tube are also in place. A central venous oximetry catheter has been inserted into the right internal jugular vein under ultrasound guidance. The central venous pressure (CVP) is 18 mm Hg and ScvO2 is 48%.
Questions, part 2
What is the relevance of ScvO2 in the management of sepsis?
What treatments may be utilised to increase ScvO2?
What other ED interventions, comprising the sepsis resuscitation bundles, have been shown to improve outcomes in severe sepsis and septic shock?
Discussion, part 2
1. An important determinant of outcome in sepsis is the relationship between oxygen delivery by the circulation and oxygen consumption at the tissues. In health, DO2 is maintained at about 1000 ml/min, negatively influenced by anaemia, hypoxaemia, and heart failure. Oxygen consumption (VO2) is normally 250 ml/min and is relatively independent of DO2 above a critical level where the DO2/VO2 ratio falls to around 2:1. Below this level supply dependence occurs and, in spite of compensatory mechanisms, the cells switch to anaerobic respiration. In severe sepsis the curve is right‐shifted and supply dependence occurs at higher levels of DO2.20
There is a critical period in the natural history of severe sepsis when the tissues may be suffering the effects of global hypoxia despite relatively normal vital signs. Rivers has coined the term “cryptic shock” to describe this phenomenon.21 The aim of treatment is to restore DO2 before the onset of irreversible shock and organ failure.
The saturation of mixed venous blood (SvO2), sampled from the pulmonary artery (PA) via a Swan‐Ganz catheter, is a reliable indicator of global tissue oxygenation.22,23 It has been demonstrated that ScvO2, the saturation of blood sampled from a central vein, provides a reasonable representation of SvO2, without the need for a PA catheter.24,25,26 This relatively simple measure was used as a resuscitation end point in an important study demonstrating significant improvement in outcomes in severe sepsis and septic shock by targeting an ScvO2 at 75% during the first 6 h after ED presentation.27 The mortality in the treatment group was 30.5% and in the control group 46.5% (p = 0.009). Interestingly, the overall use of intravenous fluids, red blood cells, and vasoactive drugs was similar in both groups. The most important difference was that treatment was initiated much earlier in patients randomised to early goal directed therapy (EGDT).
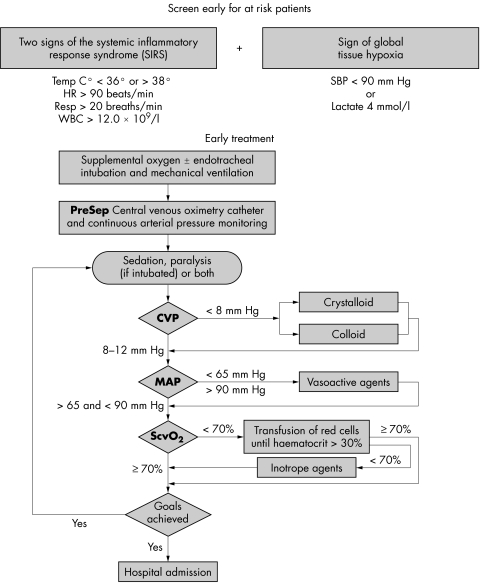
2. A suggested protocol is shown in fig 1. Protocols may be implemented using intermittent sampling from a standard CVP line. However, the use of a fibre optic device capable of continuous monitoring of ScvO2 is preferable.28,29
Figure 1 Early goal directed therapy (EGDT) protocol (reproduced by kind permission of Edwards Life Sciences Ltd).
The present patient has relatively high pressure in her central veins in any event because of chronic cardio‐respiratory disease. She should receive crystalloid or colloid to achieve a sustained increase in CVP. If the MAP is not responding to fluid she should be given noradrenaline (norepinephrine). Blood transfusion is required if the haematocrit is less than 30% or the haemoglobin falls below 7 g/dl.7
3. Guidelines for the management of severe sepsis and septic shock were published in 2004.7 Evidence based recommendations were determined following a systematic review of the literature and included resuscitation endpoints, antibiotic selection, glycaemic control, and the use of drugs such as inotropes, vasopressors, steroids, and recombinant human activated protein C (RhAPC).
Appropriate cultures must be obtained before antibiotics are administered, including at least two sets of blood cultures and, in the present case, swabs from leg ulcers. Intravenous antibiotics should be given within 1 h of diagnosis if possible. Maintaining a supply of pre‐mixed antibiotics within the ED enables prompt administration in urgent situations. The choice of drug depends on a number of factors, including recent antibiotic use, immunosuppression or proven allergy, and local resistance patterns. The antimicrobial is selected to ensure effectiveness against the likely organism(s) and penetration into the infected tissues. Broad spectrum empirical combinations such as cefuroxime and metronidazole are a reasonable choice in many circumstances; the prescription is reviewed at 48–72 h when results of cultures are to hand.
In the present case, the patient is diabetic, antibiotic experienced, and critically ill from a probable skin source. Streptococci, Staphylococci, Enterococci, Clostridia, and Pseudomonas species, and fungi must be considered. The patient has organ failures, which will adversely affect the pharmacokinetics of drugs, increasing the risk of adverse effects such as worsening renal impairment from agents, including the aminoglycosides. Specialist advice from infectious diseases and pharmacology consultants may be required. Source control includes surgery in some cases; the present patient may benefit from an assessment by a plastic surgeon and the ruling out of deep soft tissue infection.
Plasma glucose should be maintained within the reference range using insulin via a sliding scale. The patient requires stress‐dose corticosteroids because of the risk of sepsis induced adrenal suppression. High dose methylprednisolone has no role. RhAPC is usually reserved for the ICU but has been used in the ED setting when lack of intensive care beds led to delays in transfer.30 If delays are prolonged, prophylaxis against peptic ulceration and venous thromboembolism may also be initiated in the ED.
RhAPC is a naturally occurring anti‐inflammatory protein that modulates coagulation and promotes fibrinolysis. In a prospective randomised controlled trial, RhAPC was shown to reduce mortality from 30.8% to 24.7% in adult patients with severe sepsis and high risk of death.31
Case outcome
The patient was subsequently intubated and transferred to ICU where she was treated for 12 days before transfer to a medical ward. Treatment in ICU consisted of intermittent positive pressure ventilation, parenteral antibiotics (cefuroxime and gentamicin), noradrenaline (norepinephrine), intensive insulin therapy, and RhAPC. She received local antiseptic dressings to her leg ulcers. Enteral feeding was established on day 2 in the ICU and tracheostomy was performed on day 3. She was ventilated with low tidal volumes, permitting a degree of hypercarbia. Acidosis and renal impairment resolved with intravenous fluids, targeting ScvO2 at 75%. Staphylococcus aureus and Enterococcus species were isolated from the ulcers; blood cultures produced no growth. She was eventually discharged back to her nursing home after a total LOS in hospital of 28 days.
Conclusions
Severe sepsis is a common and commonly fatal disease. Recent studies have identified a number of interventions capable of producing survival benefits. Unfortunately, the uptake of many potentially useful treatments is not uniform. This situation, which reflects the difficulty of implementing evidence into practice, is a well recognised phenomenon.32 The Surviving Sepsis Campaign (http://www.survivingsepsis.org) promulgates a package of evidence based interventions known as the sepsis resuscitation bundles and the sepsis treatment bundles. The aim is to ensure that eligible patients receive all appropriate treatments in a timely fashion, utilising protocol driven prescriptions.
Many of the recommendations of the campaign are directed at ICU clinicians. However, some interventions are appropriate to the ED setting.7 Principle among these is EGDT, which is known to improve short term and long term survival.27 Implementation of the other elements of the bundles in the ED significantly reduces delays to definitive treatment and may further reduce mortality.30
Abbreviations
CVP - central venous pressure
DO2 - oxygen delivery
ED - emergency department
EGDT - early goal directed therapy
GCS - Glasgow Coma Scale
ICU - intensive care unit
IV - intravenous
LOS - length of stay
MAP - mean arterial pressure
PA - pulmonary artery
RhAPC - recombinant human activated protein C
ScvO2 - central venous oxygen saturation
SIRS - systemic inflammatory response syndrome
SvO2 - mixed venous oxygen saturation
VO2 - oxygen consumption
Footnotes
Funding: none
Competing interests: none declared
Patient details are published with consent
References
- 1.Angus D C, Linde‐Zwirble W T, Lidecker J.et al Epidemiology of severe sepsis in the United States: analysis of incidence, outcome and associated costs of care. Crit Care Med 200129(7)1303–1310. [DOI] [PubMed] [Google Scholar]
- 2.Members of the American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference Committee Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit Care Med 19922864–874. [PubMed] [Google Scholar]
- 3.Levy M M, Fink M P, Marshall J C.et al 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med 2003311250–1256. [DOI] [PubMed] [Google Scholar]
- 4.Angus D C, Wax R S. Epidemiology of sepsis: an update. Crit Care Med 200129(7)S109–S116. [DOI] [PubMed] [Google Scholar]
- 5.Padkin A, Goldfrad C, Brady A R.et al Epidemiology of severe sepsis occurring in the first 24 hours in intensive care units in England, Wales and Northern Ireland. Crit Care Med 200331(9)2332–2338. [DOI] [PubMed] [Google Scholar]
- 6.Jones G R. Assessment criteria in identifying the sick sepsis patient. J Infect 199837(suppl 1)24–29. [DOI] [PubMed] [Google Scholar]
- 7.Dellinger R P, Carlet J M, Mansur H.et al Surviving Sepsis Campaign guidelines for the management of severe sepsis and septic shock. Crit Care Med 200432(3)858–873. [DOI] [PubMed] [Google Scholar]
- 8.Kellum J A, Angus D C. Genetic variation and risk of sepsis. Minerva Anestesiol 200369245–253. [PubMed] [Google Scholar]
- 9.Gleckman R, Hibert D. Afebrile bacteremia: a phenomenon in geriatric patients. JAMA 19822481478–1481. [DOI] [PubMed] [Google Scholar]
- 10.Fontanarosa P, Kaeberlein F J, Gerson L W.et al Difficulty in predicting bacteremia in elderly emergency patients. Ann Emerg Med 199221841–848. [DOI] [PubMed] [Google Scholar]
- 11.Shapiro N I, Wolfe R E, Moore R B.et al Mortality in Emergency Department Sepsis (MEDS) score: a prospectively derived and validated clinical prediction rule. Crit Care Med 200331(3)968–969. [DOI] [PubMed] [Google Scholar]
- 12.McCabe W R, Jackson G G. Gram‐negative bacteremia. Arch Intern Med 196211092–100. [Google Scholar]
- 13.Kreger B E, Craven D E, McCabe W R. Gram‐negative bacteremia IV. Re‐evaluation of clinical features and treatment in 612 patients. Am J Med 198068344–355. [DOI] [PubMed] [Google Scholar]
- 14.Leibovici L, Shagra I, Drucker M. The benefit of appropriate empirical antibiotic treatment in patients with bloodstream infection. J Intern Med 1998244379–386. [DOI] [PubMed] [Google Scholar]
- 15.Ibrahim E H, Sherman G, Ward S. The influence of inadequate antimicrobial treatment of bloodstream infections on patient outcomes in the ICU setting. Chest 2000118146–155. [DOI] [PubMed] [Google Scholar]
- 16.Jones G R, Lowes J A. The systemic inflammatory response syndrome as a predictor of bacteraemia and outcome from sepsis. Q J Med 199689515–522. [DOI] [PubMed] [Google Scholar]
- 17.Choi P T L, Yip G, Quinonez L G. Crystalloids vs. colloids in fluid resuscitation: a systematic review, Crit Care Med 199927200–210. [DOI] [PubMed] [Google Scholar]
- 18.Cook D, Guyatt G. Colloid use for fluid resuscitation: evidence and spin. Ann Intern Med 2001135205–208. [DOI] [PubMed] [Google Scholar]
- 19.Schierhout G, Roberts G. Fluid resuscitation with colloid or crystalloid solutions in critically ill patients. A systematic review of randomised trials. BMJ 1998316(7136)961–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brealey D, Singer M. Multi‐organ dysfunction in the critically ill: epidemiology, pathophysiology and management. J R Coll Physicians Lond 200034424–427. [PMC free article] [PubMed] [Google Scholar]
- 21.Rivers E P, Nguyan B, Huang D T.et al Early goal‐directed therapy. Crit Care Med 200432(1)314–315. [DOI] [PubMed] [Google Scholar]
- 22.Krafft P, Steltzer H, Hiesmayr M.et al Mixed venous oxygen saturation in critically ill septic shock patients. The role of defined events. Chest 1993103900–906. [DOI] [PubMed] [Google Scholar]
- 23.Edwards J D. Oxygen transport in cardiogenic and septic shock. Crit Care Med 199119658–663. [DOI] [PubMed] [Google Scholar]
- 24.Reinhart K, Rudolph T, Bredle D L.et al Comparison of central venous to mixed venous oxygen saturation during changes in oxygen supply/demand. Chest 1989951216–1221. [DOI] [PubMed] [Google Scholar]
- 25.Rady M Y, Rivers E P, Nowak R M. Resuscitation of the critically ill in the ED: responses of blood pressure, heart rate, shock index, central venous oxygen saturation, and lactate. Am J Emerg Med 199614218–225. [DOI] [PubMed] [Google Scholar]
- 26.Rady M Y, Rivers E P, Martin G B. Continuous central venous oximetry and shock index in the emergency department: use in the evaluation of clinical shock. Am J Emerg Med 199210528–541. [DOI] [PubMed] [Google Scholar]
- 27.Rivers E P, Nguyen H B, Havstad S.et al Early goal‐directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med 2001345(19)1368–1377. [DOI] [PubMed] [Google Scholar]
- 28.Reinhart K, Kuhn H J, Hartog C.et al Continuous central venous and pulmonary artery oxygen saturation monitoring in the critically ill. Intensive Care Med 2004301572–1578. [DOI] [PubMed] [Google Scholar]
- 29.Rivers E P, Ander D S, Powel D. Central venous oxygen saturation monitoring in the critically ill patient. Curr Opin Crit Care 20017204–211. [DOI] [PubMed] [Google Scholar]
- 30.Nguyen H B, Klein W, Corbett S W.et al Early goal‐directed therapy, corticosteroids and activated protein C for severe sepsis/septic shock in the emergency department. Presented at the 34th Congress of the Society of Critical Care Medicine, 15–19 January 2005, Phoenix, AZ
- 31.Laterre P ‐ F, Levy H, Ball D.et al The effect of drotrecogin alpha (activated) on hospital mortality, length of stay and discharge location. Chest 2002122(suppl)S143 [Google Scholar]
- 32.Cabana M D, Rand C S, Powe N R.et al Why don't physicians follow clinical practice guidelines? A framework for improvement. JAMA 19992821458–1465. [DOI] [PubMed] [Google Scholar]