Abstract
The central governor model has recently been proposed as a general model to explain the phenomenon of fatigue. It proposes that the subconscious brain regulates power output (pacing strategy) by modulating motor unit recruitment to preserve whole body homoeostasis and prevent catastrophic physiological failure such as rigor. In this model, the word fatigue is redefined from a term that describes an exercise decline in the ability to produce force and power to one of sensation or emotion. The underpinnings of the central governor model are the refutation of what is described variously as peripheral fatigue, limitations models, and the cardiovascular/anaerobic/catastrophe model. This argument centres on the inability of lactic acid models of fatigue to adequately explain fatigue. In this review, it is argued that a variety of peripheral factors other than lactic acid are known to compromise muscle force and power and that these effects may protect against “catastrophe”. Further, it is shown that a variety of studies indicate that fatigue induced decreases in performance cannot be adequately explained by the central governor model. Instead, it is suggested that the concept of task dependency, in which the mechanisms of fatigue vary depending on the specific exercise stressor, is a more comprehensive and defensible model of fatigue. This model includes aspects of both central and peripheral contributions to fatigue, and the relative importance of each probably varies with the type of exercise.
Keywords: fatigue, electromyography, exercise, metabolism
A variety of papers have recently been published regarding the “central governor model” (CGM) of fatigue.1,2,3,4,5 In brief, the CGM “…proposes that exercise performance is regulated by the central nervous system specifically to ensure that catastrophic physiological failure does not occur during normal exercise in humans”1 (p 511). This method of regulation is based on the suggestion that, during exercise, the subconscious brain modulates the number of active motor units based on a “…pacing strategy that will allow completion of the task in the most efficient way while maintaining internal homoeostasis and a metabolic and physiological reserve capacity”3 (p 801). This series of papers has provided a number of thought provoking hypotheses and prompted renewed interest in some of the classic work concerning the role of the central nervous system (CNS) in fatigue. It is possible, however, that the current version of the CGM does not adequately explain the development of fatigue in some exercise situations, most notably in tasks that require very high forces and/or power outputs for relatively brief periods of time. The purpose of this paper is to review the scientific foundations used to formulate many aspects of the CGM. This is not a comprehensive review of fatigue. Rather, we have examined selected data from the fatigue literature to determine if the results of previous studies were consistent with what would be predicted by the CGM. Based on this review, we argue that, although some aspects of the CGM probably have validity, its current version fails to explain fatigue under many exercise conditions and is, in many ways, incomplete. Instead, it is likely that the concept of task dependency, in which fatigue is not caused uniquely by any common set of factors, but rather depends on the type of exercise that is being performed,6 provides a more valid, general model of fatigue.
Fatigue and the CGM
Traditionally, fatigue has been conceptualised as being driven by changes occurring anywhere in the chain between the brain and the muscle fibre. Effects occurring within the motor unit are considered the domain of peripheral fatigue, whereas events in the brain and spinal cord are considered to reflect central fatigue.7 The issue of central versus peripheral fatigue is not new. In the late 1880s, Waller (cited in Edwards et al,8 p 483) wrote that:
“The question is: does normal voluntary fatigue depend upon central expenditure of energy, or upon peripheral expenditure of energy, or upon both factors conjointly; and if upon both, in what proportion each? The form of this question is justified as follows; a maximum voluntary effort may decline: 1) by decline of cerebral motility; 2) by sub‐central or spinal block; Central, 3) by motor end‐plate block; 4) by decline of muscle energy; Peripheral.”
The CGM can be viewed as a special model of central fatigue. Specifically, Noakes and St Clair Gibson3 (p 801) proposed that:
“the subconscious brain sets the exercise intensity by determining the number of motor units that are activated and hence the mass of skeletal muscle that is recruited throughout the exercise bout. The extent of motor neurone activity and hence skeletal motor unit recruitment can be further influenced by sensory feedback from a variety of peripheral organs… the subconscious brain informs the conscious brain of an increasing neural effort, perhaps related to an increased difficulty in maintaining homeostasis at that exercise intensity, and this is interpreted by the brain as the increased sensation of fatigue, which may itself control further subconscious brain control processes.”
Further, the subconscious brain serves “…to regulate the power output during all forms of exercise as part of the controls necessary to maintain whole body homoeostasis”3 (p 801). From a historical perspective, many aspects of the CGM have previously been described. For example, in 1881 August Waller wrote that “central fatigue is protective from peripheral fatigue, and although normally in the body the dynamic effect of the physiological stimulus emitted by a ‘motor' centre far exceeds the maximal effect which can be elicited by direct experimental excitations, yet that physiological stimulus, by virtue of the rapid exhaustibility of the organ from which it proceeds, cannot normally ‘overdrive' and exhaust subordinate elements of the motor apparatus” (cited in Edwards et al,8 p 484).
The CGM is unique in at least two respects: (a) it limits the central regulation of performance to factors above the spinal cord (the subconscious and conscious brain), thus dismissing any role for important regulation at the level of the spinal cord or motor unit; (b) it seeks to explain fatigue under all exercise conditions. With respect to the first point, a variety of studies9,10,11,12 have reported that reflex activity in the spinal cord is altered after different types of fatiguing tasks. Furthermore, the CGM also does not acknowledge that peripheral fatigue—that is, a reduction in force/power production capability because of factors within the motor unit—can be a primary and direct influence on performance. To this end, the CGM is, in part, supported by discrepancies in what the advocates of the CGM refer to as the cardiovascular/anaerobic/catastrophe (CAC) model of fatigue, which centres on the effects of “anaerobiosis” and “lactic acidosis”2 (p 3). Fatigue at the level of the skeletal muscle, however, can be due to factors other than lactic acidosis and anaerobiosis. For example, the role of inorganic phosphate (Pi) accumulation and its effects on excitation‐contraction coupling is receiving increased attention in the muscle physiology literature.13 In addition, to be valid as proposed,1 the CGM must explain fatigue under all of the exercise conditions that have previously been attributed to task dependency as a general model of fatigue.6,14,15,16,17,18,19,20,21,22,23,24,25,26
Semantics
There are several terms that need to be clarified in order to adequately discuss the validity of the CGM. Most importantly, a centrepiece of the CGM is a redefinition of the term fatigue: “…fatigue should no longer be considered a physical event but rather a sensation or emotion, separate from an overt physical manifestation—for example, the reduction in force output by the active muscles”5 (p 121). In essence, what Noakes et al5 define as fatigue is more akin to ratings of perceived exertion which are traditionally measured with an instrument such as the Borg scale.27,28 Further, if fatigue is a sensation or emotion, rather than a physical phenomenon, then one ought to measure sensations and emotions. Yet the measures used by the advocates of the CGM are traditional measures of exercise performance—for example, oxygen uptake (V̇o2), electromyographic (EMG) amplitude, performance time, power output, etc. So, by definition, the studies generated by the advocates of the CGM are investigations not of fatigue, but of the measures that are often used to evaluate exercise performance. We further argue that, even if the other major tenets of the CGM were substantively accurate, the sensation/emotion aspect of fatiguing exercise is but one component of the regulation of muscle performance.
Although sensations/emotions are part of the milieu that affects how the CNS regulates muscle performance, fatigue has long been defined in terms of a reduction in force and/or power production capabilities.7,29 These are the physical manifestations that the advocates of the CGM5 argue do not directly limit exercise performance. For clarity, we suggest that the definition put forth by Bigland‐Ritchie and Woods,30 and adapted by Gandevia,31 be used: “any exercise‐induced reduction in the ability to exert muscle force or power, regardless of whether or not the task can be sustained” (p 1732). Further, we argue that the CGM addresses only a specific construct of fatigue. For example, factors limiting performance in a cycling time trial or a 10 km running race may include many factors besides fatigue—for example, motivation, environment, tiredness, lethargy, etc. In addition, the sensation/emotion definition of fatigue also fails to address fatigue during locomotion and cycling exercise induced by functional electrical stimulation in spinal cord injury.32,33 Indeed, one of the troubling aspects of functional electrical stimulation in spinal cord injury is the rapid decline in muscle force and power—that is, fatigue—that occurs during such exercise.33 This occurs despite a complete lack of CNS control of muscle activation and only modest changes in indices such as V̇o2 and heart rate.34 Under the definition of fatigue proposed by Noakes et al,5 however, this phenomenon is not fatigue.
In addition, Noakes et al1 implied that the term “fatigue” can be used to describe the onset of task failure or exhaustion. For example, the phrase “…there must be evidence of homoeostatic failure at fatigue”1 (p 512) suggests that fatigue occurs at a discrete point in time. Both the traditional force/power decline and sensation/emotion models of fatigue implicitly conceptualise fatigue as a process that occurs throughout the exercise task. Gandevia31 noted that “the maximal force generating capacity of muscles starts to decline once exercise commences so that fatigue really begins almost at the onset of exercise and develops progressively before the muscles fail to perform the required task” (p 1732). Typically, it is necessary to define the specific criteria for the termination of an exercise task in the study of fatigue—for example, inability to complete another repetition, the end point of a maximal graded exercise test, etc. Furthermore, for tasks that do not have a discrete point of failure—for example, time trials, Wingate anaerobic test (WAT), etc—the process of fatigue can be quantified by performance measures such as power output or force production, consistent with the operational definition of fatigue that has been used in most scientific papers.
It is also important to note that endurance—for example, time to task failure—and fatigue are separate phenomena, so one is not simply the converse of the other. Two studies that examined the effect(s) of aging and sex, respectively, on fatigue are illustrative.35,36 Bilodeau et al35 reported that elbow flexor fatigue consequent on a sustained isometric contraction (35% of maximum) resulted in similar declines in maximal force in young and older subjects, but the time to task failure (time at the target force) was ∼2.5 fold longer in the older subjects. Similarly, Hunter and Enoka36 compared men and women during continuous isometric forearm flexion contractions at 20% of maximum until task failure (inability to maintain the target force). The declines in maximal force were comparable between men and women (men ∼39% of pre‐fatigue force, women ∼34% of pre‐fatigue force), whereas time to task failure was over twofold longer in women. This distinction between fatigue, traditionally defined in terms of reductions in force and power production capability, and endurance is critically important, as the CGM has been built on observations of tasks such as running and cycling. We suggest that these tasks (in which pacing, motivation, and environmental influences are present) are not necessarily limited or defined by fatigue per se. Instead, fatigue is but one influence on endurance performance.
Another term that is often used in discussions of the CGM is “anaerobiosis”, which can be defined as “Life sustained by an organism in the absence of oxygen”.37 For example, Noakes and St Clair Gibson2 (p 1) stated that “The classical theory, since defined as the cardiovascular/anaerobic/catastrophe model of exercise physiology, postulates that fatigue during high intensity exercise of short duration results from a skeletal muscle ‘anaerobiosis' that develops when the oxygen requirement of the active skeletal muscles exceeds the heart's capacity to further augment oxygen delivery to exercising muscles by increasing the cardiac output.” Similarly, in addressing the V̇o2 plateau phenomenon—that is, the purported first hallmark of the CAC model—it is stated that “if the development of oxygen deficiency in the active muscles is the exclusive factor limiting maximum exercise performance, and if the ‘plateau phenomenon' is the external marker of this skeletal muscle anaerobiosis, then the plateau phenomenon must occur in 100% of subjects at exhaustion during progressive exercise”2 (p 6). If anaerobiosis is argued by exercise physiologists to be a proximate cause of fatigue, then we join Noakes and St Clair Gibson2 in criticising these arguments, as, under all exercise conditions, at least some ATP is derived from oxidative phosphorylation and V̇o2 is never zero. Also, we agree with Noakes and St Clair Gibson2 that the terminology advocated by Connett et al38 should be encouraged, namely, that cellular hypoxia refers to a decrease in O2 tension below resting values but “sufficient to maintain O2 and ATP flux because of adaptive change in the redox and phosphorylation drives on electron transport”38 (p 836), whereas dysoxia reflects conditions of O2 tension that restrict oxidative phosphorylation. However, we are unaware of previous studies that suggest that voluntary exercise occurs in the complete absence of oxygen in the cellular environment. Secondly, by extension, we argue that anaerobiosis as a concept is not a “classical theory” with respect to muscle fatigue. Indeed, the role of O2 insufficiency, much less fully fledged anaerobiosis, has been a point of controversy for well over 20 years—for example, Brooks.39 Furthermore, an examination of the indexes of popular academic texts in biochemistry,40 human physiology,41,42 or exercise physiology43,44,45,46,47 will fail to reveal the term “anaerobiosis.” Thus we urge the abandonment of this term.
In addition, one of the major components of the CGM is the role of homoeostasis in selecting the “optimum pacing strategy”3 (p 801). Specifically, the CGM proposes that the subconscious brain determines the metabolic cost that is required to perform the exercise task, given the environmental conditions and physical state of the organism. A pacing strategy is then selected that will allow the exercise to be completed without presenting a major threat to internal homoeostasis that would result in a “terminal metabolic crisis”3 (p 800). For example, Noakes et al5 (p 120) stated that “…the presence of homoeostasis in all organ systems at the point of exhaustion is perhaps the most robust evidence supporting the hypothesis that exercise performance is regulated centrally in the brain as part of a complex dynamic system, the principle function of which is specifically to ensure that homoeostasis is maintained under all conditions of exercise.” By definition, homoeostasis is, “a relative constancy in the internal environment of the body, naturally maintained by adaptive responses that promote healthy survival”48 (p 428). Thus this definition implies that, if homoeostasis is maintained during physical activity, then the internal environment of the body must remain relatively constant. There are, however, clear changes that occur both at the systemic and cellular level during fatiguing exercise. For example, there is an approximately 40‐fold increase in blood lactate during the transition from rest to maximal exercise.49 This is accompanied by potentially arrythmogenic changes of up to a twofold increase in plasma potassium, decreases in arterial pH of up to 0.4 unit, and up to 15‐fold increases in plasma catecholamines.50 In addition, there are dramatic increases in the concentrations of hydrogen ions (H+), ADP, and Pi in the muscle cell, all of which can contribute to immediate decreases in the amount of force that can be produced for a given level of muscle activation.20 None of these metabolic byproducts, however, permanently impair contractile function, because force production can be completely restored with adequate recovery time.51 Thus we argue that, during at least some forms of fatiguing exercise, homoeostasis as proposed by the CGM—that is, “a relative constancy in the internal environment”—is not maintained (because of rapid accumulation of lactate, H+, ADP, and Pi), yet in almost all cases, the resulting challenge cannot be considered a “terminal metabolic crisis”3 (p 800). Indeed, disruption of homoeostasis may be needed to stimulate adaptations to training.52
Task dependency
Perhaps the CGM as described by Noakes et al5 is most applicable to endurance exercise. A key consideration in the model centres on the extent of motor unit recruitment at task failure or task cessation for activities such as graded exercise tests to determine V̇o2max, cycling time trials, etc. At the end of these types of task, motor unit recruitment is usually submaximal. From this, it is argued that “…the brain does not recruit additional motor units during prolonged exercise because such additional recruitment would threaten the capacity to maintain homoeostasis…”5 (p 121). But what about other types of exercise? The second paper in the series2 addresses high intensity/short duration exercise lasting 10–90 seconds, but the discussion focuses to a large extent on the WAT, with a brief discussion of comparable treadmill exercise. In contrast, consider a 3 repetition maximum (RM) bench press task. By definition, a 3RM load allows the subject to complete three repetitions, but not four. What is causing task failure in this situation? If fatigue was due solely to limits on motor unit recruitment, as the CGM proposes, then the level of recruitment that allowed success during the third repetition should also allow completion of the fourth repetition. The more probable explanation is that fatigue at the cellular level diminished the force production capabilities of the motor units that the CNS allowed to be recruited, such that the force demands of the task exceeded the force production capability of the activated muscle.
The idea of task dependency is that “fatigue is not the consequence of a single omnipresent mechanism but rather that it can be induced by a variety of mechanisms”20 (p 1632). Further, in this model, the study of fatigue changes so that “Instead of attempting to identify a global cause of muscle fatigue, the impairment of performance resulting from an acute decline in the force capacity of muscle can be assessed by asking a more fundamental question ‘What causes task failure?'”12 (p 44). The task dependent factors that influence the fatigue response include exercise intensity, type of contraction (concentric v eccentric, dynamic v static, fast v slow), muscle group involved, environment (heat, humidity), and the physical characteristics of the exerciser (training status, muscle fibre type distribution, etc). For example, the mechanisms of fatigue are likely to be quite different when contrasting a marathon race with a 3RM weightlifting task. Indeed, the rates of change in force v muscle power are not equal.53 These differences are probably due to differential effects of metabolites on muscle performance, as Pi has been shown to reduce force output but not affect speed of shortening, whereas MgADP can potentiate force but inhibit shortening velocity.54
Peripheral fatigue and the CAC model
The advocates of the CGM create a false dichotomy in that fatigue is portrayed as either due to what they refer to as the CAC model (also known as the anaerobic/catastrophe/limitations model) or a function of the CGM. To this end, great pains are taken to note weaknesses in the ability of lactate production and H+ accumulation to explain muscle fatigue in a variety of exercise models.2 Although we agree that the CAC model, at least as presented for example by Noakes and St Clair Gibson,2 has serious limitations, we disagree that it is the “classical theory” of fatigue in exercise physiology. Indeed, Dill55 has noted that, as of 1935, workers in the Harvard Fatigue Laboratory had identified a variety of influences on fatigue besides lactic acid that varied depending on the nature of the task, an observation entirely consistent with the concept of task dependency as opposed to either the CAC or the CGM.
More recently, Westerblad et al13 observed that changes in lactate and pH probably explain little of the observations of muscle fatigue under physiological conditions. Further, much of the conventional understanding of the relation between lactate and pH is probably in error.56 However, lactic acid and H+ represent an incomplete list of potential fatigue inducing metabolites. For example, MacLaren et al57 noted over 15 years ago the potential roles for Pi and ammonia accumulation in muscle fatigue. Indeed, data indicating an important role for Pi accumulation in peripheral fatigue are rapidly growing.13,58,59 Specifically, Westerblad et al13 suggested that there are five potential mechanisms by which high concentrations of Pi can impair contractile function: (a) hindering cross bridge transition to the high force state; (b) reducing myofibrillar Ca2+ sensitivity; (c) increasing the open probability of the sarcoplasmic reticulum Ca2+ release channels; (d) inhibiting Ca2+ uptake by the sarcoplasmic reticulum; (e) precipitating with the Ca2+ in the sarcoplasmic reticulum, thereby decreasing the amount of Ca2+ available for release. Thus accumulation of Pi may influence a number of factors related to excitation‐contraction coupling.
Interestingly, the potential effect(s) of Pi accumulation appears to be related to the influence of hypoxia and hyperoxia on muscle performance. As noted by Hepple,60 mild hypoxia can decrease exercise performance even though O2 consumption is submaximal and Po2 is higher than the level necessary to inhibit mitochondrial O2 uptake. The influence of decreased O2 tension on muscle performance, even at a submaximal exercise intensity, and steady O2 consumption could be interpreted in the context of the CGM as evidence of CNS downregulation of motor unit recruitment. However, these effects of O2 tension directly influence muscle tissue itself. This effect appears to be due to the influence of O2 tension on accumulation of muscle metabolites, such as Pi. Reductions in inspired O2 can increase phosphocreatine hydrolysis, and thus Pi accumulation, at a constant submaximal power output (and therefore submaximal O2 consumption).61 Further, with incremental plantarflexion exercise at submaximal power outputs, hypoxia (induced by manipulation of fractional inspired O2 (Fio2)) produced greater declines in phosphocreatine and accumulation of Pi than during normoxia and hyperoxia, indicating that “hypoxia results in significant alterations (compared with the other Fio2 condition in muscle metabolic state before any potential O2 limitation)”62 (pp 1371–1372). At task failure, the cellular environment with respect to [Pi], [phosphocreatine], and [H+] was similar across the Fio2 conditions, but the power achieved at failure was lowest for hypoxia and greatest for hyperoxia.62 Hepple60 has suggested that “O2 may modulate the onset of fatigue by determining the rate of Pi accumulation as shown by the different slopes of the phosphocreatine v work relation with different inspired O2 concentrations…” (p 62).
Beyond Pi, other work63,64 has suggested that fatigue may even reduce the capacity of the myofibrils to produce force, regardless of the extent of muscle activation or metabolite accumulation. This phenomenon is often examined by adding caffeine to the external medium surrounding the muscle fibre, which potentiates the release of Ca2+ from the sarcoplasmic reticulum and induces contraction of the muscle fibre without membrane excitation. For example, Edman64 examined myofibrillar fatigue in single muscle fibres by stimulating the fibres until the tetanic force had been reduced by 25%. Caffeine (15 mM) was then added to the medium surrounding the muscle fibre to induce contraction. Edman reported that, even though the caffeine was sufficient to saturate the contractile system with Ca2+, the force that was produced during the contraction was nearly identical with that generated during the electrically stimulated tetanus. Thus it was suggested that “…the decrease in mechanical performance during moderate fatigue is not attributable to failure of activation of the contractile system,” and the “…fatigue process apparently makes the myofibrils less capable of producing force even though they are fully activated by calcium”64 (p 32). Edman also reported that the maximum speed of shortening is reduced in fatigued skeletal muscle. This finding is particularly important because, unlike force production, the maximum speed of shortening is independent of muscle activation.65 Furthermore, Danieli‐Betto et al63 provided evidence that myofibrillar fatigue may even occur in the absence of increases in muscle metabolite concentrations. Specifically, they found that, when the cytoplasm of fatigued rat muscle fibres was replaced with a normal medium, the effects of fatigue were still evident. They hypothesised that the impaired contractile function in the absence of high muscle metabolite concentrations may be due to modifications in muscle proteins. Regardless of the mechanism, the results from these studies63,64 strongly suggest that fatigue can affect myofibrillar function, irrespective of changes related to muscle activation (such as impaired excitation‐contraction coupling) or metabolite concentrations.
In addition to myofibrillar fatigue, previous studies57,66,67,68 have suggested that K+ may also play an important role in fatigue at the peripheral level. Specifically, K+ accumulates in the extracellular fluid during fatiguing exercise, depolarising the sarcolemma and decreasing the amplitude of the action potential.67 This reduction in action potential amplitude may result in less Ca2+ being released from the sarcoplasmic reticulum and/or a complete loss of excitability in some fibres, thereby decreasing the amount of force that is produced by the muscle.66,69 In addition, the K+ that is located in the extracellular space also diffuses into the bloodstream, and Zoladz et al68 suggested that the plasma K+ concentration may be closely related to physical working capacity. Specifically, they reported that the plasma K+ accumulation rate was greater during an incremental cycle ergometer test performed at a pedal rate of 120 rpm than when the test was performed at a pedal rate of 60 rpm. The power output at exhaustion was, however, significantly less for the 120 rpm test than for the 60 rpm test, even though the V̇o2max values were the same for both tests. The authors stated (p 585) that “…the early loss of potassium from the working muscle may indeed be directly involved in the early fatigue that occurs during cycling at 120 rev·min−1.” Thus these findings57,66,67,68 suggest that accumulation of K+ in the extracellular space may contribute to the decreases in performance that occur during sustained high intensity exercise.
Collectively, the current data on muscle fatigue suggest that a variety of cellular effects can alter muscle force and power production beyond the role of lactate and pH. Thus, although the advocates of the CGM are correct in noting the weaknesses of what they describe as the CAC model, it does not follow that peripheral effects have little to no direct influence on fatigue.
Limitations to surface EMG amplitude in the assessment of fatigue
In the development of the CGM, much has been made of the EMG amplitude responses during fatiguing exercise.2,3,5 For example, St Clair Gibson et al70 examined EMG amplitude during a simulated 100 km cycling time trial interspersed with periods of high intensity cycling. Their results showed reductions in EMG amplitude (normalised to the EMG amplitude value recorded during an isometric maximum voluntary contraction (MVC)) during cycling that tracked reductions in power output across the time trial. Similarly, Kay et al71 showed changes in EMG amplitude during periodic sprints that loosely tracked changes in cycling power over the course of a 60 minute test in a warm and humid environment. Of particular note is that the EMG amplitude values appeared to be less than what would be expected if motor unit recruitment were maximal. Noakes et al5 stated that “…peripheral factors alone cannot cause the termination of exercise when a majority of available motor units are inactive at the point of exhaustion” (p 121). However, we would like to caution the reader to not overinterpret the surface EMG amplitude data presented in these types of study. The amplitude of the surface EMG signal quantifies muscle activation, which reflects both motor unit recruitment (the number of motor units activated) and the firing rates of the activated motor units. Subsequently, there are several concerns with bipolar surface EMG signals that may warrant caution when interpreting these findings to explain fatigue: (a) surface EMG amplitude cancellation; (b) M wave area normalisation; (c) motor unit firing rate; (d) fatigue related reflex inhibition; (e) spectral compression; (f) EMG amplitude dependence on muscle action velocity.
Perhaps the strongest argument that may complicate the interpretation of surface EMG amplitude values, particularly regarding the CGM, is amplitude cancellation. The surface EMG signal is defined as an algebraic summation of all the muscle action potentials propagating within the recording areas of the surface electrodes. Thus, from the principle of summation, if two action potentials—that is, waves—of the same absolute amplitude at the same instant in time are summed, but one is a positive deflection and the other is a negative deflection, the resultant EMG amplitude would, theoretically, equal zero, because the individual muscle action potentials would cancel each other out. The concept of surface EMG amplitude cancellation has been studied.72,73 Keenen et al73 indicated that, when a muscle is near maximal activation, up to 62% of the muscle action potentials propagating within the recording areas of the surface EMG electrodes are cancelled during summation. In other words, an EMG amplitude cancellation of 62% means that 62% of the output from the spinal cord was not represented in the surface EMG. The authors indicated therefore that absolute EMG amplitude values can substantially underestimate the true amount of muscle activation—that is, neural drive. Moreover, decreases in the range of muscle action potential amplitudes and increases in the duration of the action potentials, both of which occur during fatigue, resulted in nearly 85% amplitude cancellation.73 It was concluded therefore that fatigue induced changes in action potential properties “…can contribute to the inability of EMG amplitude to reach maximal levels after a fatiguing contraction, independent of any deficit in the capacity to activate the muscle” (p 128).
Indeed the findings of Keenan et al73 showed that the interpretation of absolute EMG values was complicated by substantial amounts of amplitude cancellation; however, when EMG amplitude values were normalised to maximal levels of excitation, the differences between cancelled signals and no cancellation were generally less than 5%, with the greatest difference being a 17% overestimation at intermediate levels of excitation. These findings suggest therefore that normalising EMG amplitude values by reporting them as a percentage of maximal activation may substantially reduce the influence of amplitude cancellation, which was done for at least three studies using EMG measures by the advocates of the CGM.70,71,74 However, Keenan et al73 emphasised “…caution in interpreting different levels of normalised surface EMG in muscles where recruitment range or peak discharge rates may vary, particularly at intermediate levels of excitation” (p 129). Indeed, peak discharge rates are also known to change with fatigue (discussed below); however, the method of normalisation is also an important consideration. For example, if one is interested in describing motor control strategies with surface EMG amplitude during prolonged endurance exercise, it is wise to normalise the EMG amplitude values to changes in the size of the M wave,75 a technique that is typically not performed in the studies by advocates of the CGM.70,71,74 M waves are compound muscle action potentials generated by electrical shocks delivered to the peripheral motor nerve that innervates the muscle, and they provide information on the stability of neuromuscular propagation.76 To illustrate how M waves can contribute to the present topic, Millet et al77 showed that, after a ski skating marathon (mean duration 160 minutes), decreases in maximal isometric leg extensor torque (∼8.4%) were accompanied by a ∼30% decrease in EMG amplitude for the vastus lateralis muscle. However, motor unit recruitment was not significantly affected by the marathon, as twitch interpolation indicated that recruitment was 99.4% and 98.4% before and after the marathon respectively. Furthermore, the M wave peak to peak amplitude decreased to a similar extent to the EMG amplitude, so that the ratio of these variables did not change with fatigue. This may have indicated a fatigue induced alteration in neuromuscular propagation, which altered the surface EMG amplitude, but was independent of motor unit recruitment. An analysis of only EMG amplitude from the voluntary MVCs, without M wave normalisation, would have led to the erroneous conclusion that motor unit recruitment decreased with fatigue, which clearly did not happen in this study.77
It may be important to note that normalisation to M wave amplitude could also be confounded by the issue of amplitude cancellation, as M waves also represent the sum of many motor unit potentials. Consequently, slight changes in the timing of the potentials can cause significant changes in the M wave due to cancellation alone. Therefore it may be appropriate, although not ideal, to normalise the surface EMG to the area of the M wave. In contrast, however, a recent study by Calder et al78 reported stable and reliable peak to peak M wave amplitudes and negative phase areas with intraclass correlation coefficients ranging from 0.96 to 0.98 with no mean differences from trial 1 to trial 2. Thus the use of M wave normalisation for surface EMG amplitude values, with either peak to peak amplitude or M wave area, may be the best normalisation techniques available at this time.
It is clear that potential decreases in motor unit firing rates during fatiguing exercise may also contribute to a decline in surface EMG amplitude.79,80 Thus fatigue induced decreases in EMG amplitude can occur independently of changes in motor unit recruitment, and may have contributed to the decreases in EMG amplitude reported by St Clair Gibson et al.70 Unfortunately, it is difficult, if not impossible, to differentiate between motor unit recruitment and firing rate using traditional, bipolar surface EMG techniques, such as those used by Kay et al71,74 and St Clair Gibson et al.70
Decreases in motor unit recruitment and firing rate, and therefore EMG amplitude, can also occur because of reflex effects in the spinal cord,9,10 which would be inconsistent with the CGM, which “proposes that the subconscious brain sets the exercise intensity by determining the number of motor units that are activated…”3 (p 801). A variety of structures have been proposed for such a phenomenon. For example, type III and type IV free nerve endings are sensitive to muscle metabolites such as H+81 and are known to synapse with inhibitory interneurons in the spinal cord.82 Numerous studies have noted changes in reflex motor unit activity with a variety of exercise models (see Hunter et al12 for a brief review). For example, Avela et al9 showed decreases in H reflex amplitude (normalised to the M wave), an electrical analogue of the monosynaptic stretch reflex, as well as mechanically induced stretch reflex responses, after a marathon run. Maximal plantarflexor isometric torque and EMG amplitude (soleus and gastrocnemius) were also significantly reduced after the marathon.9 The M wave amplitude was not significantly affected after the marathon, so the differences in EMG amplitude probably reflected changes in motor unit recruitment and firing rate.9 Unfortunately, no twitch interpolation procedure was performed, so it is difficult to differentiate between a failure of recruitment, as predicted by the CGM, or an effect of reflex modulation in the spinal cord. Similarly, Duchateau et al10 reported decreased H reflex amplitude (normalised to the M wave) after sustained isometric muscle actions of the abductor pollicis brevis at 25% and 50% MVC. Thus, given the clear modulation of stretch reflex and H reflex responses with fatigue, at the least a reflex influence on EMG amplitude during the post‐marathon MVCs must be considered as a plausible explanation, in contrast with a subconscious, anticipatory regulation of motor unit recruitment from the brain, as suggested by the CGM.
Furthermore, recent work has examined fatigue, time to task failure (endurance), and EMG responses to isometric tasks with the same force requirement, but which differed in the type of feedback that was given.83 Specifically, subjects perform trials with a force task, which was a submaximal force held against a transducer (force output feedback), and a position task, which was a submaximal inertial load that elicited the same force as the force task, and it was held at the same joint position as the force task (joint position feedback). Despite comparable torque requirements (and therefore metabolic demand), the time to task failure was shorter for the position task (inability to maintain joint position) than the force task (inability to maintain target force), and resulted in a faster rate of increase in EMG amplitude, which suggested a more rapid increase in muscle activation. However, the declines in maximal voluntary force after exercise were not significantly different, which indicated comparable fatigue. It was hypothesised that the differences between the force and position tasks were “…caused by variation in synaptic input, likely involving heightened sensitivity of the stretch reflex during the position task”83 (p 389). Therefore, irrespective of the exact mechanisms, these findings revealed two observations that were inconsistent with the CGM: (a) dissociation between metabolic demand and muscle endurance and muscle activation; (b) evidence of fatigue induced muscle activation at the level of the spinal cord.
The amplitude of the surface EMG signal is also affected by muscle fatigue in ways that do not directly reflect motor unit recruitment or firing rate. The well established “spectral compression” of the surface EMG signal during fatiguing exercise, due in part to changes in muscle fibre conduction velocity,84,85,86 can also influence EMG amplitude by altering the amount of signal attenuation resulting from the low pass filter characteristics of the subcutaneous fat and skin. Furthermore, surface EMG amplitude is also influenced by contraction velocity,87,88,89,90 so in tasks such as cycling time trials, in which pedal cadence is not rigorously controlled,70,71 the interpretation of changes in EMG amplitude are problematic.
Given the limitations outlined above, such as fatigue induced spectral compression, alterations in neuromuscular propagation, and amplitude cancellation, caution must be exercised when interpreting surface EMG amplitude values during voluntary muscle actions. In addition, more in depth reviews of the limitations of surface EMG during fatigue are available.91,92 These findings do not necessarily suggest that surface EMG signals contain no useful information, but rather that the limitations of the method should be acknowledged when evaluating hypotheses based on surface EMG data. With new technologies such as linear and multidimensional arrays,93,94 perhaps the use of surface EMG can provide more conclusive information regarding the CGM. Thus until the hypothesis of the CGM1,2,3,4,5 can be tested with more sophisticated measures, such as twitch interpolation, M wave normalisation, invasive EMG, and/or multi‐electrode arrays, it may be misleading to justify the CGM with data derived from traditional, bipolar surface EMG techniques.
What is the extent of motor unit recruitment during voluntary contractions?
In the presentation of the CGM, there are numerous references to the observation that under voluntary conditions, humans are not capable of maximally activating all available motor units.2,3,5 Indeed, Noakes and St Clair Gibson2 argued that the fourth hallmark requirement of the CAC model is that “models of peripheral fatigue predict that there must always be complete recruitment of all motor units in the active limbs at fatigue” (p 15). Further, Noakes et al5 stated (p 121) that “…if there is a skeletal muscle reserve at the point of physical exhaustion, then fatigue cannot be caused by a peripherally based control but must result from CNS regulation of skeletal muscle motor unit recruitment”. It should be noted, however, that the extent of motor unit recruitment during maximal voluntary efforts has been shown to be 100% in some studies, going back to the classic study of Merton.51 Similar observations have been reported by other studies95,96,97 that have used the twitch interpolation technique (also known as twitch superimposition, twitch occlusion), in which supramaximal electrical stimuli are superimposed on MVCs.6,76 Theoretically, with twitch interpolation, increases in force above the voluntary level are indicative of electrical activation of motor units that were not recruited under purely voluntary conditions. In contrast, other studies have shown less than 100% recruitment98 (for review, see Gandevia31). Differences in recruitment may differ by muscle group examined. The dorsiflexors have been shown to be fully recruited in a variety of studies,95,98 whereas the plantarflexors appear to be more difficult to fully activate, even when not fatigued.98 Of note, however, is the fact that, even in studies showing less than complete voluntary recruitment, the extent of motor unit recruitment is quite high.98
However, recruitment of less than 100% of the available motor units is not, in itself, disproof of peripheral fatigue. The aforementioned twitch interpolation studies showed that, for some muscles, the CNS may prevent maximal recruitment for reasons other than in response to fatigue. More importantly, if one assumes that a pool of motor units is unavailable for voluntary activation, even under non‐fatiguing conditions, then a decline in muscle performance would be evidence of a peripheral limitation to performance, at least under those experimental conditions. Klass et al99 have recently made just such an observation. Subjects performed multiple sets of 30 voluntary plantarflexions (50 contractions per minute pace) until task failure, defined as range of motion <50% of control. Immediately after task failure, muscle activation (twitch interpolation) was not different from rest (98.4% v 98.3%). There was also no significant change in EMG amplitude, normalised by the M wave amplitude (2.1% v 2.0% of M wave amplitude). The authors stated (p 1518) that “Our results showed that the neural drive and M‐wave amplitude were not significantly affected by the fatiguing experiment, indicating that mechanisms located beyond the muscle cell membrane limited the force production after the fatiguing task in the present experimental conditions.” Further, electrically evoked twitch torque from a single and double impulse decreased by 23% and 16% respectively. Clearly, something happened in the periphery. Similar results have been reported after high intensity uphill running100 and a ski skating marathon.77 In contrast, Millet et al101 showed that, after a 30 km running race, central activation of the quadriceps declined by ∼7.5%, whereas maximal voluntary isometric torque declined by ∼24%, indicating at least some contribution of central fatigue to the fatigue response. The differences between the responses to uphill running and ski skating versus the 30 km running race illustrate the task dependent nature of the fatigue response and reflect differences not only in exercise volume, duration, and metabolic response, but also in the relative amount of eccentric loading.
Lanza et al95 reported that, during intermittent isometric contractions (five seconds on, five seconds off, for three minutes at 100% MVC) and dynamic contractions (90 isokinetic repetitions at 90°/s) of the dorsiflexors, there was no evidence of central fatigue because twitch interpolation showed 100% recruitment both before and after the fatigue tasks. Furthermore, the increase in relaxation time after electrical stimulation correlated with the decline in isometric (r2 = 0.17) and isokinetic (r2 = 0.56) torque, suggesting a role for altered Ca2+ handling (excitation‐contraction coupling) in the fatigue process.95 Furthermore, Kooistra et al102 reported that, after prolonged isometric contractions of the knee extensors, muscle activation (determined by twitch interpolation) at task failure was about 95% and nearly identical with the resting values before the task and immediately after exercise. Thus, although recruitment was not 100%, it was 100% of what was available at rest, indicating that lack of recruitment was not, in itself, the cause of task failure.
There are fewer studies examining activation and fatigue during dynamic muscle actions. However, Newham et al96 were able to quantify activation failure during maximal concentric isokinetic contractions of the knee extensors. Before fatiguing exercise, five of 23 subjects were unable to fully activate the quadriceps at 20°/s, and only two subjects were unable to achieve maximal activation at 150°s. All subjects, however, showed complete activation under isometric conditions. Fatigue was then induced by two two minute bouts of maximal concentric isokinetic knee extensions at 85°/s, separated by one minute of rest. Voluntary torque decreased by about 21%, 34%, and 16% under isometric, 20°/s, and 150°/s test conditions respectively. Voluntary activation was significantly reduced under isometric (∼36%) and slow (20°/s) isokinetic (∼29%) conditions. At 150°/s, however, motor unit activation increased slightly after the fatigue task. These results suggested that both central and peripheral fatigue can occur simultaneously, and the effects can vary depending on the task conditions.
Collectively, these studies95,96,99,102 indicated that, during at least some forms of voluntary exercise, it is possible to elicit fatigue responses that are not due to limitations on motor unit recruitment by the CNS. Specifically, for some exercise conditions, recruitment is complete both before and after fatiguing exercise.95 Other muscle groups, however, cannot achieve full recruitment even under non‐fatigued conditions, but the extent of recruitment is not decreased after the fatiguing task.102 Therefore it is possible that there is a cap on motor unit recruitment that cannot be exceeded voluntarily, and task failure occurs when the force production capabilities of the recruited motor units reduces performance such that the exercise intensity can no longer be maintained.
Regardless of the subtleties of the extent of motor unit recruitment during fatigue, peripheral fatigue models require that all motor units must be recruited with fatigue (as argued by Noakes and St Clair Gibson2), therefore the converse should also apply. Namely, the CGM must also require that artificially induced maximal recruitment—for example, magnetic supraspinal stimulation, twitch interpolation through electrical stimulation of the muscle or nerve—should restore muscle performance to the values before fatigue. There is substantial evidence, however, to disprove this hypothesis. For example, Bigland‐Ritchie et al16 examined peripheral fatigue during repeated (six second contraction, four second rest) submaximal (50% MVC) isometric muscle actions of the leg extensors. At the end of each minute during this fatigue test, a single supramaximal electric shock was delivered to the quadriceps femoris muscles through transcutaneous electrical stimulation. The authors reported that the leg extension MVC torque decreased throughout the fatigue test, and the supramaximal electric shock never increased torque above the level that could be produced voluntarily. In a subsequent review,103 it was concluded (p 141) that the CNS remained capable of fully activating all motor units during the fatigue test, and the decline in leg extension MVC torque, “…must have been due entirely to contractile failure within the muscle fibres.” Similarly, Merton et al104 reported that the reduction in isometric force during a sustained MVC of the adductor pollicis muscle could not be restored by direct stimulation of the muscle fibres themselves. Thus, under these conditions, the loss of force must have been due to factors within the muscle fibres.
Another piece that is missing from the CGM is how much of the decline in force/power is attributable to potential decreases in motor unit recruitment. Unfortunately, the relative contributions of central and peripheral factors to fatigue have been assessed in just a few studies. James et al105 studied power and force loss during isokinetic contractions of the knee extensors at 90°/s (one repetition per second for six minutes). Central fatigue was assessed using supramaximal electrical stimulation of the femoral nerve. In the two subjects who were tested, there was no evidence of central fatigue in one subject, whereas central fatigue accounted for ∼20% of the loss in isokinetic force in the other subject. Both subjects exhibited declines of isokinetic force of ∼50%, but only ∼20% losses in isometric force.
Kent‐Braun106 examined simultaneous changes in central activation ratio (CAR = maximal voluntary force/(maximal voluntary force + electrically elicited force)), EMG, and muscle metabolites (assessed with 31P magnetic resonance spectroscopy) during a four minute continuous contraction of the dorsiflexors at 100% of maximal voluntary force. Voluntary force declined to ∼22% of pre‐fatigue values, whereas EMG amplitude declined modestly to ∼73% of pre‐fatigue levels (with no change in M wave amplitude). The CAR declined from 94% to 78%. The ∼16% reduction in central drive and modest decrease in EMG amplitude in the face of an ∼88% reduction in force indicates that the dominant effects in this fatigue model were due to factors within the muscle cell, especially given the intracellular changes in [phosphocreatine], which declined to ∼22% of pre‐fatigue levels, [Pi], which increased about eightfold, and pH, which declined from ∼7.0 to ∼6.5. More recently, Kent‐Braun et al107 showed, using similar measurements but a different fatigue protocol, that there was no change in CAR, despite a significant fall in maximal voluntary force (12–28%, depending on age and sex), indicating little to no central fatigue. Comparable results have also been reported by Bigland‐Ritchie et al,108 Schillings et al,109 and Nordlund et al.110
Collectively, these findings suggest that, under voluntary conditions, a suboptimal level of motor unit activation (recruitment and firing rate), in combination with deterioration of cellular contractile performance, contribute to force/power decline. That is, both central and peripheral factors can simultaneously act to limit force and power production. The relative contribution of central and peripheral factors to fatigue appears to vary depending on the nature of the task. One of the challenges of subsequent research will be to identify the characteristics of exercise tasks that lend themselves to central or peripheral influences.
Does the CGM adequately explain the fatigue response in tasks such as the Wingate test (WAT)?
The WAT is a high intensity cycling test that purports to measure anaerobic exercise capacity and is typically performed for a duration of 30 seconds.111 Noakes and St Clair Gibson2 stated (p 20) that “the marked fall in cadence that characterises this form of exercise testing is not likely to be the result of peripheral skeletal muscle regulation but of an altered anticipatory skeletal muscle recruitment by the CNS”. What are the lines of evidence to support this statement? Firstly, the authors noted (p 18) several tangential observations, based primarily on a paper by Calbet et al,112 that do not preclude a peripheral contribution to performance decline per se: (a) the WAT is not purely anaerobic “as at least 20–30% of the energy utilised during the test comes from aerobic sources”, and (b) the contribution of anaerobic ATP production can be increased when the test is performed in hypoxia, indicating that “a reserve capacity for ‘anaerobic' ATP production exists that is not fully activated during exercise in normoxia”. However, neither of these observations disprove a peripheral effect on performance. In addition, it was noted2 (p 18) that, in hypoxia, WAT performance by sprint cyclists is reduced, relative to normoxia, within two to three seconds of test onset, and that “this cannot have resulted from metabolites acting directly within the exercising muscles”. It is unclear why Noakes and St Clair Gibson2 assumed that such a rapid effect (two to three seconds; although a closer examination of the paper by Calbet et al112 reveals that the actual responses represent mean values over five second periods, not two to three second point estimates) cannot be due to metabolic effects within the muscle, as no reference was included for this assertion. However, changes in muscle metabolite concentrations and force/power can occur extremely rapidly in skeletal muscle. For example, Gaitanos et al113 have shown that lactate can increase up to ∼7 mM (muscle biopsy) in just six seconds of high intensity bicycle exercise. Indeed, Calbet et al112 interpret their own findings as indicative of high rates of cellular ATP turnover, resulting in accumulation of Pi and ADP, which would “set the upper limit of ATP utilisation throughout their inhibitory effects on muscle contraction” (p 674). In addition, several studies have reported immediate decreases in maximal torque production during fatiguing isometric114 and concentric isokinetic115 muscle actions.
More generally, Noakes and St Clair Gibson2 stated (p 19) that, during the WAT, power output “begins to decline after the first 7–8 seconds of the exercise. There are two broad mechanisms that might explain the progressive fall in power output resulting from this progressive fall in cadence: either there is a reduced rate of motor unit recruitment by the central nervous system (the concept of muscle wisdom) or the rate of recruitment stays the same but the peripheral nervous system, including excitation‐contraction coupling within the active skeletal muscles, becomes increasingly refractory to such constant stimulation. To our knowledge, no one has yet offered a possible mechanism for this latter effect, which would have to be extremely effective, as cadence falls by about 50% during the Wingate test.”2 Two issues are of note here. Firstly, as discussed previously, the phenomenon of muscle wisdom describes the modulation of motor unit firing rates during fatigue, not motor unit recruitment. Theoretically, by decreasing firing rates during a fatiguing task, force production may be maximised for that set of conditions116. Increasing firing rates above the level required for full fusion may actually decrease force production.117 Thus, if muscle wisdom occurs during tasks such as the WAT, it could potentially result in decreases in EMG amplitude, which could be mistaken as changes in motor unit recruitment, as opposed to firing rate. Secondly, evidence that changes in excitation‐contraction coupling affect muscle fatigue continually accumulates. We specifically note the previously discussed influence of Pi, for example, on aspects of excitation‐contraction coupling.
Is there electromyographic evidence that CNS limitations on motor unit recruitment explain the rapid decline in pedal cadence and power output during the WAT? Interestingly, work performed by the advocates of the CGM actually indicates otherwise. Specifically, Hunter et al118 examined EMG responses from the rectus femoris during a 30 second WAT in 10 subjects. The EMG amplitude values during the test were normalised to values from maximal isometric contractions. Their results showed no significant change in EMG amplitude, despite an average fatigue index during the test of 44.5%. The authors noted that “during the short (30 second) period of WAT, the afferent command from the metabolic receptors to the central nervous system is insufficient to cause a reduction in central drive to result in an altered IEMG pattern”, and that “during a 30 second WAT, a protective mechanism may not be used”118 (p 298). Noakes and St Clair Gibson2 (p 19) subsequently tried to reconcile these results with the CGM by arguing that the results “seem to suggest that central neural drive remains unchanged and that peripheral mechanisms must explain the reduction in power output. However, the ∼50% reduction in cadence would indicate that the active limbs were generating force for ∼50% less time at the end than at the start of the test. If EMG activity were the same, then substantially more motor units must be recruited at the end than at the beginning of the Wingate test. Thus this finding implicates a central regulation of motor unit recruitment, with the initial recruitment of a smaller number of fast contracting fibres contracting more frequently at the outset of the Wingate test, with their progressive replacement by a larger number of slower contracting fibres contracting less frequently as the test progresses and the power production falls”. There are at least two problems with this argument. Firstly, the well established size principle of motor unit recruitment119 suggests the exact opposite pattern of recruitment from that suggested by Noakes and St Clair Gibson.2 More importantly, no matter what the pedal cadence, the relative duty cycle of the active muscles is the same assuming no change in the cycling biomechanics of the subjects, an assumption not challenged by Noakes and St Clair Gibson.2 That is, at any cadence, the relative amount of time generating force v relaxation (while the other limb is active) does not change, so that the active limbs were generating force for the same amount of time at the end as at the start of the test. As an aside, the choice of the rectus femoris for the EMG analysis of Hunter et al118 is particularly unfortunate, because the rectus femoris performs both hip flexion and knee extension, and it is not possible, based on the details provided, to be confident that the results are not contaminated by changes in the hip flexion effect of the rectus femoris.
In addition, a recent paper120 by the advocates of the CGM examined potential pacing strategies during the WAT. In this study, subjects performed six different WATs on a cycle ergometer: two were standard 30 second protocols, two were 33 seconds in duration, and two were 36 seconds in duration. One each of the 33 and 36 second trials were performed with the subjects deceived as to the length of the test, as they were told that these tests were actually 30 seconds long. The most striking finding was the fact that decreases in power output over the first 30 seconds of the test were the same, regardless of the total duration of the test. Indeed, examination of Figure 1 in Ansley et al120 shows that, over the common 30 second time period, the power profiles were essentially superimposed on each other. The authors interpreted this result as follows (p 311): “This finding is surprising, if it is believed that muscle energy depletion alone acting peripherally limits such exercise. Rather, it would be predicted that the same amount of chemical energy would allow a faster rate of energy expenditure during the shorter durations. Instead, this finding suggests that power output during the Wingate test is controlled by factors other than purely the magnitude of the skeletal muscle energy reserves and the maximum rate at which such reserves can be depleted”. They further stated that “the similarity in pacing strategy in all informed trials regardless of duration suggests that the pacing strategy is centrally regulated and is independent of the total work to be performed” (p 313). We suggest that the opposite interpretation is more plausible. That is, the similarities in the fatigue profiles over the first 30 seconds of the tests, regardless of the test duration, suggest a complete lack of pacing strategy or CNS modulation of power output. If the CGM and “teleoanticipation”4 (p 53) were operative, then we would expect to see a slower decline in power over the first 30 seconds of the 33 and 36 second tests performed without deception, as the central governor would need to dampen power output to adjust for the longer trials and subsequently larger metabolic stress.
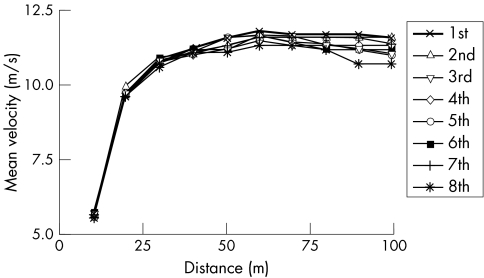
Figure 1 Individual mean velocity (m/s) for each 10 m interval of the men's 100 m finals at the 1999 World Championships.
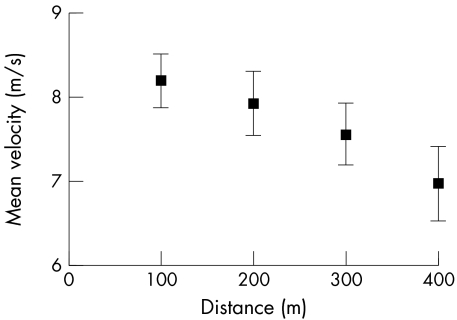
Finally, with respect to high intensity, short duration exercise, it is important to address the idea of pacing strategies in general. Noakes et al5 argued (p 122) that peripheral limitations cannot explain the “rapidity with which the successful pacing strategy is adopted: within about two to three seconds in a 100 m sprint and within the first few hundred metres in all longer running events. Clearly, these decisions cannot be based on metabolic events in the active muscles because a metabolic steady state is not achieved so expeditiously. Nor can the peripheral limitations model explain why elite athletes are able to increase their pace during the last 5–10% of the race…when fatigue should be greatest because the concentration of the ‘poisonous' intramuscular metabolites should be the highest.” With respect to pacing in a 100 m sprint, fig 1 shows the mean running velocity at 10 m intervals from the men's finals of the 100 m dash at the 1999 World Championships.121 The consistent pattern is one of initial acceleration, with maximum velocity being attained typically at about 60 m, followed by a brief period of relative stability, and then declines in velocity towards the end of the race. There is no hint of a pacing strategy or steady state in any of the competitors, metabolic or otherwise. With respect to the ability of elite athletes to increase their pace at the end of the race, Noakes et al5 cited velocity data from a 10 km race. In contrast, fig 1 clearly shows no such pacing strategy for elite athletes in a high intensity sprint. In addition, Nummela et al122 studied fatigue during 400 m sprints in competitive sprinters and hurdlers. They found that running speed declined significantly across each 100 m interval (fig 2), whereas EMG amplitude of the gastrocnemius and vastus lateralis muscles increased significantly during the race. Thus, as with the 100 m data, there was no evidence of a pacing strategy or ability of the athletes to increase speed at the end of the 400 m races. Further, the increase in EMG amplitude is in direct contradiction to the CGM, which predicts that EMG amplitude should decline as the brain decreases motor unit recruitment to avoid a terminal metabolic crisis and catastrophe (rigor).
Figure 2 Mean (SD) velocity over each 100 m interval of a 400 m sprint. Data derived from Table 2 of Nummela et al.122
Is the CNS required to prevent catastrophic ATP depletion and subsequent rigor?
The advocates of the CGM have correctly noted that exercise does not result in catastrophic reductions in muscle ATP concentrations, and therefore rigor does not ensue. Indeed, Noakes et al1 stated (p 511) that: “Perhaps the most interesting question in exercise physiology is one that is not often asked: why do muscles not develop rigor during exercise of high intensity and short duration (seconds to minutes) or when their fuel reserves are depleted during more prolonged exercise lasting two or more hours?” The answer, according to the CGM that they propose, is that the CNS regulates motor unit recruitment so that ATP consumption and production are matched, thus preventing rigor. However, the observation that ATP concentration is relatively stable during fatiguing exercise is certainly not unknown in exercise physiology.44 In fact, peripheral fatigue may be a protective mechanism to prevent catastrophe (rigor) from occurring during high intensity exercise.123 For example, the downregulation theory of muscle fatigue posits that contractile function is downregulated to preserve essential cell functions such as integrity of the ionic gradients across the sarcolemma.44 Muscle appears entirely capable of matching ATP production and consumption during fatiguing contractions, independent of any CNS involvement. Russ et al124 have shown that electrical stimulation of the medial gastrocnemius muscle, resulting in decreases in phosphocreatine concentration of about 37.5%, increases in Pi concentration of about 200%, and decreases in force of about 30%, resulted in no significant changes in ATP concentration. Thus significant fatigue and alterations in the environment of the sarcoplasm can occur with little change in ATP concentration and without any involvement of the CNS.
A variety of cellular metabolites appear to play a role in cellular regulation of ATP consumption. Any metabolite that inhibits cross bridge activity, such as Pi, will depress ATP consumption. In addition, recent evidence suggests that ADP accumulation may inhibit ATP consumption at the cross bridge.54
Further, it should be noted that, although [ATP] does not fall to a level sufficient to cause rigor, [ATP] is not perfectly stable.125 For example, [ATP] has been shown to decrease by 13% after one six second maximal sprint on a cycle ergometer, and by 32% over a series of 10 such sprints interspersed with 30 second recovery periods.113 Prolonged electrical stimulation of the knee extensors (∼22% MVC) under conditions of blood flow occlusion resulted in whole muscle [ATP] that was ∼57% of the resting value.126 In addition, single‐fibre studies have shown that decreases in [ATP] vary dramatically across fibres within a whole muscle,127 so that although the net [ATP] decline may appear relatively small, the effect on individual fibres, especially those expressing type IIX myosin heavy chain isoforms, may meaningfully impact force/power production.54
As the CGM proposes that the brain limits motor unit recruitment during fatiguing exercise to prevent catastrophic changes in [ATP], then one should predict that fatigue occurs faster under conditions where the ATP demand is greatest. However, muscle length has been shown to affect force loss during isometric testing128 and the rate of change in EMG129,130 and mechanomyographic amplitude,129 yet Baker et al131 and Sacco et al132 have shown that ATP turnover, assessed using nuclear magnetic resonance spectroscopy, is similar at long and short muscle lengths. Although the EMG and mechanomyographic amplitude data129,130 suggested that motor control properties are involved in the length dependent fatigue responses, they appear to be independent of the rate of ATP turnover.
As a final comment on [ATP] and rigor, it should be noted that rigor mortis does not occur until hours after death.133 Thus it seems extreme to suggest that a central governor is necessary to explain why rigor does not develop during voluntary exercise in pre‐mortem humans. Further, even extremely reduced [ATP] does not imply a threat of rigor because ATP turnover would still be occurring through oxidative and non‐oxidative pathways.
Central fatigue is more complex than the CGM
In previous sections, we have noted that EMG activity reflects not just motor unit recruitment, but also motor unit firing rate. It is well established that changes in firing rate79,80 can occur with fatigue. Here we will address other neurological observations that also go beyond simply regulating motor unit recruitment to prevent catastrophe, as proposed by the CGM.
Firstly, numerous studies have shown changes in reflex function with fatigue in a variety of tasks. For example, Hortobagyi et al134 had subjects perform 50 depth jumps and measured muscle voluntary and reflex responses. Surprisingly, the depth jump heights did not significantly decline over the course of the repetitions. However, squat jump height (jumps after a three second pause at a static squat position, which eliminates the stretch‐shortening cycle aspect of the task) declined ∼13%, isometric knee extension torque declined ∼20%, and EMG reflex parameters were also significantly altered. Specifically, mechanically induced patellar tendon responses from controlled taps with an instrumented reflex hammer resulted in a significantly larger EMG response (peak to peak amplitude increased ∼23%), as well as a modest increase in the time between the onset of the EMG and the mechanical response and the duration of the compound muscle action potential. In addition, the EMG burst duration of the vastus lateralis and biceps femoris muscles during the drop jumps increased by ∼13%, and ground contact time increased ∼16%. The authors suggested (p 149) that the maintenance of the stretch‐shortening performance in the face of a decline in muscle force/power production capability could be accomplished if the subjects lengthened “…the activity duration of the muscle groups primarily involved and enhanced muscle spindle sensitivity and/or used muscle fibre potentiation to compensate for the loss of muscle force.” Thus these results indicated changes in CNS control of muscle function (prolonged burst duration and increased reflex sensitivity) that are not a function of the subconscious brain simply adjusting the number of recruited motor units. In contrast, Nicol et al135 showed a reduction in gastrocnemius stretch reflex responses after two to four minutes of jumping on an instrumented sledge.
There is also evidence of depressed Golgi tendon organ activity during fatiguing exercise. Hutton and Nelson136 showed depressed firing in cats during electrically induced fatigue of the gastrocnemius muscle. Thus the general picture that emerges is that the response of the muscle spindles (group Ia and II afferents)137 and Golgi tendon organs (Ib afferents) can be altered to modify alpha motoneuron excitability during fatiguing exercise.
In addition, coactivation may play an important role in muscle fatigue. Coactivation refers to the simultaneous activation of agonist and antagonist muscles and serves to decrease torque/force production as activation of the antagonist creates an opposing torque/force. Although coactivation may have a beneficial effect by stabilising and evenly distributing force across a joint,138,139 the amount of antagonist activation varies by muscle group. For example, the quadriceps are only minimally active during knee flexion, but the hamstrings are meaningfully active during knee extension.140,141 In addition, greater coactivation occurs at faster contraction velocities.140,141 The extent of coactivation has also been shown to be affected by fatigue. Psek and Cafarelli142 and Rothmuller and Cafarelli143 have shown that the level of hamstring coactivation increases during fatiguing isometric contractions of the knee extensors. The relative amount of hamstring coactivation has also been shown to increase during fatiguing maximal isokinetic knee extension contractions at both slow (100°/s) and fast (250°/s) speeds.141 Coactivation has not been considered in the development of the CGM, as the CGM implicitly only considers agonist activity, and it is unclear how increased coactivation fits with the CGM of agonist motor unit recruitment modulation. In addition, the isokinetic coactivation data of Weir et al141 showed a decrease in EMG amplitude for the vastus lateralis during the fatiguing contractions, which is arguably consistent with the CGM. However, the relative decline in vastus lateralis EMG amplitude was greater at the fast speed than the slow speed, yet the normalised decline in force was not different between speeds. This dissociation between the force and EMG amplitude responses, along with an increase in normalised biceps femoris EMG amplitude, is not consistent with the CGM, at least as currently conceptualised, and further emphasises the difficulties involved with inferring motor unit recruitment strategies based solely on surface EMG data70,118).
What is already known on this topic
The central governor model of fatigue posits that the brain regulates/paces all exercise performance through motor unit recruitment, notably in conjunction with inhibitory signals mediated by unpleasant sensations of fatigue
In this model, the brain adjusts motor unit recruitment to prevent “catastrophic system failure” and fatigue is redefined from a physical manifestation such as a decrease in force production capability to a sensation/emotion
Conclusions
The CGM is based on arguments that the CAC model fails to satisfactorily explain fatigue phenomena. By arguing that the CAC model has been disproved—for example, by undermining the “six hallmark requirements”2 (p 5) of so‐called catastrophe models—the advocates of the CGM argue, by extension, that fatigue is not governed by changes in the contractile machinery, but rather by the brain adjusting motor unit recruitment to prevent “catastrophic system failure”5 (p 123) such as ATP depletion and subsequent rigor. For the sake of brevity, we have not substantially addressed three issues. (a) Is the CAC model really the dominant model of fatigue in exercise physiology? (b) What does catastrophe really mean in this context? (We encourage the reader to read the original work of Edwards29 from which the term catastrophe was applied to fatigue.) (c) Do the “six hallmark requirements”2 (p 5) of the CAC model flow logically from the model? What we have shown, however, is that insufficient motor unit recruitment from the brain is unable to completely explain the decline in muscle performance (force, power, etc) during a variety of fatiguing tasks, so that whatever merits are present in the CAC model, it is clear that the decline in muscle performance under some fatiguing exercise conditions can be attributed, at least partially, to factors within the muscle(s).
What this study adds
This paper reviews a variety of exercise models that do not conform with the central governor model of fatigue; in particular, numerous studies show that exercise performance can be limited not just by motor unit recruitment, but by loss of contractile performance of the skeletal muscle itself. Further, the central governor has yet to incorporate known fatigue related modulation of muscle activation at the level of the spinal cord
It is argued that task dependency, in which the mechanisms of fatigue are acknowledged to vary depending on the nature of the exercise task, is a more defensible model of fatigue
A limitation of many studies that have examined central versus peripheral fatigue is that assessments of central activation (twitch interpolation) do not typically occur during the fatiguing exercise bout. In addition, the tests of central activation failure are typically made during tests that are different from the fatiguing task. We have also noted the difficulties in inferring changes in central activation using only surface EMG data, especially with tasks where factors such as joint angle and contraction velocity are not rigorously controlled (as with isometric and isokinetic testing, and constant pedal rate cycling). Therefore it is often difficult to quantify the extent to which central activation failure contributes to the performance decline during the task. This is probably not possible with current technology for tasks such as running, but may be possible for fatigue studies of heavy resistance exercise. Future studies in this regard will be helpful.
Our arguments, in conjunction with the observations presented by the advocates of the CGM, lead us to conclude that the search for a grand unifying theory of fatigue is futile. The CGM cannot explain all observations of fatigue. Similarly, we acknowledge that “peripheral fatigue” cannot explain all performance decrements during fatiguing exercise. The concept of task dependency is that the mechanism(s) of fatigue vary depending on the type of task that is performed. Our position is that task dependency is a more logical view of fatigue than the CGM, which attempts to explain all fatigue phenomena through a common mechanism, and that both central and peripheral factors can contribute to muscle fatigue.
Acknowledgements
We are indebted to Bryan Heiderscheit and Ann York for their helpful comments on drafts of this paper.
Abbreviations
CAR - central activation ratio
CGM - central governor model
CNS - central nervous system
EMG - electromyographic
MVC - maximum voluntary contraction
RM - repetition maximum
WAT - Wingate anaerobic test
Footnotes
Competing interests: none declared
References
- 1.Noakes T D, St Clair Gibson A, Lambert E V. From catastrophe to complexity: a novel model of integrative central neural regulation of effort and fatigue during exercise in humans. Br J Sports Med 200438511–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Noakes T D, St Clair Gibson A. Logical limitations to the “catastrophe” models of fatigue during exercise in humans. Br J Sports Med 200438648–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.St Clair Gibson A, Noakes T D. Evidence for complex system integration and dynamic neural regulation of skeletal muscle recruitment during exercise in humans. Br J Sports Med 200438797–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lambert E V, St Clair Gibson A, Noakes T D. Complex systems model of fatigue: integrative homoeostatic control of peripheral physiological systems during exercise in humans. Br J Sports Med 20053952–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Noakes T D, St Clair Gibson A, Lambert E V. From catastrophe to complexity: a novel model of integrative central neural regulation of effort and fatigue during exercise in humans: summary and conclusions. Br J Sports Med 200539120–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bigland‐Ritchie B, Rice C L, Garland S J.et al Task‐dependent factors in fatigue of human voluntary contractions. Adv Exp Med Biol 1995384361–380. [DOI] [PubMed] [Google Scholar]
- 7.Asmussen E. Muscle fatigue. Med Sci Sports 197911313–321. [PubMed] [Google Scholar]
- 8.Edwards R H T, Toescu V, Gibson H. Historical perspective: a framework for interpreting pathobiological ideas on human muscle fatigue. In: Gandevia SC, Enoka RM, McComas AJ, et al, eds. Fatigue: neural and muscular mechanisms. New York: Plenum Press, 1995481–494. [DOI] [PubMed]
- 9.Avela J, Kyrolainen H, Komi P V.et al Reduced reflex sensitivity persists several days after long‐lasting stretch‐shortening cycle exercise. J Appl Physiol 1999861292–1300. [DOI] [PubMed] [Google Scholar]
- 10.Duchateau J, Balestra C, Carpentier A.et al Reflex regulation during sustained and intermittent submaximal contractions in humans. J Physiol 2002541959–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Levenez M, Kotzamanidis C, Carpentier A.et al Spinal reflexes and coactivation of ankle muscles during a submaximal fatiguing contraction. J Appl Physiol 2005991182–1188. [DOI] [PubMed] [Google Scholar]
- 12.Hunter S K, Duchateau J, Enoka R M. Muscle fatigue and the mechanisms of task failure. Exerc Sport Sci Rev 20043244–49. [DOI] [PubMed] [Google Scholar]
- 13.Westerblad H, Allen D G, Lannergren J. Muscle fatigue: lactic acid or inorganic phosphate the major cause? News Physiol Sci 20021717–21. [DOI] [PubMed] [Google Scholar]
- 14.Bellemare F, Garzaniti N. Failure of neuromuscular propagation during human maximal voluntary contraction. J Appl Physiol 1988641084–1093. [DOI] [PubMed] [Google Scholar]
- 15.Bigland‐Ritchie B. EMG/force relations and fatigue of human voluntary contractions. Exerc Sport Sci Rev 1981975–117. [PubMed] [Google Scholar]
- 16.Bigland‐Ritchie B, Furbush F, Woods J J. Fatigue of intermittent submaximal voluntary contractions: central and peripheral factors. J Appl Physiol 198661421–429. [DOI] [PubMed] [Google Scholar]
- 17.Bigland‐Ritchie B, Kukulka C G, Lippold O C.et al The absence of neuromuscular transmission failure in sustained maximal voluntary contractions. J Physiol 1982330265–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Duchateau J, Hainaut K. Behaviour of short and long latency reflexes in fatigued human muscles. J Physiol 1993471787–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Enoka R M, Robinson G A, Kossev A R. Task and fatigue effects on low‐threshold motor units in human hand muscle. J Neurophysiol 1989621344–1359. [DOI] [PubMed] [Google Scholar]
- 20.Enoka R M, Stuart D G. Neurobiology of muscle fatigue. J Appl Physiol 1992721631–1648. [DOI] [PubMed] [Google Scholar]
- 21.Fuglevand A J, Zackowski K M, Huey K A.et al Impairment of neuromuscular propagation during human fatiguing contractions at submaximal forces. J Physiol 1993460549–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gandevia S C. Neural control in human muscle fatigue: changes in muscle afferents, motoneurones and motor cortical drive. Acta Physiol Scand 1998162275–283. [DOI] [PubMed] [Google Scholar]
- 23.Hunter S K, Flagg D M, Fleshner M.et al The endurance time of a submaximal fatiguing contraction is reliable for men and women. Med Sci Sports Exerc 200032S184 [Google Scholar]
- 24.Kranz H, Williams A M, Cassell J.et al Factors determining the frequency content of the electromyogram. J Appl Physiol 198355392–399. [DOI] [PubMed] [Google Scholar]
- 25.Milner‐Brown H S, Miller R G. Muscle membrane excitation and impulse propagation velocity are reduced during muscle fatigue. Muscle Nerve 19869367–374. [DOI] [PubMed] [Google Scholar]
- 26.Walsh M L. Whole body fatigue and critical power: a physiological interpretation. Sports Med 200029153–166. [DOI] [PubMed] [Google Scholar]
- 27.Morgan W, Borg G A. Perception of effort in the prescription of physical activity. In: Nelson T, ed. Mental health and emotional aspects of sports. Chicago: American Medical Association, 1976126–129.
- 28.Noble B J, Borg G A, Jacobs I.et al A category‐ratio perceived exertion scale: relationship to blood and muscle lactates and heart rate. Med Sci Sports Exerc 198315523–528. [PubMed] [Google Scholar]
- 29.Edwards R H T. Biomechanical bases for fatigue in exercise performance: catastrophe theory in muscular exercise. In: Knuttgen HG, Vogel JA, Poortmans J, eds. Biochemistry of exercise. Champaign, IL: Human Kinetics, 19831–28.
- 30.Bigland‐Ritchie B, Woods J J. Changes in muscle contractile properties and neural control during human muscular fatigue. Muscle Nerve 19847691–699. [DOI] [PubMed] [Google Scholar]
- 31.Gandevia S C. Spinal and supraspinal factors in human muscle fatigue. Physiol Rev 2001811725–1789. [DOI] [PubMed] [Google Scholar]
- 32.Jacobs P L, Mahoney E T. Peak exercise capacity of electrically induced ambulation in persons with paraplegia. Med Sci Sports Exerc 2002341551–1556. [DOI] [PubMed] [Google Scholar]
- 33.Winslow J, Jacobs P L, Tepavac D. Fatigue compensation during FES using surface EMG. J Electromyogr Kinesiol 200313555–568. [DOI] [PubMed] [Google Scholar]
- 34.Bhambani Y, Tuchak C, Burnham R.et al Quadriceps muscle oxygenation during functional electrical stimulation in adults with spinal cord injury. Spinal Cord 200038630–638. [DOI] [PubMed] [Google Scholar]
- 35.Bilodeau M, Henderson T K, Nolta B E.et al Effect of aging on fatigue characteristics of elbow flexor muscles during sustained submaximal contraction. J Appl Physiol 2001912654–2664. [DOI] [PubMed] [Google Scholar]
- 36.Hunter S K, Enoka R M. Sex differences in the fatigability of arm muscles depends on absolute force during isometric contractions. J Appl Physiol 2001912686–2694. [DOI] [PubMed] [Google Scholar]
- 37.The American Heritage Steadman's medical dictionary. Boston: Houghton Mifflin Company, 2002
- 38.Connett R J, Honig C R, Gayeski T E.et al Defining hypoxia: a systems view of VO2, glycolysis, energetics, and intracellular PO2. J Appl Physiol 199068833–842. [DOI] [PubMed] [Google Scholar]
- 39.Brooks G A. Anaerobic threshold: review of the concept and directions for future research. Med Sci Sports Exerc 19851722–34. [PubMed] [Google Scholar]
- 40.Garrett R, Grisham C M.Biochemistry. 2nd ed. Fort Worth: Saunders College Publishers, 1999
- 41.Ganong W F.Review of medical physiology. 20th ed. New York: Lange Medical Books/McGraw‐Hill Medical Publishing Division, 2001
- 42.Guyton A C, Hall J E.Human physiology and mechanisms of disease. 6th ed. Philadelphia: Saunders, 1997
- 43.ACSM Resource manual for guidelines for exercise testing and prescription. 5th ed. Philadelphia: Lippincott Williams & Wilkins, 2005
- 44.Brooks G A, Fahey T D, Baldwin K M.Exercise physiology: human bioenergetics and its applications. 4th ed. Boston: McGraw‐Hill, 2005
- 45.DeVries H A.Physiology of exercise for physical education and athletics. 4th ed. Dubuque, IA: WC Brown, 1986
- 46.Housh T J, Housh D J, deVries H A.Applied exercise & sport physiology. Scottsdale: Holcomb Hathaway Publishers, 2003
- 47.Powers S K, Howley E T.Exercise physiology: theory and application to fitness and performance. Boston: McGraw Hill, 2004
- 48.Pocket dictionary of medicine, nursing, & allied health. St Louis: The CV Mosby Company, 1990428
- 49.Osnes J B, Hermansen L. Acid‐base balance after maximal exercise of short duration. J Appl Physiol 19723259–63. [DOI] [PubMed] [Google Scholar]
- 50.Paterson D J. Antiarrhythmic mechanisms during exercise. J Appl Physiol 1996801853–1862. [DOI] [PubMed] [Google Scholar]
- 51.Merton P A. Voluntary strength and fatigue. J Physiol 1954123553–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hansen A K, Fischer C P, Plomgaard P.et al Skeletal muscle adaptation: training twice every second day vs. training once daily. J Appl Physiol 20059893–99. [DOI] [PubMed] [Google Scholar]
- 53.Jones D A. How far experiments in the laboratory explain the fatigue of athletes in the field? In: Sargeant AJ, Kernell D, eds. Neuromuscular fatigue. Amsterdam: Elsevier, 1993100–108.
- 54.Myburgh K H. Can any metabolites partially alleviate fatigue manifestations at the cross‐bridge? Med Sci Sports Exerc 20043620–27. [DOI] [PubMed] [Google Scholar]
- 55.Dill D B. The Harvard Fatigue Laboratory. Its development, contributions, and demise. Circ Res 196720–21(suppl I)I161–I170. [Google Scholar]
- 56.Robergs R A, Ghiasvand F, Parker D. Biochemistry of exercise‐induced metabolic acidosis. Am J Physiol Regul Integr Comp Physiol 2004287R502–R516. [DOI] [PubMed] [Google Scholar]
- 57.Maclaren D P, Gibson H, Parry‐Billings M.et al A review of metabolic and physiological factors in fatigue. Exerc Sport Sci Rev 19891729–66. [PubMed] [Google Scholar]
- 58.Allen D G, Kabbara A A, Westerblad H. Muscle fatigue: the role of intracellular calcium stores. Can J Appl Physiol 20022783–96. [DOI] [PubMed] [Google Scholar]
- 59.Giannesini B, Cozzone P J, Bendahan D. Non‐invasive investigations of muscular fatigue: metabolic and electromyographic components. Biochimie 200385873–883. [DOI] [PubMed] [Google Scholar]
- 60.Hepple R T. The role of O2 supply in muscle fatigue. Can J Appl Physiol 20022756–69. [DOI] [PubMed] [Google Scholar]
- 61.Haseler L J, Richardson R S, Videen J S.et al Phosphocreatine hydrolysis during submaximal exercise: the effect of FIO2. J Appl Physiol 1998851457–1463. [DOI] [PubMed] [Google Scholar]
- 62.Hogan M C, Richardson R S, Haseler L J. Human muscle performance and PCr hydrolysis with varied inspired oxygen fractions: a 31P‐MRS study. J Appl Physiol 1999861367–1373. [DOI] [PubMed] [Google Scholar]
- 63.Danieli‐Betto D, Germinario E, Esposito A.et al Effects of fatigue on sarcoplasmic reticulum and myofibrillar properties of rat single muscle fibers. J Appl Physiol 200089891–898. [DOI] [PubMed] [Google Scholar]
- 64.Edman K A. Myofibrillar fatigue versus failure of activation. Adv Exp Med Biol 199538429–43. [DOI] [PubMed] [Google Scholar]
- 65.Edman K A. The velocity of unloaded shortening and its relation to sarcomere length and isometric force in vertebrate muscle fibres. J Physiol 1979291143–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Juel C, Pilegaard H, Nielsen J J.et al Interstitial K(+) in human skeletal muscle during and after dynamic graded exercise determined by microdialysis. Am J Physiol Regul Integr Comp Physiol 2000278R400–R406. [DOI] [PubMed] [Google Scholar]
- 67.Sejersted O M, Sjogaard G. Dynamics and consequences of potassium shifts in skeletal muscle and heart during exercise. Physiol Rev 2000801411–1481. [DOI] [PubMed] [Google Scholar]
- 68.Zoladz J A, Duda K, Majerczak J.et al Effect of different cycling frequencies during incremental exercise on the venous plasma potassium concentration in humans. Physiol Res 200251581–586. [PubMed] [Google Scholar]
- 69.Allen D, Westerblad H. Physiology. Lactic acid: the latest performance‐enhancing drug, Science 20043051112–1113. [DOI] [PubMed] [Google Scholar]
- 70.St Clair Gibson A, Schabort E J, Noakes T D. Reduced neuromuscular activity and force generation during prolonged cycling. Am J Physiol Regul Integr Comp Physiol 2001281R187–R196. [DOI] [PubMed] [Google Scholar]
- 71.Kay D, Marino F E, Cannon J.et al Evidence for neuromuscular fatigue during high‐intensity cycling in warm, humid conditions. Eur J Appl Physiol 200184115–121. [DOI] [PubMed] [Google Scholar]
- 72.Day S J, Hulliger M. Experimental simulation of cat electromyogram: evidence for algebraic summation of motor‐unit action‐potential trains. J Neurophysiol 2001862144–2158. [DOI] [PubMed] [Google Scholar]
- 73.Keenan K G, Farina D, Maluf K S.et al Influence of amplitude cancellation on the simulated surface electromyogram. J Appl Physiol 200598120–131. [DOI] [PubMed] [Google Scholar]
- 74.Kay D, St Clair Gibson A, Mitchell M J.et al Different neuromuscular recruitment patterns during eccentric, concentric and isometric contractions. J Electromyogr Kinesiol 200010425–431. [DOI] [PubMed] [Google Scholar]
- 75.Hug F, Decherchi P, Marqueste T.et al EMG versus oxygen uptake during cycling exercise in trained and untrained subjects. J Electromyogr Kinesiol 200414187–195. [DOI] [PubMed] [Google Scholar]
- 76.Enoka R M.Neuromechanics of human movement. 3rd ed. Champaign, IL: Human Kinetics, 2002
- 77.Millet G Y, Martin V, Maffiuletti N A.et al Neuromuscular fatigue after a ski skating marathon. Can J Appl Physiol 200328434–445. [DOI] [PubMed] [Google Scholar]
- 78.Calder K M, Hall L A, Lester S M.et al Reliability of the biceps brachii M‐wave. J Neuroengineering Rehabil 2005233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mottram C J, Jakobi J M, Semmler J G.et al Motor‐unit activity differs with load type during a fatiguing contraction. J Neurophysiol 2005931381–1392. [DOI] [PubMed] [Google Scholar]
- 80.Rubinstein S, Kamen G. Decreases in motor unit firing rate during sustained maximal‐effort contractions in young and older adults. J Electromyogr Kinesiol 200515536–543. [DOI] [PubMed] [Google Scholar]
- 81.Fisher M, Schafer S S. Effects of changes in pH on the afferent impulse activity of isolated cat muscle spindles. Brain Res 20051043163–178. [DOI] [PubMed] [Google Scholar]
- 82.Cleland C L, Rymer W Z, Edwards F R. Force‐sensitive interneurons in the spinal cord of the cat. Science 1982217652–655. [DOI] [PubMed] [Google Scholar]
- 83.Maluf K S, Enoka R M. Task failure during fatiguing contractions performed by humans. J Appl Physiol 200599389–396. [DOI] [PubMed] [Google Scholar]
- 84.Lowery M M, Vaughan C L, Nolan P J.et al Spectral compression of the electromyographic signal due to decreasing muscle fiber conduction velocity. IEEE Trans Rehabil Eng 20008353–361. [DOI] [PubMed] [Google Scholar]
- 85.Merletti R, Roy S. Myoelectric and mechanical manifestations of muscle fatigue in voluntary contractions. J Orthop Sports Phys Ther 199624342–353. [DOI] [PubMed] [Google Scholar]
- 86.Ravier P, Buttelli O, Jennane R.et al An EMG fractal indicator having different sensitivities to changes in force and muscle fatigue during voluntary static muscle contractions. J Electromyogr Kinesiol 200515210–221. [DOI] [PubMed] [Google Scholar]
- 87.Barnes W S. The relationship of motor‐unit activation to isokinetic muscular contraction at different contractile velocities. Phys Ther 1980601152–1158. [DOI] [PubMed] [Google Scholar]
- 88.Cramer J T, Housh T J, Johnson G O.et al Mechanomyographic amplitude and mean power output during maximal, concentric, isokinetic muscle actions. Muscle Nerve 2000231826–1831. [DOI] [PubMed] [Google Scholar]
- 89.Cramer J T, Housh T J, Weir J P.et al Gender, muscle, and velocity comparisons of mechanomyographic and electromyographic responses during isokinetic muscle actions. Scand J Med Sci Sports 200414116–127. [DOI] [PubMed] [Google Scholar]
- 90.Cramer J T, Housh T J, Weir J P.et al Power output, mechanomyographic, and electromyographic responses to maximal, concentric, isokinetic muscle actions in men and women. J Strength Cond Res 200216399–408. [PubMed] [Google Scholar]
- 91.Dimitrova N A, Dimitrov G V. Interpretation of EMG changes with fatigue: facts, pitfalls, and fallacies. J Electromyogr Kinesiol 20031313–36. [DOI] [PubMed] [Google Scholar]
- 92.Farina D, Merletti R, Enoka R M. The extraction of neural strategies from the surface EMG. J Appl Physiol 2004961486–1495. [DOI] [PubMed] [Google Scholar]
- 93.Farina D, Merletti R. Estimation of average muscle fiber conduction velocity from two‐dimensional surface EMG recordings. J Neurosci Methods 2004134199–208. [DOI] [PubMed] [Google Scholar]
- 94.Lapatki B G, Van Dijk J P, Jonas I E.et al A thin, flexible multielectrode grid for high‐density surface EMG. J Appl Physiol 200496327–336. [DOI] [PubMed] [Google Scholar]
- 95.Lanza I R, Russ D W, Kent‐Braun J A. Age‐related enhancement of fatigue resistance is evident in men during both isometric and dynamic tasks. J Appl Physiol 200497967–975. [DOI] [PubMed] [Google Scholar]
- 96.Newham D J, McCarthy T, Turner J. Voluntary activation of human quadriceps during and after isokinetic exercise. J Appl Physiol 1991712122–2126. [DOI] [PubMed] [Google Scholar]
- 97.Rutherford O M, Jones D A, Newham D J. Clinical and experimental application of the percutaneous twitch superimposition technique for the study of human muscle activation. J Neurol Neurosurg Psychiatry 1986491288–1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Belanger A Y, McComas A J. Extent of motor unit activation during effort. J Appl Physiol 1981511131–1135. [DOI] [PubMed] [Google Scholar]
- 99.Klass M, Guissard N, Duchateau J. Limiting mechanisms of force production after repetitive dynamic contractions in human triceps surae. J Appl Physiol. 2004;96: 1516–21; discussion, [DOI] [PubMed]
- 100.Lattier G, Millet G Y, Martin A.et al Fatigue and recovery after high‐intensity exercise. Part I: neuromuscular fatigue. Int J Sports Med 200425450–456. [DOI] [PubMed] [Google Scholar]
- 101.Millet G Y, Martin V, Lattier G.et al Mechanisms contributing to knee extensor strength loss after prolonged running exercise. J Appl Physiol 200394193–198. [DOI] [PubMed] [Google Scholar]
- 102.Kooistra R D, de Ruiter C J, de Haan A. Muscle activation and blood flow do not explain the muscle length‐dependent variation in quadriceps isometric endurance. J Appl Physiol 200598810–816. [DOI] [PubMed] [Google Scholar]
- 103.Bigland‐Ritchie B, Cafarelli E, Vollestad N K. Fatigue of submaximal static contractions. Acta Physiol Scand Suppl 1986556137–148. [PubMed] [Google Scholar]
- 104.Merton P A, Hill D K, Morton H B. Indirect and direct stimulation of fatigued human muscle. In: Porter R, Whelan J, eds. Human muscle fatigue: physiological mechanisms. London: Pitman Medical Ltd, 1981120–129. [DOI] [PubMed]
- 105.James C, Sacco P, Jones D A. Loss of power during fatigue of human leg muscles. J Physiol 1995484237–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kent‐Braun J A. Central and peripheral contributions to muscle fatigue in humans during sustained maximal effort. Eur J Appl Physiol Occup Physiol 19998057–63. [DOI] [PubMed] [Google Scholar]
- 107.Kent‐Braun J A, Ng A V, Doyle J W.et al Human skeletal muscle responses vary with age and gender during fatigue due to incremental isometric exercise. J Appl Physiol 2002931813–1823. [DOI] [PubMed] [Google Scholar]
- 108.Bigland‐Ritchie B, Jones D A, Hosking G P.et al Central and peripheral fatigue in sustained maximum voluntary contractions of human quadriceps muscle. Clin Sci Mol Med 197854609–614. [DOI] [PubMed] [Google Scholar]
- 109.Schillings M L, Hoefsloot W, Stegeman D F.et al Relative contributions of central and peripheral factors to fatigue during a maximal sustained effort. Eur J Appl Physiol 200390562–568. [DOI] [PubMed] [Google Scholar]
- 110.Nordlund M M, Thorstensson A, Cresswell A G. Central and peripheral contributions to fatigue in relation to level of activation during repeated maximal voluntary isometric plantar flexions. J Appl Physiol 200496218–225. [DOI] [PubMed] [Google Scholar]
- 111.Bar Or O.A new anaerobic capacity test: characteristics and applications. Brasilia: 21st World Congress in Sports Medicine, 1978
- 112.Calbet J A, De Paz J A, Garatachea N.et al Anaerobic energy provision does not limit Wingate exercise performance in endurance‐trained cyclists. J Appl Physiol 200394668–676. [DOI] [PubMed] [Google Scholar]
- 113.Gaitanos G C, Williams C, Boobis L H.et al Human muscle metabolism during intermittent maximal exercise. J Appl Physiol 199375712–719. [DOI] [PubMed] [Google Scholar]
- 114.Moritani T, Muro M, Nagata A. Intramuscular and surface electromyogram changes during muscle fatigue. J Appl Physiol 1986601179–1185. [DOI] [PubMed] [Google Scholar]
- 115.Horita T, Ishiko T. Relationships between muscle lactate accumulation and surface EMG activities during isokinetic contractions in man. Eur J Appl Physiol Occup Physiol 19875618–23. [DOI] [PubMed] [Google Scholar]
- 116.Marsden C D, Meadows J C, Merton P A. “Muscular wisdom” that minimizes fatigue during prolonged effort in man: peak rates of motoneuron discharge and slowing of discharge during fatigue. Adv Neurol 198339169–211. [PubMed] [Google Scholar]
- 117.Binder‐Macleod S A, Guerin T. Preservation of force output through progressive reduction of stimulation frequency in human quadriceps femoris muscle. Phys Ther 199070619–625. [DOI] [PubMed] [Google Scholar]
- 118.Hunter A M, St Clair Gibson A, Lambert M I.et al Effects of supramaximal exercise on the electromyographic signal. Br J Sports Med 200337296–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Henneman E, Somjen G, Carpenter D O. Functional significance of cell size in spinal motoneurons. J Neurophysiol 196528560–580. [DOI] [PubMed] [Google Scholar]
- 120.Ansley L, Robson P J, St Clair Gibson A.et al Anticipatory pacing strategies during supramaximal exercise lasting longer than 30 s. Med Sci Sports Exerc 200436309–314. [DOI] [PubMed] [Google Scholar]
- 121.Ferro A, Rivera A, Pagola I.et al Biomechanical analysis of the 7th World Championships Athletics Seville 1999. New Studies in Athletics 20011625–60. [Google Scholar]
- 122.Nummela A, Vuorimaa T, Rusko H. Changes in force production, blood lactate and EMG activity in the 400‐m sprint. J Sports Sci 199210217–228. [DOI] [PubMed] [Google Scholar]
- 123.Sargeant A J. Human power output and muscle fatigue. Int J Sports Med 199415116–121. [DOI] [PubMed] [Google Scholar]
- 124.Russ D W, Vandenborne K, Walter G A.et al Effects of muscle activation on fatigue and metabolism in human skeletal muscle. J Appl Physiol 2002921978–1986. [DOI] [PubMed] [Google Scholar]
- 125.Taylor D J, Styles P, Matthews P M.et al Energetics of human muscle: exercise‐induced ATP depletion. Magn Reson Med 1986344–54. [DOI] [PubMed] [Google Scholar]
- 126.Spriet L L, Soderlund K, Bergstrom M.et al Anaerobic energy release in skeletal muscle during electrical stimulation in men. J Appl Physiol 198762611–615. [DOI] [PubMed] [Google Scholar]
- 127.Karatzaferi C, de Haan A, Ferguson R A.et al Phosphocreatine and ATP content in human single muscle fibres before and after maximum dynamic exercise. Pflugers Arch 2001442467–474. [DOI] [PubMed] [Google Scholar]
- 128.Fitch S, McComas A. Influence of human muscle length on fatigue. J Physiol 1985362205–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Weir J P, Ayers K M, Lacefield J F.et al Mechanomyographic and electromyographic responses during fatigue in humans: influence of muscle length. Eur J Appl Physiol 200081352–359. [DOI] [PubMed] [Google Scholar]
- 130.Weir J P, McDonough A L, Hill V J. The effects of joint angle on electromyographic indices of fatigue. Eur J Appl Physiol Occup Physiol 199673387–392. [DOI] [PubMed] [Google Scholar]
- 131.Baker A J, Carson P J, Green A T.et al Influence of human muscle length on energy transduction studied by 31P‐NMR. J Appl Physiol 199273160–165. [DOI] [PubMed] [Google Scholar]
- 132.Sacco P, McIntyre D B, Jones D A. Effects of length and stimulation frequency on fatigue of the human tibialis anterior muscle. J Appl Physiol 1994771148–1154. [DOI] [PubMed] [Google Scholar]
- 133.McCance K L, Heuther S E.Pathophysiology: the biological basis for disease in adults and children. 4th ed. St Louis: Mosby, 2002
- 134.Hortobagyi T, Lambert N J, Kroll W P. Voluntary and reflex responses to fatigue with stretch‐shortening exercise. Can J Sport Sci 199116142–150. [PubMed] [Google Scholar]
- 135.Nicol C, Komi P V, Horita T.et al Reduced stretch‐reflex sensitivity after exhausting stretch‐shortening cycle exercise. Eur J Appl Physiol Occup Physiol 199672401–409. [DOI] [PubMed] [Google Scholar]
- 136.Hutton R S, Nelson D L. Stretch sensitivity of Golgi tendon organs in fatigued gastrocnemius muscle. Med Sci Sports Exerc 19861869–74. [PubMed] [Google Scholar]
- 137.Nelson D L, Hutton R S. Dynamic and static stretch responses in muscle spindle receptors in fatigued muscle. Med Sci Sports Exerc 198517445–450. [DOI] [PubMed] [Google Scholar]
- 138.Baratta R, Solomonow M, Zhou B H.et al Muscular coactivation. The role of the antagonist musculature in maintaining knee stability. Am J Sports Med 198816113–122. [DOI] [PubMed] [Google Scholar]
- 139.Solomonow M, Baratta R, Zhou B H.et al The synergistic action of the anterior cruciate ligament and thigh muscles in maintaining joint stability. Am J Sports Med 198715207–213. [DOI] [PubMed] [Google Scholar]
- 140.Snow C J, Cooper J, Quanbury A O.et al Antagonist cocontraction of the knee extensors during constant velocity muscle shortening and lengthening. J Electromyogr Kinesiol 19953185–195. [DOI] [PubMed] [Google Scholar]
- 141.Weir J P, Keefe D A, Eaton J F.et al Effect of fatigue on hamstring coactivation during isokinetic knee extensions. Eur J Appl Physiol Occup Physiol 199878555–559. [DOI] [PubMed] [Google Scholar]
- 142.Psek J A, Cafarelli E. Behavior of coactive muscles during fatigue. J Appl Physiol 199374170–175. [DOI] [PubMed] [Google Scholar]
- 143.Rothmuller C, Cafarelli E. Effect of vibration on antagonist muscle coactivation during progressive fatigue in humans. J Physiol 1995485857–864. [DOI] [PMC free article] [PubMed] [Google Scholar]