Abstract
Age-related change in the difference between left- and right-side speed on motor examination may be an important indicator of maturation. Cortical maturation and myelination of the corpus callosum are considered to be related to increased bilateral skill and speed on timed motor tasks. We compared left minus right foot, hand, and finger speed differences using the Revised Physical and Neurological Assessment for Subtle Signs (PANESS; Denckla, 1985); examining 130 typically developing right-handed children (65 boys, 65 girls) ages 7−14. Timed tasks included right and left sets of 20 toe taps, 10 toe-heel alternation sequences, 20 hand pats, 10 hand pronate-supinate sets, 20 finger taps, and 5 sequences of each finger-to-thumb apposition. For each individual, six difference scores between left- and right-sided speeded performances of timed motor tasks were analyzed. Left-right differences decreased significantly with age on toe tapping, heel-toe alternations, hand pronation-supination, finger repetition, and finger sequencing. There were significant gender effects for heel-toe sequences (boys showing a greater left-right difference than girls), and a significant interaction between age and gender for hand pronation-supination, such that the magnitude of the left-right difference was similar for younger, compared with older girls, while the difference was significantly larger for younger, compared to older boys. Speed of performing right and left timed motor tasks equalizes with development; for some tasks, the equalization occurs earlier in girls than in boys.
Keywords: Motor, PANESS, Laterality, Gender, Corpus callosum
The majority of studies examining motor development in children suggests that hand preference emerges for a variety of skills between ages 3 and 6 years. By Kindergarten, most typically developing children consistently demonstrate a clear hand preference, with approximately 90% showing right-hand preference for most activities (Best, 1988; Bryden, Pryde, & Roy, 2000; Oeztuerk et al., 1999; Pryde, Bryden, & Roy, 2000). The remaining 10% of Kindergarten children show either left- or mixed-hand preference, or delayed manifestation of handedness, suggesting age-related changes that may continue throughout the school-age years. Hand preference implies that the preferred hand is more proficient than the nonpreferred hand in terms of coordination and speed. During the first five years of life, the preferred hand begins to emerge as superior to the nonpreferred hand in terms of speed and coordination. Typically developing toddlers demonstrate an increasingly lateralized hand preference for activities such as reaching, manipulation of toys, and drawing, with more children at age 2 years showing consistent hand preference than those at 9 and 13 months of age, particularly on crayon tasks (Cornwell, Harris, & Fitzgerald, 1991). After age 5 years, however, although hand preference persists, these lateral differences diminish; this is likely to be due (at least in part) to the rapid development and myelination of the corpus callosum that allows for more efficient communication from the dominant to nondominant motor cortex (Barnea-Goraly et al., 2005; Denckla, 1973).
Asymmetries in motor speed and coordination are thought to emerge at the same time as hand preference. Denckla (1973) found that right-handed finger repetition was faster than left-handed finger repetition in typically developing 5- to 7-year-old children. While the percentage of children performing faster with the right hand increased with age, the magnitude of this right-hand superiority decreased with age. Thus, lateral hand preference tends to become clearer during the elementary school years. An additional manifestation of dominance, however, is that nondominant-hand speed/efficiency also improves and may ultimately equalize with dominant-hand speed/skill during this same time period, potentially reflecting connectivity between hemispheres, or different rates of cortical maturation. A similar asymmetric developmental pattern has been observed among dichotic listening tasks, in which a right-ear advantage emerges early in life, followed by rapid increase in right-ear proficiency, and finally by a rapid increase (but not equalization in) left-ear skill (Geffner & Hochberg, 1971). These data highlight relative left-side (i.e., right hemisphere) disadvantage in young, right-handed children and suggest that developmental models of cerebral maturation should also examine the rate of improvement of left-sided skill in relation to right-side skill as a manifestation of the ability of the dominant left hemisphere to transmit motor skill “programs” to the nondominant right hemisphere. Little research, however, has examined the age-related changes in intraindividual left-right differences among school-aged children. This information is critical, as it may provide greater insight into the maturation and myelination of the corpus callosum.
Corpus Callosum Development
The corpus callosum is the main bundle of fibers that interconnects the corresponding neocortical areas of the two cerebral hemispheres (Fix, 2002) and is the largest fiber tract in the brain (Innocenti, 1994). It is an integral part of the neural network that supports higher cognitive functioning and is among the last white matter tracts to mature (Roessner et al., 2004), with an anterior-to-posterior myelination pattern (Hannay, 2000; Thompson et al., 2000). The first stages of development of the corpus callosum begin between the 10th and 25th week of gestation and may extend into the third decade of life (Giedd, Blumenthal, Jeffries, Rajapakse, et al., 1999b; Roessner et al., 2004). Differences in fiber types along the corpus callosum suggest functional differences in interhemispheric communication between different cortical areas (Aboitiz & Montiel, 2003).
Corpus callosum development may occur at different rates in boys and girls. De Bellis et al. (2001) found that boys had a 45.1% increase in white matter and a 58.5% increase in corpus callosum area between ages 6 and 18 years, while girls had a 17.1% and 27.4% increase in white matter and corpus callosum area, respectively. Giedd and colleagues found that the genu of the corpus callosum was significantly larger in boys ages 5 to 18 years than girls (with a trend for larger anterior midbody in boys). Boys also had 11% greater cerebral volumes. When correcting for total cerebral volume, there were no significant gender differences in corpus callosum size; however, there were trends for girls to have larger genu, posterior midbody, and isthmus (Giedd, Blumenthal, Jeffries, Rajapakse, et al., 1999b).
Motor skill development, particularly the developmental pattern of asymmetries in left- versus right-sided performance, may be a marker for maturation of the corpus callosum or for the different rates of development of the cerebral hemispheres. Performance difference between preferred- and nonpreferred-sided activities appears to follow a developmental trajectory in which there are initially small performance differences (infancy), then more pronounced differences in favor of a developing preferred side (preschool through adolescence; De Bellis et al., 2001), and finally a reduction and even “equalization” of right and left skills after age 9 years—for repetitive finger tapping, sets of finger sequences, and repetitive foot taps (Denckla, 1985). This pattern of maturation appears to occur differently in comparisons between healthy children and those with neurodevelop-mental disorders, and between healthy boys and girls (Barnea-Goraly et al., 2005). Measurement of motor skill asymmetries (speed, coordination) may allow for a deeper understanding of motor development in children and may have implications for better understanding corpus callosum as well as hemispheric maturation.
Neurobehavioral Assessment of Motor Function
A variety of standardized tests of motor function (speed, coordination, strength) with published normative data is available for school-aged children. Examination of the age- and gender-related changes in these normative data may begin to shed some light on the behavioral manifestations of corpus callosum development. The pattern of “equalization” of motor speed (which occurs around age 9 for some skills and during adolescence for other skills) is observed in the normative data for some, but not all, clinically available motor tests. To illustrate, the Purdue Pegboard (Tiffin, 1968) requires the examinee to place cylindrical pegs in vertically arranged holes, first with the preferred hand, then with the nonpreferred hand, and then with both hands together. Gardner and Broman (1979) reported data on the Purdue Pegboard that were derived from 1,334 healthy schoolchildren ages 5 to 15 years stratified on the basis of age and sex. In this age range, the preferred/ nonpreferred differences in number of pegs placed in 30 seconds remained constant (i.e., more pegs placed by preferred hand), with no differences between boys and girls in lateral time difference. In contrast, the Grooved Pegboard (Lafayette Instrument Company, 1989) requires the examinee to fill all 25 slotted holes in a five-by-five array with key-like pegs—matching the groove of the peg with the groove of the board—first with the preferred, then with the nonpreferred hand, as quickly as possible. For age range 9−14 (i.e., for the age range in which the subjects are required to fill the whole board), the preferred/ nonpreferred hand difference declined steadily from 12.6 seconds (age 9) to 3.6 seconds (age 14; Knights & Moule, 1968). The Finger Tapping Test (Reitan, 1969) uses a specially adapted finger tapper for which the subject is instructed to tap as fast as possible using the index finger of the preferred hand, and then again with the nonpreferred hand. The maximal tapping speed of each index finger is tested during five 10-second trials. Published normative data indicate that the left-right difference in tapping speed actually increases in boys between the ages of 6 and 14, whereas it remains fairly constant in girls during the same age range (Finlayson & Reitan, 1976).
Summary
In summary, the available normative data suggest that some motor functions (speed, coordination) develop at different rates for preferred- and nonpreferred-sided activities, such that for some tasks, by age 9 years, and for many by adolescence, the differences may be small or negligible. Because boys and girls have different patterns of neurological and neuroanatomic development during the school-age years, they are likely to have different patterns of motor skill asymmetry (Thompson et al., 2000). If “equalization” of motor skill efficiency is a marker for maturation of the motor system, then girls and boys may achieve this set of milestones at different times, which coincide with differential rates of maturation of the corpus callosum as well as cortical systems supporting motor speed. The purpose of the present study was to examine this pattern of preferred versus nonpreferred (foot, finger, hand) speed differences in typically developing children to determine how these differences change throughout childhood. We hypothesized that the magnitude of the differences, reflecting connectivity between hemispheres as a vehicle for the dominant left to “pace and program” the nondominant right, would decrease between ages 7 and 14 (coinciding with myelination of the corpus callosum). We further hypothesized that this “equalization” of speeds/skills would occur earlier in girls than in boys.
METHODS
Participants
A total of 130 typically developing, right-handed children (65 girls, 65 boys) ages 7−14 participated in this study. Participants were recruited from local area schools, social/ service organizations (e.g., Boy/Girl Scouts), and from advertisements in the community (e.g., postings at libraries). Children with a history of seizures, traumatic brain injury, mental retardation, pervasive developmental disorder, visual or hearing impairment, or other neurological illness were excluded from participation. Participants involved in this study were recruited between 1990 and 2005, as such, intellectual level was assessed using the Wechsler Intelligence Scale for Children, Revised (WISC-R; Wechsler, 1974), Third Edition (WISC-III; Wechsler, 1991), or Fourth Edition (WISC-IV; Wechsler, 2003). Only children with Full Scale IQ of 80 or greater were included in the study.
Inclusion criteria for the study were established through administration of the Diagnostic Interview for Children and Adolescents-Revised, Parent Form (DICA-R; Welner, Reich, Herjanic, Jung, & Amado, 1987), or the Diagnostic Interview for Children and Adolescents-IV (DICA-IV; Reich, Welner, & Herjanic, 1997). Additionally, word reading skills were assessed using the Basic Reading subtest from the Wechsler Individual Achievement Test (WIAT; Wechsler, 1992), or the Word Reading subtest from the WIAT-II (Wechsler, 2002), in order to rule out a learning disorder in basic reading skills. Children were excluded from participation if they had a statistically significant discrepancy between their Basic/Word Reading and WISC Full Scale IQ scores or Basic/Word Reading subtest scores below 85, regardless of IQ score. Children with psychiatric diagnoses (based on DICA interview with parent) were excluded, as were children who were currently taking or had previously taken any psychotropic medication.
Procedures
Parents of each participant had an initial phone screen to determine the child's eligibility based on inclusion criteria. Participants completed PANESS and IQ testing as part of a larger research study. Parents completed structured diagnostic interviews before or while the child was tested.
Measures
Wechsler Intelligence Scales for Children
Measures of Full Scale IQ were obtained for all participants using the Wechsler Intelligence Scale for Children, Revised (WISC-R; Wechsler, 1974) for children tested prior to 1996, or the Wechsler Intelligence Scale for Children, Third Edition (WISC-III; Wechsler, 1991), for children tested from 1996 to 2002 or the Wechsler Intelligence Scale for Children, Fourth Edition (WISC-IV; Wechsler, 2003) for children tested from 2003 to present.
Diagnostic Interview for Children and Adolescents (DICA)
This is a semistructured interview that is designed for determining selected current and retrospective psychiatric diagnoses. The parent version was administered to parents about their child. The DICA-R (Welner et al., 1987) was administered to parents before 1998 and the DICA-IV (Reich et al., 1997) was administered from 1998 on. For each, the modules administered included those assessing present and retrospective reports of: Attention deficit/hyperactivity disorder (ADHD>, Conduct Disorder, Oppositional Defiant Disorder, Major Depressive Disorder, Bipolar Disorders, Dysthymic Disorder, Separation Anxiety Disorder, Panic Disorder, Generalized Anxiety Disorder, Specific Phobia, Obsessive-Compulsive Disorder, and Adjustment Disorders.
Physical and Neurological Assessment of Subtle Signs (PANESS)
The revised PANESS (Denckla, 1985) includes a lateral preference, untimed and timed motor tasks. Untimed motor tasks include gaits on heels, toes, and sides of feet; tandem gait forward/backward; standing/hopping on one foot; standing heel-to-toe with eyes closed; standing both feet together, arms outstretched with eyes closed. The six timed motor tasks included toe tapping, alternating heel-toe tapping, repetitive hand patting, hand pronation-supination, repetitive finger tapping, and finger sequencing—each of which was performed on the right and left sides. The PANESS has been found to have adequate test-retest reliability (Holden, Tarnowski, & Prinz, 1982), interrater reliability, and internal consistency (Vitiello, Ricciuti, Stoff, Behar, & Denckla, 1989). Although the entire PANESS was administered to each participant, only the timed motor examination component was analyzed for the present study. Timed movements were measured with a stopwatch and scored according to how long it took the child to complete 20 actions. For toe tapping, hand patting, and finger tapping, “time to do 20” was 20 repetitive taps. For toe-heel alternation sets and hand pronate-supinate sets, “time to do 20” was 10 pairs of alternations. For finger sequencing, “time to do 20” was based on 5 sequences of 4 touches (i.e., each digit taps the thumb one time).
During the timed motor examination, the examiner demonstrates the task while giving the directions. The child is allowed to practice as the examiner instructs the child. For each task the child is initially seated facing the examiner with hands placed on lap with palms down. Once the action is demonstrated, the examiner then directs the child to complete the task as fast as possible. Timing begins when the child has reached a steady pace and continues until 20 movements have been made. Time to complete 20 movements for each of the 12 tasks was recorded. Observations of overflow and dysrhythmia during timed tasks were also recorded, but they were not analyzed for the present study.
Hand preference was determined based on the child's performance on a set of 11 pantomimed tasks. A participant was considered right- (left-) handed if he/she used right (left) hand to perform 9 or more of the 11 pantomimed tasks. If the child used the nondominant hand to perform 3 or more of the 11 tasks, he/she was considered “mixed” handed. Only children who were scored as right-handed (i.e., performing at least 9 of 11 tasks with the right hand) were included in the present study.
Analyses
Performance for each of the 12 PANESS timed movements (six pairs, right and left) was calculated separately for boys and girls. To analyze left-right differences on the timed movements, a series of six multiple regression analyses were used to examine the effects of age, gender, and age-by-gender interaction, following the methods described by Holmbeck (2002). The left-right difference scores (absolute value of left-side minus right-side time) per individual for toe tapping, heel-toe sequences, hand patting, hand pronation-supination, finger repetition, and finger sequencing were used as dependent measures. The continuous predictor variable (age in years) was centered by subtracting the mean age from each participant's age. Centering reduces the multicollinearity between predictors and interaction terms without altering the significant of the interaction or values of simple slopes (Holmbeck, 2002). Using hierarchical entry, age was entered first, followed by gender, and then the age-by-gender interaction. When indicated, post hoc examination of the moderating effects of group on the interaction between age and gender were examined. In order to control for multiple comparisons, significance level was set at a more conservative p = .01.
RESULTS
Demographic Information
Demographic information for the sample is provided in Table 1. The sample was drawn from a largely middle-class socioeconomic status (SES) and was predominantly Caucasian (85% Caucasian, 9.2% African American, 2.3% Asian, 0.8% Hispanic, 0.8% Native American, and 1.6% other). Children ranged in age from 7 to 14 years (M = 10.1, SD = 2.0). There were equal numbers of boys and girls in the sample. There were no significant differences in racial composition of the boys versus the girls (χ2 = 4.7, p = .45), nor any significant differences in proportion of boys versus girls tested with each IQ test version (χ2 = 2.3, p = .32). There were also no significant differences in gender ratios at each age.
Table 1.
Demographic and Screening Information.
| Boys (n = 65) |
Girls (n = 65) |
|||||
|---|---|---|---|---|---|---|
| M | SD | Range | M | SD | Range | |
| Age | 10.0 | 2.0 | 7−14 | 10.1 | 2.0 | 7−14 |
| SES | 51.8 | 8.8 | 17−66 | 50.6 | 9.4 | 18−66 |
| FSIQ | 115.0 | 11.4 | 87−134 | 112.5 | 12.7 | 87−139 |
Note. SES = Hollingshead Index; FSIQ = Full Scale IQ. All comparisons between boys and girls are nonsignificant.
Time Differences on PANESS
Means and standard deviations for boys, girls, and totals at each age level for time difference scores (i.e., absolute value of right compared with left-side time) on the six PANESS timed motor tasks are presented in Table 2, and results of regression analyses are presented in Table 3. There were significant main effects for age on toe tapping (ΔR2 = .088, p = .001), toe-heel alternation sets (ΔR2 = .077, p = .001), hand pronation-supination (ΔR2 = .052, p = .009), finger repetition (ΔR2 = .076, p = .002), and finger sequencing (ΔR2 = .177, p < .001); in all cases, the magnitude of the left-right difference decreased with age (Figure 1). There was also a significant main effect for gender on toe-heel alternation sets (ΔR2 = .048, p = .009) and a trend on hand pronation-supination sets (ΔR2 = .032, p = .037); in both cases boys had greater overall left-right differences than girls. There was also a significant interaction effect between age and gender for hand pronation-supination sets (ΔR2 = .047, p = .01). Post hoc analysis of the simple slopes of regression lines was calculated separately for boys and girls. For girls, the slope for age-related change in hand pronation-supination left-right difference score was not significant (b = −.005, t = −.115, p = .91); however, for boys, the slope was highly significant (b = −.169, t = −3.736, p < .001), indicating that for girls the magnitude of the left-right difference score changed little from age 7 to 14, whereas for boys, the magnitude of the difference decreased over that time period. This interaction suggests that girls’ left-sided speed “equalizes” with their right-sided speed earlier in life than boys for the hand pronation-supination task.
Table 2.
PANESS Left-Right Difference Scores by Age and Gender.
| 7 (n = 12) | 8 (n = 27) | 9 (n = 19) | 10 (n=18) | 11 (n=21) | 12 (n=16) | 13 (n=10) | 14 (n=7) | Total (n=130) | |
|---|---|---|---|---|---|---|---|---|---|
| TT-B | 1.6 (1.9) | 1.4 (1.0) | .79 (.75) | .67 (.47) | .59 (.56) | .51 (.53) | .58 (.32) | .82 (1.0) | .90 (.96) |
| TT-G | 1.4 (1.4) | 1.2 (.99) | .59 (.31) | .41 (.19) | .76 (.78) | .79 (.41) | .48 (.34) | .72 (.55) | .83 (.77) |
| TT-Tot | 1.5 (1.7) | 1.3 (.99) | .68 (.56) | .58 (.41) | .68 (.67) | .67 (.47) | .53 (.32) | .75 (.62) | .87 (.86) |
| HT-B | 2.7 (2.8) | 2.3 (2.6) | 1.6 (1.1) | 1.1 (1.0) | 1.1 (.75) | .81 (.35) | 1.3 (.73) | .91 (.36) | 1.5 (1.7) |
| HT-G | 1.4 (.79) | 1.3 (1.9) | .65 (.47) | .53 (.51) | .84 (.58) | .54 (.41) | .71 (.62) | .65 (.43) | .87 (1.0) |
| HT-Tot | 2.1 (2.2) | 1.7 (2.2) | 1.1 (.95) | .93 (.92) | .92 (.66) | .66 (.40) | 1.1 (.74) | .76 (.39) | 1.2 (1.4) |
| HP-B | 1.1 (.78) | .56 (.43) | .33 (.35) | .81 (.59) | .64 (.40) | .47 (.33) | .29 (.31) | .11 (.18) | .60 (.52) |
| HP-G | .62 (.50) | .57 (.39) | .47 (.27) | .46 (.50) | .49 (.61) | .44 (.40) | .85 (1.4) | .41 (.15) | .52 (.55) |
| HP-Tot | .91 (.70) | .57 (.40) | .40 (.31) | .69 (.57) | .56 (.51) | .46 (.36) | .57 (1.0) | .28 (.22) | .56 (.53) |
| HPS-B | 1.8 (1.1) | 1.2 (1.1) | .84 (.87) | .99 (.76) | .73 (.59) | .65 (.45) | .38 (.39) | .49 (.11) | .95 (.86) |
| HPS-G | .53 (.30) | .62 (.51) | .64 (.48) | .71 (.38) | .86 (1.0) | .83 (.77) | .39 (.24) | .36 (.22) | .66 (.61) |
| HPS-T | 1.3 (1.0) | .87 (.87) | .74 (.68) | .90 (.66) | .81 (.82) | .75 (.64) | .39 (.31) | .41 (.18) | .80 (.76) |
| FR-B | 8.1 (.53) | .98 (.55) | .61 (.33) | .69 (.44) | 1.1 (.73) | .58 (.43) | .32 (.17) | .17 (.04) | .75 (.54) |
| FR-G | 1.2 (.89) | .88 (.54) | .52 (.41) | .22 (.38) | .61 (.51) | .48 (.24) | .49 (.29) | .73 (.47) | .65 (.51) |
| FR-Tot | .98 (.70) | .93 (.54) | .56 (.36) | .53 (.43) | .85 (.66) | .53 (.33) | .41 (.24) | .49 (.45) | .70 (.53) |
| FS-B | 3.7 (2.8) | 1.6 (1.2) | 1.1 (.75) | 1.2 (1.0) | 2.1 (2.4) | .90 (.61) | .30 (.09) | .80 (.14) | 1.5 (1.7) |
| FS-G | 2.8 (1.8) | 1.8 (1.4) | 1.8 (1.4) | 1.1 (.63) | .96 (.61) | .36 (.34) | .65 (.40) | .31 (.17) | 1.3 (1.2) |
| FS-Tot | 3.3 (2.4) | 1.7 (1.3) | 1.4 (1.2) | 1.1 (.88) | 1.5 (1.8) | .60 (.54) | .47 (.33) | .52 (.30) | 1.4 (1.5) |
Note. TT = Toe-tapping; HT = Heel-toe sequences; HP = Hand patting; HPS = Hand pronation-supination sequences; FR = Finger repetition; FS = Finger sequencing; B = Time differences for boys; G = Time differences for girls; Times are absolute value of mean raw score differences between right and left side in seconds, standard deviations in ().
Table 3.
Effects of Age and Gender on Left-Right Time Differences.
| Movement | Predictor Entered | β | ΔR2 | ΔF | P |
|---|---|---|---|---|---|
| Toe Tapping | Age | −.19 | .088 | 12.20 | .001 |
| Gender | .02 | .001 | 0.09 | .757 | |
| Age × Gender | −.15 | .012 | 1.64 | .203 | |
| Toe-Heel Tap | Age | −.16 | .077 | 10.73 | .001 |
| Gender | .22 | .048 | 7.03 | .009 | |
| Age × Gender | −.16 | .014 | 2.00 | .160 | |
| Hand Patting | Age | −.02 | .024 | 3.18 | .077 |
| Gender | .06 | .003 | 0.43 | .514 | |
| Age × Gender | −.19 | .019 | 2.57 | .112 | |
| Hand Pronation-Supination | Age | −.01 | .052 | 7.07 | .009 |
| Gender | .18 | .032 | 4.45 | .037 | |
| Age × Gender | −.30 | .047 | 6.84 | .010 | |
| Finger Repetition | Age | −.27 | .076 | 10.48 | .002 |
| Gender | .08 | .007 | 0.96 | .328 | |
| Age × Gender | .00 | .000 | 0.00 | .991 | |
| Finger Sequencing | Age | −.43 | .177 | 27.45 | .000 |
| Gender | .07 | .005 | 0.74 | .393 | |
| Age × Gender | .02 | .000 | 0.05 | .829 |
Note. Analyses indicate hierarchical regression with age entered first, followed by gender and then the age-by-gender interaction. Times are absolute value of mean raw score differences between right and left side in seconds. Significant effects in bold.
Figure 1.
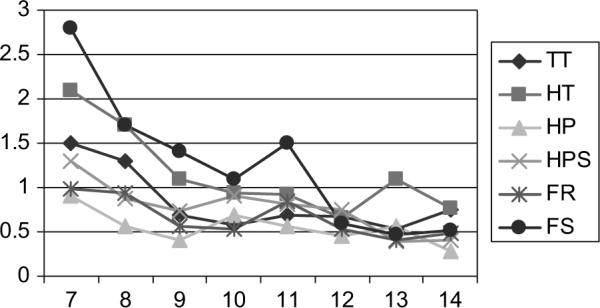
Mean left-right differences (seconds) by age (n = 130). Note: TT = Toe-tapping; HT = Heel-toe sequences; HP = Hand patting; HPS = Hand pronation-supination sequences; FR = Finger repetition; FS = Finger sequencing.
DISCUSSION
Results of the present study are consistent with previous research that has demonstrated consistent decreases in left-right differences among timed unimanual and unipedal tasks during the elementary and middle school years. By age 14, the magnitude of the left-right time difference for performance of 20 foot, hand, and finger movements is negligible. On some tasks, especially those requiring patterned/sequenced movements, this “equalization” of lateralized speed occurs earlier in girls than in boys.
The equalization of left- versus right-side speed on timed motor activities in this age range is considered to reflect the myelination and maturation of cortical brain systems supporting motor skill, as well as the rapid myelination of the corpus callosum. The maturation of brain systems supporting speed may also occur simultaneously with the development of related motor functions; in school-aged children the amount of mirror overflow (i.e., associated movements) decreases with the greatest change occurring between the ages of 6 and 8 (Lazarus & Todor, 1991; Mostofsky, Newschaffer, & Denckla, 2003). Equalization in unilateral speed appears to occur in parallel with decreased overflow, which is probably because of development of cortical brain systems that support both increased speed and collateral inhibition of overflow. Additionally, myelination of corpus callosum between the ages of 7 and 14 would allow for both a decrease in overflow due to the reciprocal influence of the contralateral cerebral cortex, and a decrease in right/left speed differences (Hannay, 2000; Klaas, Hannay, Caroselli, & Fletcher, 1999).
Motor and executive systems depend on the functional integrity and maturation of related brain regions, suggesting a parallel neural circuitry that includes not only the corpus callosum but also frontostriatal systems (Castellanos, Sonuga-Barke, Milham, & Tannock, 2006) and frontocerebellar pathways (Denckla, 2005; Denckla & Reiss, 1997; Diamond, 2000; Pennington & Ozonoff, 1996). Indeed, studies of clinical groups highlight the parallel developmental differences in these related systems. For example, compared to age-related controls, children with ADHD demonstrate smaller midsagittal anterior corpus callosum areas (Baumgardner et al., 1996), as well as reduced premotor, prefrontal (Mostofsky, Cooper, Kates, Denckla, & Kaufmann, 2002), and cerebellar volumes (Mostofsky, Reiss, Lockhart, & Denckla, 1998). At the same time, children with ADHD also show reduced motor speeds (Denckla, 1973; Mahone, Prahme, Koth, Morris, & Denckla, 2004; Schuerholz, Cutting, Mazzocco, Singer, & Denckla, 1997), increased motor overflow (Mostofsky, Newschaffer, & Denckla, 2003), and decreased motor inhibition (Mahone et al., 2006; Wodka et al., in press). Thus, the reduction in left-right speed differences between ages 7 and 14 may be a marker for maturation of neural pathways linking basic motor control with higher order “executive” control. Motor and executive systems display a similar protracted developmental trajectory, with periods of rapid growth in elementary years (Denckla, 1973; Diamond, 2000).
The relative decrease with age in lateral speed differences among typically developing children may reflect the normal progression of the availability of the brain systems that support both motor speed and motor inhibition (i.e., those that are deficient or delayed in children with ADHD). Simultaneous “equalization” of left-right speed and reduction in mirror overflow movements may be due to callosal maturation involving the normal interplay between transcallosal facilitation and transcallosal inhibition. In healthy individuals, activation in one hemisphere during unilateral timed motor tasks facilitates activation of the same neural area in the opposite hemisphere via connections of the corpus callosum (Baliz et al., 2004; Ikeda, Luders, Burgess, & Shibasaki, 1992). Motor overflow occurs via this transcallosal facilitation early in life, until it becomes inhibited by (callosally mediated) interhemispheric inhibitory mechanisms arising from the contralateral hemisphere (Aranyi & Rosler, 2002; Baliz et al., 2004; Sohn, Jung, & Kaelin-Lang, 2003). Transcallosal inhibition increases as the spread of cortical activation increases, which in turn allows for more efficient unilateral movement (Hoy, Fitzgerald, Bradshaw, Armatas, & Georgiou-Karistianis, 2004).
On two of the six motor tasks examined in the current study, girls showed smaller left-right time difference than boys; additionally, on the hand pronation supination task, they “equalized” significantly earlier than boys. These findings point to the earlier maturation of brain structures supporting motor coordination and speed in girls. Giedd and colleagues demonstrated that gray matter volume increases, peaks and begins to decline earlier in girls than in boys, in reciprocal with increase in white matter. After age 5, for example, girls have smaller total cerebral volume than boys (Giedd, Castellanos, Rajapakske, Vaisuzis, & Rapoport, 1997). Further, in girls, frontal gray matter volumes peak at age 11.0 for girls and 12.1 for boys (Giedd, Blumenthal, Jeffries, Castellanos, et al., 1999a). These gray matter reductions occur at the same time white matter myelination advances throughout the brain. Interestingly, while both girls and boys have rapid increases in cerebral white matter between the ages of 7 and 14, boys increase toward peak volume at a more rapid rate during these years (Giedd, Blumenthal, Jeffries, Castellanos, et al., 1999a). Corpus callosum myelination occurs in a rostro-caudal wave of growth, with this wave occurring earlier in girls than in boys (Thompson et al., 2000). Both these findings highlight the earlier development of white matter structures in girls, including those supporting motor speed and efficiency. Further, we observed gender differences for timed patterned movements (i.e., toe-heel taps), but not for timed repetitive movements, suggesting that the neural pathways and motor systems that underlie patterned movement may mature differently in girls than in boys, perhaps as a function of earlier left hemisphere maturation in girls (Chen, Gerloff, Hallett, & Cohen, 1997; Grafton, Hazeltine, & Ivry, 2002). In addition to structural differences observed in girls and boys, it is important to recognize that, during the age range studied, more girls than boys will likely have reached puberty (Rosenfield et al., 2000). During puberty, changes in androgen levels across the menstrual cycle in women appear to influence functional asymmetries of cerebral activation (Kimura & Hampson, 1994).
Our findings highlight the utility of quantified motor assessment in children as a means of examining age-related changes in functions related to cortical and corpus callosum maturation in the brain. The PANESS is particularly useful for assessment of motor speed in children because it is brief, minimizes the need for equipment, provides lateralized data, and is applicable to children as young as 5 years. Strengths of this study include careful screening of participants for psychopathology, reading disorders, and ADHD, as well as the standardized administration of timed motor battery for children, the relatively large sample size, SES- and gender-matching. There were also several limitations with the current study. First, although we inferred from our data that our findings coincided with maturation of certain cortical structures and the corpus callosum, we did not directly link our motor findings to imaging studies in these children. Additionally, the relationships among age-related increases in speed, declines in overflow movements, and reductions in left-right differences in, speed were not directly explored. Further, the association between “degree of hand preference” and motor asymmetries was not directly examined in the present study.
In addition to more direct links with imaging and correlations with overflow movements, future research involving lateral motor function should investigate patterns of left-right differences in clinical populations, especially those known to have motor dys-function (e.g., ADHD, traumatic brain injury, congenital hydrocephalus). We expect that the pattern of left-right equalization would occur later in life (or perhaps fail to occur) in these clinical groups. Secondly, the association between mirror overflow movements and patterns of motor speeds should be examined more carefully in both control and clinical populations, especially examining the effects of stimulant medications on these motor characteristics in clinical groups (e.g., ADHD). Finally, the longitudinal examination of motor speed/efficiency in conjunction with analysis of imaging findings (anatomic MRI diffusion tensor imaging) will provide the greatest insight into both typical and atypical development of neuromotor function in children.
Acknowledgments
A portion of this paper was presented at the 34th annual meeting of the International Neuropsychological Society in Boston, Massachusetts, on February 3, 2006. This work was supported by NS-25806 (Neurodevelop-mental Pathways to Learning Disabilities); HD-24061 (Mental Retardation and Developmental Disabilities Research Center); U.S. Congressionally Directed Materiel and Medical Command (DAMD17-00-1-0548); K08 NS02039, K01 MH 01824, MH 52432R29, P01 HD35468, K02 NS04485, R01 NS047781, R01 NS047781 and the Rita Rudel Foundation. The authors also wish to thank Rebecca Landa, Ph.D., for her assistance in obtaining some of the data.
REFERENCES
- Aboitiz F, Montiel J. One hundred million years of interhemispheric communication: The history of the corpus callosum. Brazilian Journal of Medical and Biological Research. 2003;36:409–420. doi: 10.1590/s0100-879x2003000400002. [DOI] [PubMed] [Google Scholar]
- Aranyi Z, Rosler KM. Effort-induced mirror movements: A study of transcallosal inhibition in humans. Experimental Brain Research. 2002;145:76–82. doi: 10.1007/s00221-002-1101-1. [DOI] [PubMed] [Google Scholar]
- Baliz Y, Armatas C, Farrow M, Hoy KE, Fitzgerald PB, Bradshaw JL, Georgiou-Karistianis N. The influence of attention and age on the occurrence of mirror movements. Journal of the International Neuropsychological Society. 2004;11:855–862. doi: 10.1017/s1355617705051003. [DOI] [PubMed] [Google Scholar]
- Barnea-Goraly N, Menon V, Eckert M, Tamm L, Bammer R, Karchemskiy A, Dant CC, Reiss AL. White matter development during childhood and adolescence: A cross-sectional diffusion tensor imaging study. Cerebral Cortex. 2005;15:1848–1854. doi: 10.1093/cercor/bhi062. [DOI] [PubMed] [Google Scholar]
- Baumgardner TL, Singer GS, Denckla MB, Rubin MA, Abrams MT, Colli MJ, Reiss AL. Corpus callosum morphology in children with Tourette syndrome and attention deficit hyperactivity disorder. Neurology. 1996;47:1–6. doi: 10.1212/wnl.47.2.477. [DOI] [PubMed] [Google Scholar]
- Best CT. Emergence of cerebral asymmetries in early human development: A literature review and a neuroembryological model. In: Molfese DL, Segalowitz SJ, editors. Brain lateralization in children: Developmental implications. Guilford Press; New York: 1988. pp. 5–34. [Google Scholar]
- Bryden PJ, Pryde KM, Roy EA. A developmental analysis of the relationship between hand preference and performance: II. A performance-based method of measuring hand preference in children. Brain and Cognition. 2000;43:60–64. [PubMed] [Google Scholar]
- Castellanos FX, Sonuga-Barke EJS, Milham MP, Tannock R. Characterizing cognition in ADHD: Beyond executive dysfunction. Trends in Cognitive Sciences. 2006;10(3):117–123. doi: 10.1016/j.tics.2006.01.011. [DOI] [PubMed] [Google Scholar]
- Chen R, Gerloff C, Hallett M, Cohen L. Involvement of the ipsilateral motor cortex in finger movements of different complexities. Annals of Neurology. 1997;41:247–254. doi: 10.1002/ana.410410216. [DOI] [PubMed] [Google Scholar]
- Cornwell KS, Harris LJ, Fitzgerald HE. Task effects in the development of hand preference in 9-, 13-, and 20-month-old infant girls. Developmental Neuropsychology. 1991;7:19–34. [Google Scholar]
- De Bellis MD, Keshavan MS, Beers SR, Hall J, Frustaci K, Masalehdan A, Noll J, Boring AM. Sex differences in brain maturation during childhood and adolescence. Cerebral Cortex. 2001;11:552–557. doi: 10.1093/cercor/11.6.552. [DOI] [PubMed] [Google Scholar]
- Denckla MB. Development of speed in repetitive and successive finger-movements in normal children. Developmental Medicine and Child Neurology. 1973;15:635–645. doi: 10.1111/j.1469-8749.1973.tb05174.x. [DOI] [PubMed] [Google Scholar]
- Denckla MB. Revised neurological examination for subtle signs. Psychopharmacology Bulletin. 1985;21:773–779. [PubMed] [Google Scholar]
- Denckla MB. Executive Function. In: Gozal D, Molfese D, editors. In attention deficit hyperactivity disorder: From genes to patients. Humana Press; Totowa, NJ: 2005. pp. 165–183. [Google Scholar]
- Denckla MB, Reiss AL. Prefrontal-subcortical circuits in developmental disorders. In: Krasnegor NA, Lyon GR, Goldman-Rakic PS, editors. Development of the prefrontal cortex: Evolution, neurobiology, and behavior. Brookes Publishing Co; Baltimore: 1997. pp. 283–293. [Google Scholar]
- Diamond A. Close Interrelation of motor development and cognitive development and of the cerebellum and prefrontal cortex. Child Development. 2000;71:44–56. doi: 10.1111/1467-8624.00117. [DOI] [PubMed] [Google Scholar]
- Finlayson MA, Reitan RM. Handedness in relation to measures of motor and tactile-perceptual functions in normal children. Perceptual and Motor Skills. 1976;43:475–481. doi: 10.2466/pms.1976.43.2.475. [DOI] [PubMed] [Google Scholar]
- Fix JD. Neuroanatomy. 3rd ed. Lippincott, Williams, & Williams; Baltimore, MD: 2002. [Google Scholar]
- Gardner RA, Broman M. The Purduc Pegboard: Normative data on 1334 school children. Journal of Clinical Child Psychology. 1979;8:156–162. [Google Scholar]
- Geffner DS, Hochberg I. Ear laterality performance of children from low and middle socioeconomic levels on a verbal dichotic listening task. Cortex. 1971;7(2):193–203. doi: 10.1016/s0010-9452(71)80016-3. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, Paus T, Evans AC, Rapoport JL. Brain development during childhood and adolescence: A longitudinal MRI study. Nature Neuroscience. 1999a;2:861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Rajapakse JC, Vaituzis AC, Liu H, Berry YC, Tobin M, Nelson J, Castellanos FX. Development of the human corpus callosum during childhood and adolescence: A longitudinal MRI study. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 1999b;23:571–588. doi: 10.1016/s0278-5846(99)00017-2. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Castellanos FX, Rajapakske JC, Vaisuzis AC, Rapoport JL. Sexual dimorphism of the developing human brain. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 1997;21:1185–1201. doi: 10.1016/s0278-5846(97)00158-9. [DOI] [PubMed] [Google Scholar]
- Grafton S, Hazeltine E, Ivry RB. Motor sequence learning with the nondominent left hand: A PET functional imaging study. Experimental Brain Research. 2002;146:369–378. doi: 10.1007/s00221-002-1181-y. [DOI] [PubMed] [Google Scholar]
- Hannay HJ. Functioning of the corpus callosum in children with early hydrocephalus. Journal of the International Neuropsychological Society. 2000;6:351–361. doi: 10.1017/s1355617700633106. [DOI] [PubMed] [Google Scholar]
- Holden E, Tarnowski K, Prinz R. Reliability of neurological soft signs in children: Reevaluation of the PANESS. Journal of Abnormal Child Psychology. 1982;10:163–172. doi: 10.1007/BF00915938. [DOI] [PubMed] [Google Scholar]
- Holmbeck GN. Post-hoc probing of significant moderational and mediational effects in studies on pediatric populations. Journal of Pediatric Psychology. 2002;27:87–96. doi: 10.1093/jpepsy/27.1.87. [DOI] [PubMed] [Google Scholar]
- Hoy K, Fitzgerald P, Bradshaw J, Armatas C, Georgiou-Karistianis N. Investigating the cortical origins of motor overflow. Brain Research Reviews. 2004;46:315–327. doi: 10.1016/j.brainresrev.2004.07.013. [DOI] [PubMed] [Google Scholar]
- Ikeda A, Luders HO, Burgess RC, Shibasaki H. Movement related potentials recorded from supplementary motor area and primary motor area. Brain. 1992;115:1017–1043. doi: 10.1093/brain/115.4.1017. [DOI] [PubMed] [Google Scholar]
- Innocenti GM. Some new trends in the study of the corpus callosum. Behavioural Brain Research. 1994;64:1–8. doi: 10.1016/0166-4328(94)90113-9. [DOI] [PubMed] [Google Scholar]
- Kimura D, Hampson E. Cognitive pattern in men and women is influenced by fluctuations in sex hormones. Current Directions in Psychological Science. 1994;3:57–61. [Google Scholar]
- Klaas PA, Hannay HJ, Caroselli JS, Fletcher JM. Interhemispheric transfer of visual, auditory, tactile, and visuomotor information in children with hydrocephalus and partial agenesis of the corpus callosum. Journal of Clinical and Experimental Neuropsychology. 1999;21:837–850. doi: 10.1076/jcen.21.6.837.851. [DOI] [PubMed] [Google Scholar]
- Knights RM, Moule AD. Normative data on the motor steadiness battery for children. Perceptual and Motor Skill. 1968;26:643–650. doi: 10.2466/pms.1968.26.2.643. [DOI] [PubMed] [Google Scholar]
- Lafayette Instrument Company . Manual for the Grooved Pegboard. Author; Lafayette, IN: 1989. [Google Scholar]
- Lazarus JC, Todor JI. The role of attention in the regulation of associated movement in children. Developmental Medicine and Child Neurology. 1991;33:32–39. doi: 10.1111/j.1469-8749.1991.tb14783.x. [DOI] [PubMed] [Google Scholar]
- Mahone EM, Powell S, Loftis CW, Goldberg M, Denckla MB, Mostofsky SH. Motor persistence and inhibition in autism and ADHD. Journal of the International Neuropsychological Society. 2006;12:622–631. doi: 10.1017/S1355617706060814. [DOI] [PubMed] [Google Scholar]
- Mahone EM, Prahme C, Koth C, Morris M, Denckla MB. Effects of age and verbal IQ on timed motor performance in children [Abstract]. Journal of the International Neuropsychological Society. 2004;10(S1):181. [Google Scholar]
- Mostofsky SH, Cooper KL, Kates WR, Denckla MB, Kaufmann WE. Smaller prefrontal and premotor volumes in boys with attention-deficit hyperactivity disorder. Biological Psychiatry. 2002;52:785–794. doi: 10.1016/s0006-3223(02)01412-9. [DOI] [PubMed] [Google Scholar]
- Mostofsky SH, Newschaffer CJ, Denckla MB. Overflow movements predict impaired response inhibition in children with ADHD. Perceptual and Motor Skills. 2003;97:1315–1331. doi: 10.2466/pms.2003.97.3f.1315. [DOI] [PubMed] [Google Scholar]
- Mostofsky SH, Reiss AL, Lockhart P, Denckla MB. Evaluation of cerebellar size in attention-deficit hyperactivity disorder. Journal of Child Neurology. 1998;13:434–439. doi: 10.1177/088307389801300904. [DOI] [PubMed] [Google Scholar]
- Oeztuerk C, Durmazlar N, Ural B, Karaagaoglu E, Yalaz K, Anlar B. Hand and eye preference in normal preschool children. Clinical Pediatrics. 1999;38:677–680. doi: 10.1177/000992289903801109. [DOI] [PubMed] [Google Scholar]
- Pennington BF, Ozonoff S. Executive functions and developmental psychopathology. Journal of Child Psychology and Psychiatry. 1996;37:51–87. doi: 10.1111/j.1469-7610.1996.tb01380.x. [DOI] [PubMed] [Google Scholar]
- Pryde KM, Bryden PJ, Roy EA. A developmental analysis of the relationship between hand preference and performance: I. Preferential reaching into hemispace. Brain and Cognition. 2000;43:370–374. [PubMed] [Google Scholar]
- Reich W, Welner Z, Herjanic B. The Diagnostic Interview for Children and Adolescents-IV. Multi-Health Systems; North Tonawanda: 1997. [Google Scholar]
- Reitan R. Manual for Administration of Neuropsychological Test Batteries for Adults and Children. Reitan Neuropsychology Laboratories; Indianapolis: 1969. [Google Scholar]
- Roessner V, Banaschewski T, Uebel H, Becker A, Rothenberger A. Neuronal network models of ADHD – Lateralization with respect to interhemispheric connectivity reconsidered. European Child & Adolescent Psychiatry. 2004;13:71–79. doi: 10.1007/s00787-004-1007-5. [DOI] [PubMed] [Google Scholar]
- Rosenfield RL, Bachrach LK, Chernausek SD, Gertner JM, Gottschalk M, Hardin DS, Pescovitz OH, Saenger P. Current age at onset of puberty. Pediatrics. 2000;106:622–623. doi: 10.1542/peds.106.3.622. [DOI] [PubMed] [Google Scholar]
- Schuerholz LJ, Cutting L, Mazzocco MM, Singer HS, Denckla MB. Motor abnormalities in children with Tourette syndrome with and without attention deficit hyperactivity disorder. Journal of Child Neurology. 1997;12:438–442. doi: 10.1177/088307389701200705. [DOI] [PubMed] [Google Scholar]
- Sohn YH, Jung HY, Kaelin-Lang A. Excitability of the ipsilateral motor cortex during phasic voluntary hand movements. Experimental Brain Research. 2003;148:176–185. doi: 10.1007/s00221-002-1292-5. [DOI] [PubMed] [Google Scholar]
- Thompson PM, Giedd JN, Woods RP, MacDonald D, Evans AC, Toga AW. Growth patterns in the developing brain detected by using continuum-mechanical tensor maps. Nature. 2000;404:190–193. doi: 10.1038/35004593. [DOI] [PubMed] [Google Scholar]
- Tiffin J. Purdue Pegboard: Examiner Manual. Science Research Associates; Chicago: 1968. [Google Scholar]
- Vitiello B, Ricciuti A, Stoff D, Behar D, Denckla MB. Reliability of subtle (soft) neurological signs in children. Journal of the American Academy of Child and Adolescent Psychiatry. 1989;28:749–753. doi: 10.1097/00004583-198909000-00017. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Intelligence Scale for Children-Revised. Psychological Corporation; New York: 1974. [Google Scholar]
- Wechsler D. Wechsler Intelligence Scale for Children-III. Psychological Corporation; San Antonio: 1991. [Google Scholar]
- Wechsler D. Wechsler Individual Achievement Test. The Psychological Corporation; San Antonio, Texas: 1992. [Google Scholar]
- Wechsler D. Wechsler Individual Achievement Test, Second Edition. The Psychological Corporation; San Antonio, TX: 2002. [Google Scholar]
- Wechsler DL. Wechsler Intelligence Scale for Children, Fourth Edition. The Psychological Corporation; San Antonio, TX: 2003. [Google Scholar]
- Welner Z, Reich W, Herjanic B, Jung KG, Amado H. Reliability, validity and parent-child agreement studies of the Diagnostic Interview for Children and Adolescents (DICA). Journal of American Academy of Child and Adolescent Psychiatry. 1987;26:649–653. doi: 10.1097/00004583-198709000-00007. [DOI] [PubMed] [Google Scholar]
- Wodka EL, Mahone EM, Blankner JG, Gidley Larson JC, Fotedar S, Denckla MB, Mostofsky SH. Evidence that response inhibition is a primary deficit in ADHD. Journal of Clinical and Experimental Neuropsychology. doi: 10.1080/13803390600678046. (in press) [DOI] [PubMed] [Google Scholar]


