Abstract
Tlg1p and Tlg2p, members of the syntaxin family of SNAREs in yeast, have been implicated in both endocytosis and the retention of late Golgi markers. We have investigated the functions of these and the other endocytic syntaxins Pep12p and Vam3p. Remarkably, growth is possible in the absence of all four proteins. In the absence of the others, Pep12p and Tlg1p can each create endosomes accessible to the endocytic tracer dye FM4-64. However, although Pep12p is required for the ligand-induced internalization of the α factor receptor and its passage via Pep12p-containing membranes to the vacuole, Tlg1p is not. In contrast, Tlg1p is required for the efficient localization of the catalytic subunit of chitin synthase III (Chs3p) to the bud neck, a process that involves endocytosis and polarized delivery of Chs3p. In wild-type cells, internalized Chs3p cofractionates with Tlg1p and Tlg2p, and in a strain lacking the other endocytic syntaxins, either Tlg1p or Tlg2p is sufficient for correct localization of the enzyme. Pep12p is neither necessary nor sufficient for this process. We conclude that there are two endocytic routes in yeast that can operate independently and that Tlg1p is located at the junction of one of these with the polarized exocytic pathway.
INTRODUCTION
Secretion and endocytosis are the mechanisms by which eukaryotic cells control membrane flow to and from the cell surface. An equilibrium is maintained by the fusion of secretory vesicles and by the invagination of endocytic vesicles. Hence, traffic along the secretory and endocytic pathways must be continuously balanced to maintain the appropriate lipid and protein composition of the plasma membrane and to coordinate cell surface expansion during growth and morphogenesis.
A full understanding of membrane traffic requires knowledge of the individual steps and routes between organelles. Analysis of these has been helped by the realization that specific membrane proteins, termed SNAREs, mediate the various fusion events (Bennett and Scheller, 1993; Rothman, 1994; Nichols and Pelham, 1998). In general, fusion requires SNAREs on vesicles (v-SNAREs) to bind to SNAREs on target organelles (t-SNAREs), although because t-SNAREs also enter vesicles this distinction is blurred.
We have focused on the syntaxin family of t-SNAREs, because these proteins are the easiest to identify by sequence homology (Weimbs et al., 1997; Holthuis et al., 1998). In addition, all known SNARE-dependent fusion steps studied to date use a syntaxin homologue as an essential component. The yeast genome encodes eight identifiable syntaxins, two of which (Sso1p and Sso2p) are closely related and functionally indistinguishable. Cell growth and secretion show an absolute requirement for three syntaxins: Ufe1p in the ER, Sed5p in the cis-Golgi, and Sso1p or Sso2p on the plasma membrane (Hardwick and Pelham, 1992; Aalto et al., 1993; Lewis and Pelham, 1996). The nonessential syntaxins comprise Vam3p on the vacuole, Pep12p in a late endosomal or prevacuolar compartment, and Tlg1p and Tlg2p, which have overlapping distributions in organelles that seem to correspond to the yeast equivalent of the trans-Golgi network (TGN) and perhaps early endosomes (Becherer et al., 1996; Darsow et al., 1997; Nichols et al., 1997; Abeliovich et al., 1998; Holthuis et al., 1998).
Vam3p is required for homotypic fusion of vacuoles, and loss of Vam3p or Pep12p blocks protein transport through distinct biosynthetic routes to the vacuole (Becherer et al., 1996; Darsow et al., 1997; Nichols et al., 1997). The roles of Tlg1p and Tlg2p are less clear. They are required for efficient degradation of the α factor receptor and for the retrieval of TGN resident proteins from the endocytic pathway (Holthuis et al., 1998), but they are not absolutely required either for secretion, for delivery of proteins to the vacuole, or for at least some endocytosis. This suggests that alternative pathways exist for some or all of these processes, complicating the analysis. It is also possible that different syntaxins can substitute for another syntaxin’s function. Indeed overexpressed Pep12p can to some extent perform the role of Vam3p when this is absent and vice versa (Darsow et al., 1997; Gotte and Gallwitz, 1997), and all four of the endocytic syntaxins bind the same v-SNARE, Vti1p (Fischer von Mollard et al., 1997; Holthuis et al., 1998).
One role for endocytosis in yeast is to redirect plasma membrane proteins to the sites of polarized growth. This has been well studied in the case of Chs3p, the catalytic subunit of chitin synthase III. Chs3p deposits chitin in a ring around the base of the bud as well as in the lateral wall (Shaw et al., 1991) and is localized on either side of the bud neck in large-budded cells, apparently because of its interaction with other proteins present at these sites (Chuang and Schekman, 1996; DeMarini et al., 1997; Santos and Snyder, 1997). Movement of the enzyme to the forming bud neck occurs by its uptake into intracellular organelles, followed by its incorporation into secretory vesicles that are directed to the forming bud (Chuang and Schekman, 1996; Ziman et al., 1996; Santos and Snyder, 1997). Mutants that lack Chs3p or that fail to deliver it to the plasma membrane have a characteristic phenotype with large misshapen cells and abnormal bud necks and budding patterns (Shaw et al., 1991). Cells lacking Tlg1p have a somewhat similar phenotype (Holthuis et al., 1998), suggesting that Tlg1p might be involved in the recycling of Chs3p either directly or more indirectly because of its effects on endocytosis in general.
In this manuscript we have investigated the roles of Tlg1p and Tlg2p in more detail, avoiding the problems of redundancy by constructing strains lacking multiple syntaxins. We show that simultaneous removal of Tlg1p, Tlg2p, Pep12p, and Vam3p does not prevent secretion. Both Tlg1p and Pep12p can allow fusion of endocytic vesicles, but unlike Pep12p, Tlg1p cannot generate a stable compartment containing endocytosed and vacuolar markers. However, Tlg1p cofractionates with internalized Chs3p and is required for the targeting of Chs3p to the bud neck. Tlg2p can also perform this function, although with lower efficiency, but Pep12p cannot. We propose that Tlg1p is located at the junction of the endocytic and exocytic pathways, allowing selected proteins from the plasma membrane to reenter post-Golgi transport vesicles.
MATERIALS AND METHODS
Plasmids
The open reading frames of TLG1, TLG2, and PEP12 were subcloned into pRS316 (CEN, URA3 [Sikorski and Hieter, 1989]) behind the PHO5 (TLG1, TLG2) or TPI1 (PEP12) promoter. These constructs gave 4-fold (TLG1), 10-fold (TLG2), or 8-fold (PEP12) higher expression levels than did the endogenous genes, as determined by immunoblotting. Expression plasmids encoding myc-tagged invertase or green fluorescent protein (GFP)-tagged Sft2p have been described previously (Banfield et al., 1995; Wooding and Pelham, 1998). All enzymes for manipulation of DNA were from New England Biolabs (Beverly, MA).
Antibodies
Rabbit polyclonal antibodies to carboxypeptidase Y (CPY), Sed5p, Tlg1p, Tlg2p, Pep12p, and Vam3p have been described previously (Hardwick and Pelham, 1992; Banfield et al., 1995; Nichols et al., 1997; Holthuis et al., 1998). Rabbit polyclonal antibodies to Sso2p (also recognizing Sso1p) and Chs3p were kindly provided by S. Keränen (Biotechnology and Food Research, Espoo, Finland) and R. Schekman (University of California, Berkeley, Berkeley, CA), respectively. Rabbit polyclonal antibodies recognizing the myc or hemagglutinin (HA) epitopes were from Santa Cruz Biotechnology (Santa Cruz, CA), and the mouse monoclonal antibody to Vma1p was from Molecular Probes (Eugene, OR).
Yeast Strains
Unless indicated, all yeast strains were grown at 25°C in synthetic dextrose medium (SDM). Standard yeast genetic techniques for sporulation, tetrad analysis, and gene disruption were used as described by Guthrie and Fink (1991). Yeast transformations were performed as described by Elble (1992). Deletion phenotypes were characterized in SEY6210 (MATα ura3-52 his3-Δ200 leu2-3, -112 trp1-Δ901 suc2-Δ9 lys2-801) or in haploid strains established after sporulation of SEY6210/SEY6211 diploids (SEY6211 genotype: MATa ura3-52 his3-Δ200 leu2-3, -112 trp1-Δ901 suc2-Δ9 ade2-101). Mating types of strains derived from isolated spores were determined by pheromone halo assays (Guthrie and Fink, 1991). The Δtlg1 (JHY016, MATa), Δtlg2 (JHY004, MATα), Δtlg1 Δtlg2 (JHY014, MATa), and Δpep12 (JHY005, MATα) strains used in this study have been described elsewhere (Holthuis et al., 1998). As a first step to generate a Δtlg1 Δtlg2 Δpep12 Δvam3 strain, JHY004 was mated with SEY6211, and a PEP12 allele in the diploid was replaced with a loxP-HIS3- loxP cassette (as described in Holthuis et al. 1998). Sporulation yielded a haploid Δtlg2 Δpep12 strain (JHY009, MATa) that was mated with SEY6210. After replacement of a TLG1 allele with TRP1, the diploid was transformed with the TLG1 expression plasmid and sporulated, allowing the isolation of a haploid Δtlg1 Δtlg2 Δpep12 strain (JHY043, MATα) that was capable of losing the TLG1 expression plasmid and that grew at 25°C. The VAM3 allele in JHY043 was replaced with a loxP-URA3-loxP cassette, and the HIS3 and URA3 markers were removed by excisive recombination at the loxP sites after transient expression of Cre recombinase (Sauer, 1987). This yielded a Δtlg1 Δtlg2 Δpep12 Δvam3 strain (JHY046, MATα) that grew at 25°C.
Three copies of the HA epitope were inserted at the C terminus of endogenously expressed Ste2p (α factor receptor) in SEY6211 by recombination with a PCR-generated cassette that contained the appropriate homologous regions, the tag, and the HIS5 gene of Schizosaccharomyces pombe. Strains expressing Ste2p-GFP (RAY 1284, a kind gift from R. Arkowitz, Medical Research Council Laboratory of Molecular Biology, Cambridge, United Kingdom) or Ste2p-(HA)3 (JHY037) were used to monitor ligand-induced internalization of α factor receptor as described below. Both modified versions of Ste2p retained activity, as judged by mating. Ste2p-GFP was introduced into Δtlg1 and Δpep12 strains by crossing. The end4-1 internalization mutant (strain RHY1597) was kindly provided by Howard Riezman (University of Basel, Basel, Switzerland).
Pulse-Chase Studies
Pulse-chase analysis of CPY and secretory glycoproteins was performed exactly as described by Stepp et al. (1997), although the incubation temperature was 25 instead of 30°C. To analyze secretion of radiolabeled glycoproteins, we collected the medium of pulsed and pulse-chased cells by centrifugation, diluted the medium 10-fold in phosphate-buffered saline (PBS), and incubated the diluted medium for 1 h with concanavalin A–coupled sepharose beads (Pharmacia, Piscataway, NJ). The beads were washed three times with PBS containing 0.2% SDS, and bound glycoproteins were eluted by boiling in SDS-PAGE sample buffer.
Electron Microscopy
Cells were grown in SDM and harvested at a density of 1 OD600 per ml. Permanganate fixation, dehydration, and embedding in Spurr’s resin (Agar Scientific, Stansted, United Kingdom) were performed as described by Kaiser and Schekman (1990). Thin sections were cut and then stained by incubation in 5% uranyl acetate for 10 min at 60°C followed by 5 min in Reynold’s lead citrate at room temperature.
Immunofluorescence
Cells grown to midlogarithmic phase (∼1 OD600 per ml) were fixed and mounted on slides as described previously by Hardwick and Pelham (1992). Both primary and secondary antibody incubations were performed in PBS supplemented with 2% dried milk for 2 h at room temperature. Primary antibodies to Sed5p, Vma1p, and Chs3p were used at a dilution of 1:2000, 1:200, and 1:500, respectively. Fluorescein- or Cy3-conjugated secondary antibodies (Amersham, Arlington Heights, IL) were used for visualization at a dilution of 1:100. Images were obtained with an MRC-600 confocal laser scanning microscope (Bio-Rad, Richmond, CA) using excitation at the appropriate wave lengths.
FM4-64 Uptake Studies
Cells grown to midlogarithmic phase were harvested and resuspended at 10–20 OD per ml in SDM. FM4-64 (Molecular Probes) was added to 40 μM from a 32 mM stock in DMSO. The cells were then either mounted on glass slides for immediate inspection or incubated with shaking for another 150 min at 25°C before mounting. To immobilize cells, we coated glass slides with concanavalin A, applied a 3-μl cell suspension, and gently layered a coverslip on top of the cells (Vida and Emr, 1995). Slide preparations were suitable for viewing up to 60 min. To follow GFP-Ste2p uptake simultaneously, we exposed cells to 1 μM α-Factor (Sigma, St. Louis, MO) and immediately mounted the cells as described above. FM4-64 was added either at the same time as α-Factor or 30 min previously. Images were collected with an MRC-600 confocal laser scanning microscope using a 546-nm laser line for FM4-64 fluorescence and a 468-nm line for GFP fluorescence.
Subcellular Fractionation
Subcellular fractionation studies with wild-type (SEY6210) and JHY046 cells (see Figures 4 and 9A) were performed exactly as described in Holthuis et al. (1998). For subcellular fractionation of HA-tagged Ste2p (α factor receptor; see Figure 8), exponentially growing JHY037 cells were harvested and resuspended in SDM at 10 OD per ml, cycloheximide was added to 10 μg/ml, and cells were incubated for 10 min at 30°C with shaking, cooled on ice to 15°C, and then incubated at 15°C with shaking. After 10 min, 40 ml of cells was removed to a tube on ice containing NaF and NaN3 (each at 20 mM final concentration), and α factor was added to the culture to a final concentration of 1 μM. At 7.5- and 30-min time points after α factor addition, 40 ml of cells was removed from the culture and transferred to tubes on ice as described above. Collected cells were spheroplasted, lysed in a hypo-osmotic buffer, and subjected to differential centrifugation as described in Holthuis et al. (1998). The 100,000 × g membrane pellets were resuspended in 1 ml of hypo-osmotic buffer and loaded on top of a 22–60% (w/w) sucrose step gradient that was prepared in 10 mM HEPES-KOH, pH 7.6, and 1 mM EDTA using the following steps: 1.0 ml of 60%, 1.5 ml of 40%, 1.5 ml of 37%, 1.5 ml of 34%, 2.0 ml of 32%, 2.0 ml of 29%, 1.0 ml of 27%, and 1.0 ml of 22%. For subcellular fractionation with the end4-1 internalization mutant (see Figure 9B), RHY1597 cells were grown to midlogarithmic phase in YEPD medium at 25°C, harvested, resuspended to 10 OD per ml in prewarmed YEPD, and incubated with shaking at 37°C. After 30 min of incubation, NaF and NaN3 were added to 20 mM each. Cells were incubated for another 10 min at 37°C, collected, then spheroplasted, lysed, and subjected to differential centrifugation as described (Holthuis et al., 1998). The 100,000 × g membrane pellet was resuspended in 2.0 ml of hypo-osmotic buffer and loaded on top of a 32–60% (w/w) sucrose step gradient consisting of the following steps: 1.0 ml of 60%, 1.0 ml of 50%, 1.5 ml of 46%, 1.5 ml of 43%, 1.5 ml of 40%, 1.5 ml of 37%, 1.5 ml of 34%, and 1.0 ml of 32%. Gradients were spun in a Beckman SW40Ti rotor (Fullerton, CA) at 170,000 × g for 17–18 h at 4°C. Sixteen 0.78-ml fractions were collected from the top, and the sucrose concentration was determined by refractive index. Equal volumes per fraction were subjected to SDS-PAGE, transferred to nitrocellulose, and probed with the appropriate antisera. All antibody incubations were performed in PBS containing 5% dried milk and 0.5% Tween 20. After incubation with peroxidase-conjugated secondary antibodies (Bio-Rad), detection was performed using enhanced chemiluminescence (ECL kit; Amersham). Bands on ECL Western blots were quantified using a scanning densitometer (Molecular Dynamics, Sunnyvale, CA) with ImageQuant software.
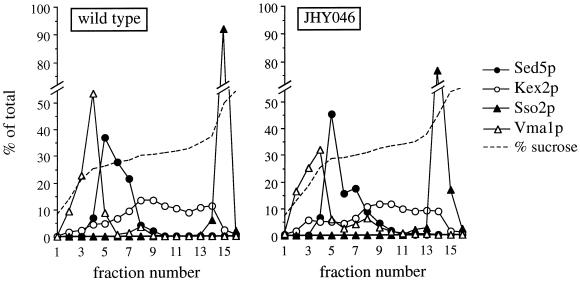
Figure 4.
Subcellular fractionation of JHY046 cells. Membranes from a high-speed pellet fraction were separated on equilibrium sucrose gradients, and the indicated markers were detected by immunoblotting or, for Kex2p, by enzyme assay. Quantitation was by densitometric scanning of fluorograms produced by immunoblots.
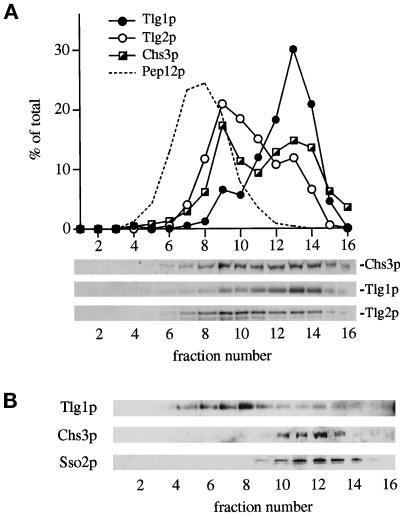
Figure 9.
Distribution of Chs3p assessed by subcellular fractionation. (A) A high-speed membrane pellet from wild-type cells was fractionated on an equilibrium sucrose gradient, and proteins were detected by immunoblotting. The plasma membrane marker Sso2p was primarily (∼80%) restricted to fraction 15 on this gradient. (B) Cells carrying the end4-1 mutation were incubated for 30 min at 37°C before fractionation on an equilibrium sucrose gradient. The gradient profile was adjusted to maximize separation of Tlg1p from the plasma membrane marker Sso2p.
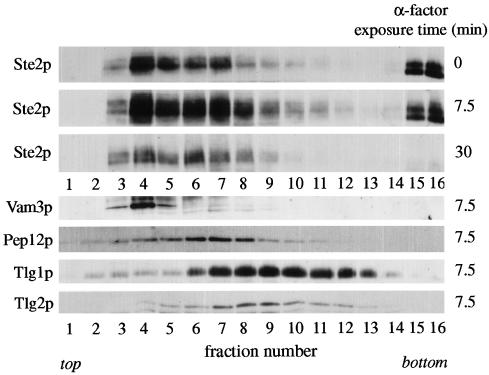
Figure 8.
Subcellular fractionation of internalized Ste2p (α factor receptor). Wild-type cells expressing HA-tagged Ste2p were preincubated with cycloheximide for 10 min at 30°C and then exposed to α factor at 15°C for the indicated times. After addition of azide and fluoride, the cells were spheroplasted and lysed, and membranes from a high-speed pellet fraction were separated on equilibrium sucrose gradients. Ste2p and the endocytic syntaxins were detected by immunoblotting. Note that for syntaxins only the immunoblots of one gradient (7.5-min α factor exposure) are shown; α factor treatment did not lead to obvious changes in the fractionation profiles of the syntaxins.
RESULTS
Secretory Pathway Function in Cells Lacking Late Golgi and Endosomal Syntaxins
We reported previously that removal of the late Golgi syntaxin Tlg2p, or of both Tlg1p and Tlg2p, had remarkably little effect on cargo transport through the secretory pathway in yeast (Holthuis et al., 1998). However, we did not exclude the possibility that Pep12p, or even Vam3p, might provide secretory functions lacking in tlg mutants. This prompted us to investigate to what extent secretion can occur independently of all four syntaxins present in late Golgi or endocytic organelles. To address this issue, we mated a haploid Δtlg2 Δpep12 mutant with a wild-type strain (SEY6211). After disruption of one TLG1 allele in the resultant diploid, the cells were transformed with a Tlg1p-encoding plasmid and sporulated, yielding a haploid strain (JHY043) that carried the disrupted tlg1, tlg2, and pep12 genes, together with the TLG1-containing plasmid. This triple-deletion strain readily lost the Tlg1p-encoding plasmid, and the absence of any detectable Tlg1p, Tlg2p, or Pep12p protein was confirmed by immunoblotting. The ease with which we were able to make this strain was surprising because we had found previously that a tlg1 pep12 double mutant was almost inviable, with only rare cells escaping (Holthuis et al., 1998). The difference in viability was not simply caused by the tlg2 mutation, because complementation of this in the triple mutant had no ill effect, but it may be that adaptation of the cells to growth without Tlg2p or Pep12p facilitated subsequent loss of the TLG1 gene. Although viable, JHY043 cells grew more slowly than did wild-type cells at 25°C, and at a temperature of 30°C or above, growth was blocked. Genetic disruption of VAM3 in JHY043 did not increase the severity of the growth phenotype. Thus we were able to establish a strain (JHY046) that lacked Tlg1p, Tlg2p, Pep12p, and Vam3p and that grew at 25°C.
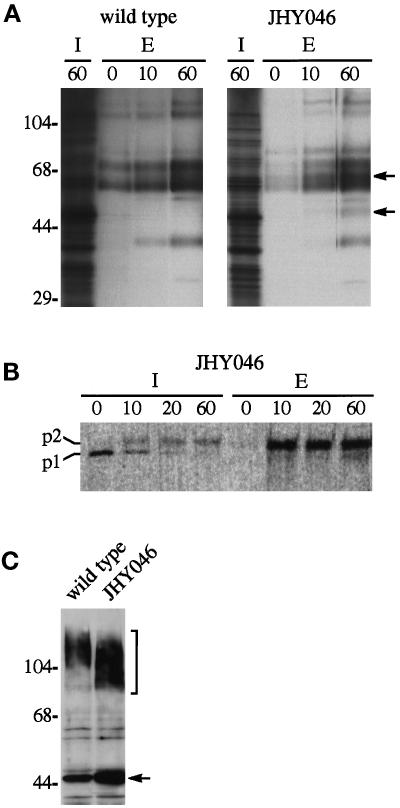
As a further test of secretory competence of JHY046 cells, we pulsed them with 35S-labeled amino acids and monitored the appearance of newly synthesized, concanavalin A–binding glycoproteins in the medium. Figure 1A shows that JHY046 cells secrete several glycoproteins whose overall abundance and kinetics of secretion are similar to those released by wild-type cells, with at most a short delay in the release of proteins from the mutant. However, some glycoprotein species secreted by JHY046 were less evident in the culture medium of wild-type cells (Figure 1A, arrows). These might represent vacuolar enzymes that are missorted to the plasma membrane. Indeed the vacuolar protease CPY, which is produced as a p1 precursor in the ER and subsequently glycosylated to a larger p2 form in the Golgi, fails to undergo normal vacuolar processing in JHY046 cells and instead is secreted in the p2 form (Figure 1B). The kinetics of p2 CPY secretion was similar to that found for other secretory glycoproteins, consistent with the notion that forward transport of proteins through the Golgi is not seriously impaired in JHY046 cells. Whereas glycosylation of p1 CPY in the Golgi appeared unaffected, we found that the periplasmic secretory enzyme invertase produced in JHY046 cells had shorter outer polysaccharide chains than normal (Figure 1C). A similar degree of invertase underglycosylation can be observed in Δtlg1 cells, in which it has been ascribed to a loss of late-acting Golgi enzymes whose efficient retrieval from the endosomal system requires Tlg1p (Holthuis et al., 1998).
Figure 1.
Secretion by cells lacking Tlg1p, Tlg2p, Pep12p, and Vam3p. (A) Secretion of glycoproteins. Cultures from wild-type and quadruple-deletion (JHY046) strains were pulsed with 35S-labeled amino acids for 10 min and then chased for the times indicated (in minutes). The cultures were separated into intracellular (I) and extracellular (E) fractions, and glycoproteins were precipitated from extracellular fractions as described in MATERIALS AND METHODS. Arrows mark radioactive bands present in the medium from JHY046 cells only. Molecular weight markers (kDa) are indicated on the left. (B) Secretion of CPY. JHY046 cells were pulse-labeled for 10 min, chased for the indicated times (in minutes), and CPY precipitated from lysed cells (I) and the medium (E). The p1 and p2 precursors are indicated; no mature CPY was detected. (C) Glycosylation of invertase. Cells expressing myc-tagged invertase were analyzed by immunoblotting. Because the myc tag is removed from secreted molecules by proteolysis, this shows mostly internal invertase. The mutant cells show a reduction in the size of mature invertase (bracket) and a slight increase in the proportion of invertase that fails to be inserted into the ER (arrow), as observed for a Δtlg1 strain (Holthuis et al., 1998).
Taken together, the above results indicate that protein secretion in yeast can occur independently of late Golgi and endosomal syntaxins. Our previous observation that secretion continues in the absence of Tlg1p and Tlg2p cannot therefore be explained by their function being provided by Pep12p or by Vam3p.
Subcellular Distribution of Organellar Markers
To investigate the consequences of the simultaneous loss of endosomal and late Golgi syntaxins for the compartmental organization of a cell, we compared the ultrastructural appearance of JHY046 with that of wild-type cells. No obvious abnormalities were found with respect to the size and structure of the ER and nuclear envelope. JHY046 cells also contained horseshoe-like membrane structures characteristic of Golgi cisternae (Figure 2). However, the cells lacked recognizable vacuoles and contained a considerable number of darkly stained circular structures of unknown identity, as observed previously in a tlg1 mutant (Holthuis et al., 1998). Most strikingly, the cells contained large numbers of small vesicles, which often appeared in clusters (see Figure 2). These may represent Golgi-derived transport vesicles that would normally have been destined for endocytic compartments and/or plasma membrane–derived endocytic vesicles.
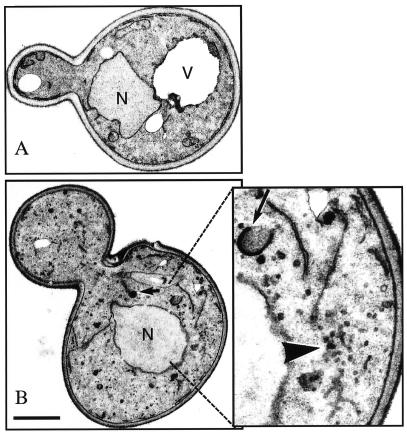
Figure 2.
Electron microscopy of JHY046 cells. (A) A wild-type (SEY6210) cell. (B) A quadruple-mutant (JHY046) cell. Note the abundant vesicles. The long arrow indicates a typical Golgi cisterna; the short arrow indicates one of the darkly staining structures previously described in Δtlg1 cells. The large arrowhead indicates a cluster of 40-nm vesicles. Bar, 1 μm; the right-hand panel in B is enlarged threefold. N, nucleus; V, vacuole.
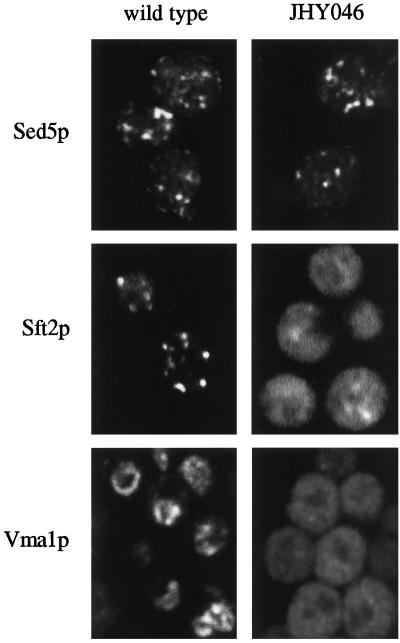
In agreement with this, immunofluorescent staining of the vacuolar ATPase component Vma1p in JHY046 cells showed the diffuse distribution expected for many small vesicles, in contrast to the ring-like vacuolar staining observed in wild-type cells (Figure 3). The late Golgi marker GFP-Sft2p (Wooding and Pelham, 1998) also showed a predominantly diffuse pattern, together with some more punctate fluorescence. The diffuse fluorescence may represent GFP-Sft2p that has entered endosome-directed vesicles and become trapped in them. In contrast, the cis-Golgi marker Sed5p gave a typical punctate Golgi pattern in both wild-type and JHY046 cells (Figure 3).
Figure 3.
Distribution of organelle markers in JHY046 cells. Sed5p and Vma1p were detected by immunofluorescence. Sft2p localization was determined using a GFP-tagged construct (Wooding and Pelham, 1998). Cells were imaged in one plane by confocal microscopy.
As a complementary method to assess the intracellular distribution of organellar markers, we also separated membranes on equilibrium sucrose density gradients (Figure 4). As expected, Sed5p fractionated similarly in membranes from wild-type and JHY046 cells. More surprisingly, Vma1p and the late Golgi marker Kex2p also showed broadly similar profiles in wild-type and quadruple-mutant cells, being separated not only from plasma membrane and cis-Golgi but also from each other. Although both are thought normally to pass or cycle through a prevacuolar compartment (Stack et al., 1995; Bryant and Stevens, 1997), it seems that they can segregate from each other and enter membranes of characteristic density, even without the SNAREs required to form late endosomes and vacuoles.
Syntaxins Tlg1p and Pep12p Independently Mediate Endocytic Traffic
The results described above complement our previous indications that the primary roles of Tlg1p and Tlg2p are not in secretion but rather in mediating retrieval of late Golgi proteins from the endocytic system and/or in endocytosis itself (Holthuis et al., 1998). To investigate endocytosis, we exposed a series of syntaxin mutant strains to the lipid dye FM4-64, an endocytic tracer that allows visualization of membrane traffic from the plasma membrane to the vacuole. After internalization, the dye is delivered to the vacuole via intermediate endosomal structures in a time-, temperature-, and energy-dependent manner (Vida and Emr, 1995).
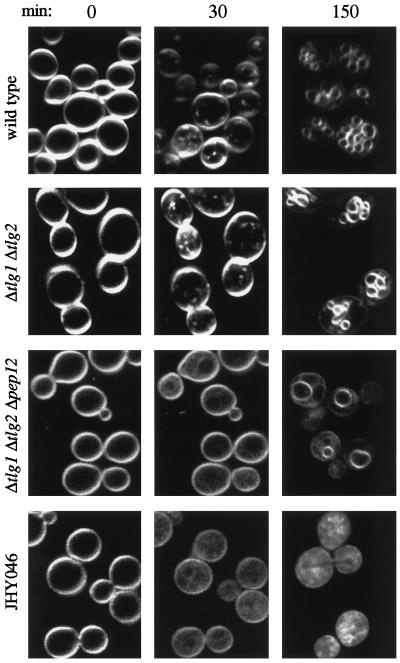
Wild-type cells visualized immediately upon exposure to FM4-64 displayed a bright fluorescent staining of the plasma membrane (Figure 5, 0-min time point). Within 10 min of incubation at 25°C, small fluorescent dots appeared in the cytoplasm that became increasingly brighter over the next 20 min (Figure 5, 30-min time point). The staining of these endosomal intermediates subsequently decreased concomitantly with the appearance of FM4-64 in vacuolar membranes, and after 150 min, staining was nearly exclusive to the vacuoles (Figure 5). In cells lacking both Tlg1p and Tlg2p, FM4-64–labeled endosomal intermediates appeared with kinetics similar to that seen in wild-type cells, and subsequent delivery to the vacuole was not impaired in any obvious way (Figure 5). Additional removal of Pep12p completely abolished the formation of FM4-64–stained endosomal structures; instead we found a diffuse cytoplasmic staining whose intensity increased over time together with a gradual loss of plasma membrane staining, indicating considerable endocytic traffic from the plasma membrane (Figure 5). Internalized FM4-64 eventually reached the vacuole, although at a considerably slower rate than that in wild-type or Δtlg1 Δtlg2 cells. Uptake of FM4-64 by JHY046 cells occurred with an efficiency similar to that in the Δtlg1 Δtlg2 Δpep12 mutant and gave rise to a diffuse cytoplasmic staining whose intensity increased with time. However, even after prolonged incubation, JHY046 cells remained devoid of vacuolar membrane structures, as expected given our failure to detect such organelles by electron microscopy (EM) and immunofluorescence microscopy.
Figure 5.
Uptake of FM4-64 by syntaxin-deletion mutants. Cells were grown to exponential phase, harvested, and exposed to FM4-64 for the indicated times (in minutes) at 25°C. Note that in each case the 0- and 30-min time points show confocal images of the same cells.
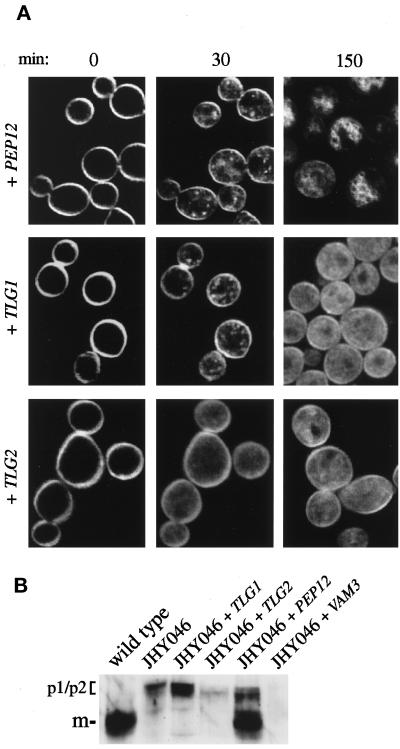
The above findings show that Pep12p has a critical role in the formation and/or maintenance of an endosomal compartment accessible to membrane traffic from the plasma membrane but that Tlg1p and Tlg2p are not essential for traffic to this compartment. These findings do not, however, eliminate the possibility that Tlg1p and/or Tlg2p can mediate endocytic trafficking independently of Pep12p. To assess the contribution of individual syntaxins to this process, we performed FM4-64 uptake experiments in JHY046 cells expressing Pep12p, Tlg1p, or Tlg2p from single copy plasmids. The levels of these proteins, as estimated by immunoblotting, were elevated compared with that in wild-type cells: ∼4-fold for Tlg1p and 5- to 10-fold for Tlg2p and Pep12p. As shown in Figure 6A, expression of Pep12p restored, at least partially, the ability of cells to form endosomal intermediates that were accessible to FM4-64. These structures became visible within 10 min of exposure to the dye. After 2–3 h, many cells contained FM4-64–labeled rings, structures reminiscent of the small vacuole-like organelles seen in a Δvam3 mutant (Darsow et al., 1997; Nichols et al., 1997; Wada et al., 1997). JHY046 cells expressing Tlg1p were also capable of forming endosomal intermediates (Figure 6A, 30-min time point) that were labeled with FM4-64 within 10 min of its addition to the cells. However, staining of these structures appeared transient and seemed to fade over time, being replaced by a diffuse and homogenous staining of the cytoplasm (Figure 6A, 150-min time point). Tlg2p expressed in JHY046 cells failed to generate endosomal intermediates that were accessible to FM4-64; even after prolonged incubation, the dye only gave a diffuse cytoplasmic staining indistinguishable from that found in JHY046 control cells.
Figure 6.
Functions of single syntaxins expressed in JHY046 cells. (A) Uptake of FM4-64. FM4-64 uptake was performed as described in Figure 5. (B) Immunoblotting of intracellular CPY. Tlg1p, Tlg2p, and Pep12p were expressed from single copy plasmids described in MATERIALS AND METHODS. Vam3p was expressed from its normal chromosomal location. The positions of the precursor (p1/p2) and mature (m) forms of CPY are indicated.
These results suggest that Tlg1p and Pep12p play independent roles in endocytic trafficking. Both induce endosomal intermediates that are accessible to membrane traffic from the plasma membrane. However, whereas Tlg1p generates only transiently labeled structures, Pep12p can also create inflated vacuole-like organelles, consistent with its ability to partially suppress vam3 mutations (Darsow et al., 1997; Gotte and Gallwitz, 1997). In agreement with this, expression of Pep12p resulted in the intracellular accumulation and proteolytic processing of CPY in JHY046 cells, whereas expression of Tlg1p caused at most a slight accumulation of unprocessed precursor (Figure 6B). This property of Pep12p was specific. Tlg2p had little effect, and expression of Vam3p at normal levels, although sufficient to create morphologically recognizable vacuoles, did not restore CPY sorting or maturation (Figure 6B).
Pep12p and Tlg1p Cofractionate with Distinct Endocytic Markers
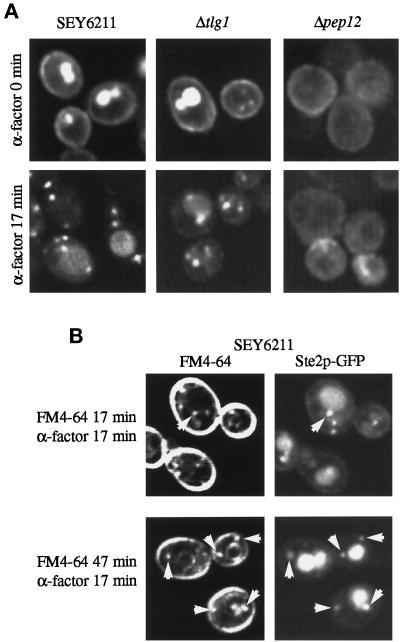
As an approach to further define the transport routes mediated by Pep12p and Tlg1p, we compared their subcellular distributions with those of specific endocytic markers. Ste2p, the α factor receptor, is constitutively endocytosed at a slow rate. Internalization of Ste2p is stimulated 5- to 10-fold when cells are exposed to α factor. After internalization, the receptor is transported to the vacuole where it is degraded (Schandel and Jenness, 1994). Endosomal intermediates containing the receptor can be visualized by immunofluorescence as punctate structures induced by the addition of α factor.
We used a version of the receptor tagged with green fluorescent protein (Ste2p-GFP) to follow its endocytosis in living cells and to compare the labeled structures directly with those accessible to FM4-64. Before addition of α factor, GFP fluorescence was visible at the plasma membrane, as well as in the vacuole where constitutive turnover of the receptor occurs. Addition of α factor induced the formation of punctate fluorescent structures, some but not all of which were adjacent to vacuoles (Figure 7A). These likely correspond to a Pep12p-containing compartment, because in a Δpep12 strain, the GFP dots were abolished and only hazy fluorescence was observed (Figure 7A). In cells lacking Tlg1p, there were some punctate structures containing Ste2p-GFP even before the addition of α factor. However, many cells still displayed clear surface fluorescence. After addition of α factor, labeling of the plasma membrane was no longer apparent, and punctate endosomal intermediates were more prominent. Thus, endocytosis of Ste2p-GFP to the vacuole requires Pep12p but not Tlg1p.
Figure 7.
Comparison of Ste2p-GFP and FM4-64 uptake. (A) Ste2p-GFP visualized in the indicated strains at 25°C before and 17 min after addition of α factor. Large bright structures are vacuoles. (B) Double label of Ste2p-GFP and FM4-64 17 min after addition of α factor. FM4-64 was added either with the α factor or 30 min before. Arrows indicate structures labeled with both markers.
In wild-type cells, FM4-64 added at the same time as the α factor also labeled punctate structures, but many of these did not contain GFP-Ste2p, and conversely there were GFP-labeled structures that contained little FM4-64 (see Figure 7B for examples). Only when the dye was added 30 min before the α factor did we observe good correspondence between the FM4-64– and GFP-labeled structures. It seems that the receptor accumulates in a subset of endosomes that are preferentially labeled by FM4-64 at later times after its addition. The other FM4-64–positive structures must represent endosomes either that do not receive Ste2p or through which it passes rapidly. It may be that they contain Tlg1p, given that this syntaxin can create such structures but is not required for Ste2p internalization.
Others have reported that two distinct endosomal intermediates can be detected when α factor internalization is followed by subcellular fractionation (Singer-Kruger et al., 1993; Hicke et al., 1997), raising the possibility that the receptor normally passes through the Tlg1p compartment before reaching late endosomes. We therefore analyzed receptor distribution using the same incubation conditions as Singer-Kruger et al. (1993) (addition of α factor at 15°C in the presence of cycloheximide) and a fractionation procedure that separates Tlg1p from Pep12p. To aid detection of Ste2p, we used a yeast strain in which the endogenously expressed receptor carried three copies of the HA epitope at its C terminus. The epitope-tagged receptor was functional and underwent ligand-induced internalization and degradation with normal kinetics (our unpublished observations).
Figure 8 shows that before α factor addition Ste2p was found in three peaks on the sucrose density gradient. One (fractions 15 and 16) was at the position of plasma membrane markers; this material is underrepresented because the plasma membrane is partially removed by the preliminary 10,000 × g centrifugation step in our fractionation procedure. The remaining two peaks coincided with the endosomal and vacuolar markers Pep12p (fraction 7) and Vam3p (fraction 4). Very little receptor was found in the position of Tlg1p (or Tlg2p). After addition of α factor, there was a progressive shift of the receptor from the plasma membrane peak to the Pep12p and Vam3p peaks, followed by the disappearance of the receptor because of degradation. Although there was some receptor detectable in fractions 9–12, we did not observe a substantial peak associated with the Tlg1p compartment at any stage. It appears that Ste2p passed from the cell surface to Pep12p-containing endosomes and then to the vacuole, either bypassing the Tlg1p compartment or passing through it rapidly so that it did not accumulate there. These results are consistent with our observations of the behaviour of GFP-Ste2p.
As a second endocytic marker, we used the chitin synthase subunit Chs3p. Delivery of this protein to the bud neck has been reported previously to involve its mobilization from a specialized compartment, called the chitosome (Chuang and Schekman, 1996; Ziman et al., 1996). Maintenance of this compartment requires endocytosis, and Chs3p is thought to be continuously recycled from the plasma membrane. Chs3p thus provides an endocytic marker whose trafficking diverges from that of Ste2p. This prompted us to compare the subcellular distribution of Chs3p with that of Pep12p, Tlg1p, and Tlg2p. Figure 9A shows that the intracellular pool of Chs3p fractionated in two peaks coincident with the peaks of Tlg1p and Tlg2p; these were distinct from the peak of Pep12p. Hence it appears that internalized Chs3p resides in compartments defined by Tlg1p and Tlg2p. Note that in this experiment little Chs3p was detected at the density of plasma membrane (fraction 15; see Figure 4); the cell surface Chs3p was efficiently removed during the 10,000 × g precentrifugation.
We used the temperature-sensitive end4-1 mutant that is defective for internalization from the plasma membrane (Raths et al., 1993) to verify that the internal pool of Chs3p is recycling via the cell surface. After a 30-min incubation of this mutant at the nonpermissive temperature, all the Chs3p detectable on the sucrose gradient was at a density corresponding to the plasma membrane, marked by Sso2p (Figure 9B). A portion of the Tlg1p also cofractionated with Sso2p, in contrast to the normal situation in which Tlg1p is well separated from plasma membrane markers (see, for example, Figure 8). This suggests that there is some transport of Tlg1p to the surface, although the bulk was segregated from Chs3p and remained at its normal density.
Tlg1p Mediates Trafficking of Chs3p to Polarized Growth Sites
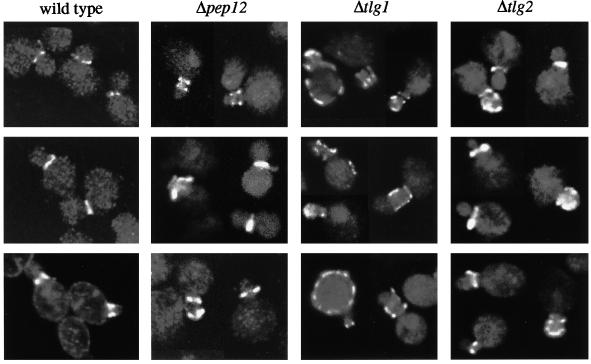
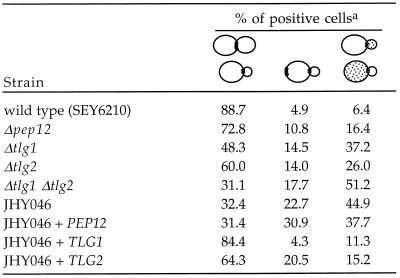
Because Chs3p appeared to pass through the compartments containing Tlg1p and Tlg2p, we sought to determine to what extent Chs3p trafficking relies on these syntaxins. We analyzed the location of Chs3p in our wild-type strain (SEY6210) and in a series of syntaxin mutants, using indirect immunofluorescence. In almost 90% of budding SEY6210 cells in which cell surface staining of Chs3p was visible, it was restricted to a narrow strip or ring at the neck between the mother cell and the emerging bud (Figure 10 and Table 1). A few cells, presumably those in the process of relocalizing the enzyme to the new bud, showed bipolar or only partially localized staining. In cells lacking Tlg1p, the discrete staining pattern was partially lost; of the budding cells showing surface staining for Chs3p, fewer than one-half had a clear neck ring. A substantial number displayed patches of cell surface staining distributed around the mother cell or bud, or sometimes both, whereas others showed staining adjacent to the bud or on the opposite pole of the mother cell, indicating inefficient extraction of Chs3p from previous bud sites (Figure 10 and Table 1). After removal of Pep12p or Tlg2p, we also found some mislocalization of Chs3p but at a much lower frequency than that found in Δtlg1 cells (Figure 10 and Table 1). Δtlg1 Δtlg2 double mutants, on the other hand, displayed a severe Chs3p mislocalization phenotype, with a considerable rise in the portion of budding cells exhibiting randomly distributed patches of Chs3p on the surface of mothers and/or buds (Table 1).
Figure 10.
Location of Chs3p in syntaxin mutants. Collages of immunofluorescence images are shown. In wild-type and Δpep12 cells, surface staining is primarily restricted to a single or double ring at the neck; Δtlg1 and to a lesser extent Δtlg2 cells show more delocalized staining of the bud and, especially in the Δtlg1 strain, the mother cells. Note that images were selected to illustrate surface staining; many medium-budded cells did not show any of this.
Table 1.
Distribution of Chs3p surface staining
At least 200 budding cells showing clear surface staining were counted for each strain, and the patterns categorized into localized neck staining, bipolar staining, and dislocalised patches in mother and/or bud, as indicated.
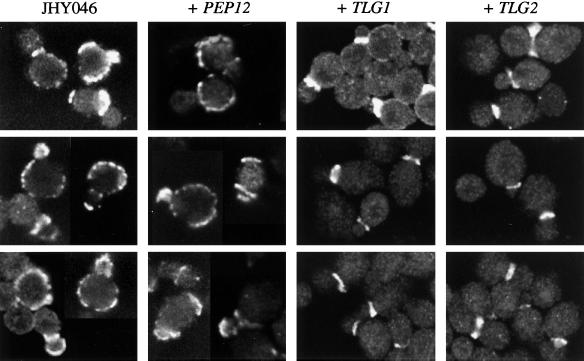
A very substantial degree of Chs3p mislocalization was also observed in JHY046 cells (with less than one-third of budding cells showing clear neck rings [Figure 11 and Table 1]). Expression of Pep12p failed to suppress this mislocalization phenotype of JHY046. However, expression of Tlg1p resulted in a dramatic recovery, with nearly 85% of Chs3p-positive cells displaying a discrete ring at the boundary of the mother and emerging bud (Figure 11 and Table 1). Tlg2p also partially restored Chs3p localization in JHY046, although less efficiently than did Tlg1p.
Figure 11.
Rescue of the Chs3p localization phenotype of JHY046 cells by Tlg1p and Tlg2p but not by Pep12p. Chs3p was visualized by immunofluorescence, using the strains described in Figure 6.
The mislocalization of Chs3p was also reflected in the distribution of chitin in the cell walls of the various strains, as revealed by visual inspection of cells stained with calcofluor white. Mutants lacking Tlg1p and Tlg2p showed less prominent staining of bud necks and scars above the background of general cell wall staining, an effect that could be reversed by expression of Tlg1p in the quadruple mutant (our unpublished observations). However, this assay proved harder to quantify than the immunofluorescence assay described above.
Collectively, these findings indicate that Tlg1p and to a lesser extent Tlg2p play a critical role in the timely and efficient delivery of Chs3p to its site of action in budding cells. Pep12p, despite its ability to promote endocytosis, cannot perform this function.
DISCUSSION
In this manuscript we have sought to clarify the roles of Tlg1p and Tlg2p in the exocytic and endocytic pathways. Perhaps surprisingly, we found that yeast cells can grow without any of the four syntaxins implicated in endocytosis; syntaxins in the ER (Ufe1p), cis-Golgi (Sed5p), and plasma membrane (Sso1/2p) are sufficient. Secretion and segregation of early and late Golgi markers can still occur, although at least one late Golgi marker appears to be primarily vesicular, consistent with a role for Tlg1p and Tlg2p in retrieval of such proteins. As we have pointed out previously, a cisternal maturation model would allow segregation of Golgi markers with only a single Golgi syntaxin (Nichols and Pelham, 1998; Pelham, 1998). We can now say with certainty that the lack of an essential requirement for Tlg1p or Tlg2p is not because their function was taken over by Pep12p or Vam3p.
The dispensability of the endocytic syntaxins has allowed an assessment of the function of each one in the absence of the others. Our results confirm the central role of Pep12p in the delivery of material to the vacuole both from the Golgi complex and from the plasma membrane. Vam3p alone is sufficient to create vacuoles, which presumably receive membrane via the specialized direct route from the Golgi (Cowles et al., 1997; Piper et al., 1997), but these vacuoles lack CPY, and the endocytic tracer FM4-64 reaches them from the cell surface only slowly. Pep12p, on the other hand, creates endocytic structures that both contain mature CPY and are efficiently labeled by FM4-64. In the absence of the other endocytic syntaxins, it is likely that vesicles derived from the plasma membrane fuse directly with Pep12p-induced structures. This is presumably mediated by a plasma membrane–derived v-SNARE such as Snc1p (Protopopov et al., 1993), and indeed we have observed coprecipitation of Pep12p and Snc1p (our unpublished observations). A direct route from the cell surface to Pep12p-containing membranes is also indicated by our analysis of cells containing both Pep12p and Vam3p but lacking Tlg1p and Tlg2p. In such cells FM4-64 is internalized and passes through a punctate endocytic compartment en route to the vacuole. Furthermore, although Ste2p is degraded less efficiently in the absence of Tlg1p (Holthuis et al., 1998), we have shown that endocytosis itself is not blocked; the effect may at least in part be an indirect one, at the level of vacuolar proteolysis.
Tlg1p is able to induce the formation of structures accessible to FM4-64, consistent with our previous observation that it binds to Snc1p. Because there is considerable overlap between the distributions of Tlg2p and Tlg1p, this fits the observation of Abeliovich et al. (1998) that structures containing GFP-tagged Tlg2p can be labeled with FM4-64. Indeed, although Tlg1p and Tlg2p tend to be concentrated in membranes of different densities, we have found no marker that is completely restricted to one or the other density, and complexes containing both Tlg1p and Tlg2p can be detected by immunoprecipitation (Nichols et al., 1998). Because proteins likely to be in the yeast equivalent of the TGN (such as Kex2p) colocalize with these syntaxins, Tlg1p and Tlg2p can perhaps both be considered to be components of the TGN, although this may be heterogeneous in nature. On the other hand, the accessibility of these membranes to at least some endocytic tracers means that it is equally valid to call them endosomes. We suggest that this ambiguity exists because the Tlg proteins cycle between structures at the junction between the endocytic and exocytic pathways; for simplicity, we use the term TGN. Because TGN and early endosomal membranes are constantly turning over, it is perhaps not surprising that the FM4-64 labeling of such structures is only transient.
The protein that most clearly recycles through the TGN is the major chitin synthase Chs3p. It has been well established that this protein is recovered from the cell surface and dwells in an endocytic compartment before being delivered to the bud neck at the appropriate stage of the cell cycle (Chuang and Schekman, 1996; Ziman et al., 1996). Although the internal structures in which it is found, termed chitosomes, have been suggested to be specialized storage compartments, an alternative view would be that the enzyme recycles continuously and is localized at the bud neck only when appropriate interactions trap it there (DeMarini et al., 1997; Ziman et al., 1996). Indeed, the internalized material can be chased to late secretory vesicles (Chuang and Schekman, 1996) or to the plasma membrane when vesicle fusion or endocytosis is blocked. We find that it cofractionates with Tlg1p and Tlg2p, in agreement with previous immunofluorescence studies showing coincidence with Kex2p (Santos and Snyder, 1997). Most strikingly, we have shown that Tlg1p, in the absence of the other endocytic syntaxins, is sufficient to direct Chs3p to its correct location. Tlg2p can also perform this function, although less well. In addition, Tlg1p is necessary for efficient relocalization of Chs3p, explaining our previous finding that Δtlg1 cells have morphological defects reminiscent of chs3 mutants (Holthuis et al., 1998).
Whether all the structures that have been referred to as chitosomes are equivalent remains to be seen. Two other proteins, Chs5p and Chs6p, are required for Chs3p to reach the surface, and in their absence Chs3p accumulates in intracellular patches (Santos and Snyder, 1997; Ziman et al., 1998). While these may correspond to the TGN, it is also possible that the transport-incompetent forms of Chs3p move to a later endosomal compartment or lead to the creation of a novel structure. Detailed comparisons with the locations of the syntaxins or other markers will be required to address this issue.
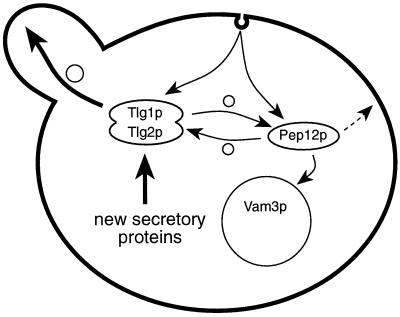
Recycling of Chs3p through the TGN is probably required for Chs3p to enter vesicles that are directed to the appropriate part of the plasma membrane. In the absence of such recycling, only newly synthesized Chs3p would be delivered to the bud. Although Pep12p can sustain endocytosis, Chs3p either is not efficiently directed to the Pep12p compartment or, if Chs3p recycles through it, is not redelivered to the cell surface in a polarized manner. Indeed, two classes of late secretory vesicles have been identified, one of which may carry the small GTPase Ypt1p rather than Sec4p (Harsay and Bretscher, 1995; Mulholland et al., 1997); it is possible that these vesicles are derived from endosomes and are not delivered specifically to the bud, a process that is likely to be mediated by Sec4p (Walch-Solimena et al., 1997). Figure 12 outlines, in simple form, the possible pathways that proteins might take through the endocytic system.
Figure 12.
Simplified diagram of the endocytic pathways proposed. Uptake can occur to organelles containing either Tlg1p or Pep12p, but only Tlg1p gives access to the polarized exocytic pathway. Segregation of proteins destined for the vacuole from those directed to the TGN could occur in principle at the plasma membrane, in the Tlg1p or Pep12p compartments, or in an earlier endocytic structure. Note that the syntaxins themselves can move between compartments and may populate multiple structures at different stages of maturation. The dashed arrow indicates a possible nonpolarized route from endosomes to the surface. Small circles indicate vesicular traffic.
Other proteins may also recycle via the TGN. An obvious example is the v-SNARE Snc1p itself, which after mediating fusion of post-Golgi vesicles with the plasma membrane (Protopopov et al., 1993) and subsequently of endocytic vesicles has to be recycled for reuse in the exocytic pathway. We have recently observed that the normal distribution of Snc1p is perturbed in tlg1 mutants; instead of being primarily on the plasma membrane in the steady state, a significant portion appears to be trapped in late endosomes, consistent with a failure of endocytosed molecules to reach the TGN (Lewis and Pelham, unpublished observations). Evidently the rate of synthesis of Snc1p and Snc2p is sufficient to sustain secretion even when recycling is inhibited.
A question that remains is where proteins such as Ste2p and Chs3p separate during endocytosis. We have shown that Pep12p is not required for Chs3p to reach the TGN and that Tlg1p is not required for Ste2p to reach late endosomes. Furthermore, we could not detect substantial quantities of Ste2p cofractionating with Tlg1p or of Chs3p cofractionating with Pep12p. This contrasts with an earlier study in which internalized Ste2p and Chs3p were found to be present in membranes of similar density (Ziman et al., 1996), although because other TGN or late endosomal markers were not examined it is hard to deduce which compartment(s) these represent. In any case, at least two possibilities can be considered. One is that the two endocytic pathways operate independently, with proteins being sorted into different classes of endocytic vesicle at the cell surface and transported to different destinations. An alternative is that endocytic vesicles normally fuse with the Tlg1p compartment or randomly with this and with Pep12p-containing membranes, and the proteins are then rapidly sorted out and redistributed between these compartments (Figure 12). If so, the system must be flexible, because mutants such as vps45 that block Golgi-to-endosome transport do not prevent endocytic traffic to the vacuole (Bryant et al., 1998).
Yet another possibility is that endocytic vesicles fuse with each other, and with TGN-derived vesicles bearing Tlg1p, to form early endosomes. The resultant structures could then segregate distinct classes of endocytic carrier that fuse with the TGN and with late endosomes. This would be quite similar to the sorting processes observed in mammalian cells (Gruenberg and Maxfield, 1995) and is consistent with morphological studies of yeast endocytosis at the EM level (Prescianotto-Baschong and Riezman, 1998). Implicit in this model is the likelihood that Tlg1p and perhaps also Pep12p will be present in a variety of structures of different composition. Indeed, we have found that procedures used previously to separate early and late endosomes yield fractions that each contain both Pep12p and Tlg1p (Singer-Kruger et al., 1993; Hicke et al., 1997) (our unpublished observations).
Our experiments have not revealed a clear role that is specific for Tlg2p. In several ways, Tlg2p behaves as a less effective version of Tlg1p. Thus, both Tlg1p and Tlg2p are required for retrieval of TGN proteins, and both can mediate Chs3p recycling, although Tlg2p is less efficient at this and does not yield FM4-64–labeled endosomes. One possibility is that Tlg2p specializes in traffic from late endosomes, whereas Tlg1p is better at receiving endocytic vesicles. Another possibility is that Tlg2p cycles more readily through early Golgi cisternae and thus provides a target for the subsequent delivery of other TGN proteins, including Tlg1p, from endosomes. This might provide a role for the Tlg1p/Tlg2p complexes that we have recently observed (Nichols et al., 1998). Further clues may come from studies of the homologues of these proteins in higher cells. The best candidate for a Tlg1p equivalent is syntaxin 6, which although quite divergent is more closely related to Tlg1p than to other yeast syntaxins, is present on TGN membranes, and binds to the Snc1p homologue cellubrevin (Bock et al., 1997). Syntaxin 6 is present on immature secretory granules and appears to be retrieved from them in vesicles destined for late endosomes (Klumperman et al., 1998). A mammalian protein, syntaxin 16, with pronounced similarity to Tlg2p has also been shown to be localized to the Golgi complex by immunofluorescence (Simonsen et al., 1998), but its distribution has not yet been compared with that of syntaxin 6 at the EM level.
In conclusion, our studies have delineated two distinct endocytic pathways in yeast that can operate independently, one passing through Pep12p-containing membranes to the vacuole and one delivering material to the TGN for reexport. The α factor receptor Ste2p follows the first of these, whereas Chs3p follows the second, with the delivery of Chs3p to the exocytic pathway being mediated by Tlg1p and Tlg2p.
ACKNOWLEDGMENTS
We are grateful to Randy Schekman for anti-Chs3p antibodies, Sirkka Keränen for anti-Sso2p antibodies, Rob Arkowitz for the strain expressing Ste2p-GFP, and Howard Riezman for the end4-1 mutant. We also thank Douglas Kershaw for expert assistance with the electron microscopy and Sean Munro for comments on the manuscript. J.C.M.H. was supported by a training fellowship from the European Commission.
REFERENCES
- Aalto MK, Ronne H, Keränen S. Yeast syntaxins Sso1p and Sso2p belong to a family of related membrane proteins that function in vesicular transport. EMBO J. 1993;12:4095–4104. doi: 10.1002/j.1460-2075.1993.tb06093.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abeliovich H, Grote E, Novick P, Ferro-Novick S. Tlg2p, a yeast syntaxin homolog that resides on the Golgi and endocytic structures. J Biol Chem. 1998;273:11719–11727. doi: 10.1074/jbc.273.19.11719. [DOI] [PubMed] [Google Scholar]
- Banfield DK, Lewis MJ, Pelham HRB. A SNARE-like protein required for traffic through the Golgi complex. Nature. 1995;375:806–809. doi: 10.1038/375806a0. [DOI] [PubMed] [Google Scholar]
- Becherer KA, Rieder SE, Emr SD, Jones EW. Novel syntaxin homologue, Pep12p, required for the sorting of lumenal hydrolyses to the lysosome-like vacuole in yeast. Mol Biol Cell. 1996;7:579–594. doi: 10.1091/mbc.7.4.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett MK, Scheller RH. The molecular machinery for secretion is conserved from yeast to neurons. Proc Natl Acad Sci USA. 1993;90:2559–2563. doi: 10.1073/pnas.90.7.2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bock JB, Klumperman J, Davanger S, Scheller RH. Syntaxin 6 functions in trans-Golgi network vesicle trafficking. Mol Biol Cell. 1997;8:1261–1271. doi: 10.1091/mbc.8.7.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant NJ, Piper RC, Gerrard SR, Stevens TH. Traffic into the prevacuolar/endosomal compartment of Saccharomyces cerevisiae: a VPS45-dependent intracellular route and a VPS45-independent, endocytic route. Eur J Cell Biol. 1998;76:43–52. doi: 10.1016/S0171-9335(98)80016-2. [DOI] [PubMed] [Google Scholar]
- Bryant NJ, Stevens TH. Two separate signals act independently to localize a yeast late Golgi membrane protein through a combination of retrieval and retention. J Cell Biol. 1997;136:287–297. doi: 10.1083/jcb.136.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang JS, Schekman RW. Differential trafficking and timed localization of two chitin synthase proteins, Chs2p and Chs3p. J Cell Biol. 1996;135:597–610. doi: 10.1083/jcb.135.3.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowles CR, Snyder WB, Burd CG, Emr SD. Novel Golgi to vacuole delivery pathway in yeast: identification of a sorting determinant and required transport component. EMBO J. 1997;16:2769–2782. doi: 10.1093/emboj/16.10.2769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darsow T, Rieder SE, Emr SE. A multi-specificity syntaxin-homologue, Vam3p, essential for autophagy and biosynthetic protein transport to the vacuole. J Cell Biol. 1997;138:517–529. doi: 10.1083/jcb.138.3.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMarini DJ, Adams AE, Fares H, De Virgilio C, Valle G, Chuang JS, Pringle JR. A septin-based hierarchy of proteins required for localized deposition of chitin in the Saccharomyces cerevisiae cell wall. J Cell Biol. 1997;139:75–93. doi: 10.1083/jcb.139.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elble R. A simple and efficient procedure for transformation of yeast. Biotechniques. 1992;13:18–20. [PubMed] [Google Scholar]
- Fischer von Mollard G, Nothwehr SF, Stevens T. The yeast v-SNARE Vti1p mediates two vesicle transport pathways through interactions with the t-SNAREs Sed5p and Pep12p. J Cell Biol. 1997;137:1511–1524. doi: 10.1083/jcb.137.7.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotte M, Gallwitz D. High expression of the yeast syntaxin-related Vam3 protein suppresses the protein transport defects of a pep12 null mutant. FEBS Lett. 1997;411:48–52. doi: 10.1016/s0014-5793(97)00575-9. [DOI] [PubMed] [Google Scholar]
- Gruenberg J, Maxfield FR. Membrane transport in the endocytic pathway. Curr Opin Cell Biol. 1995;7:552–563. doi: 10.1016/0955-0674(95)80013-1. [DOI] [PubMed] [Google Scholar]
- Guthrie C, Fink GR, editors. Guide to Yeast Genetics and Molecular Biology. San Diego, CA: Academic Press; 1991. [Google Scholar]
- Hardwick KG, Pelham HRB. SED5 encodes a 39 kD integral membrane protein required for vesicular transport between the ER and the Golgi complex. J Cell Biol. 1992;119:513–521. doi: 10.1083/jcb.119.3.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harsay E, Bretscher A. Parallel secretory pathways to the cell surface in yeast. J Cell Biol. 1995;131:297–310. doi: 10.1083/jcb.131.2.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicke L, Zanolari B, Pypaert M, Rohrer J, Riezman H. Transport through the yeast endocytic pathway occurs through morphologically distinct compartments and requires an active secretory pathway and Sec18p/N-ethylmaleimide-sensitive fusion protein. Mol Biol Cell. 1997;8:13–31. doi: 10.1091/mbc.8.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holthuis JCM, Nichols BJ, Dhruvakumar S, Pelham HRB. Two syntaxin homologues in the TGN/endosomal system of yeast. EMBO J. 1998;17:113–126. doi: 10.1093/emboj/17.1.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser CA, Schekman R. Distinct sets of SEC genes govern transport vesicle formation and fusion early in the secretory pathway. Cell. 1990;61:723–733. doi: 10.1016/0092-8674(90)90483-u. [DOI] [PubMed] [Google Scholar]
- Klumperman J, Kuliawat R, Griffith JM, Geuze HJ, Arvan P. Mannose 6-phosphate receptors are sorted from immature secretory granules via adaptor protein AP-1, clathrin, and syntaxin 6-positive vesicles. J Cell Biol. 1998;141:359–371. doi: 10.1083/jcb.141.2.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis MJ, Pelham HRB. SNARE-mediated retrograde transport from the Golgi complex to the ER. Cell. 1996;85:205–215. doi: 10.1016/s0092-8674(00)81097-1. [DOI] [PubMed] [Google Scholar]
- Mulholland J, Wesp A, Riezman H, Botstein D. Yeast actin cytoskeleton mutants accumulate a new class of Golgi-derived secretory vesicle. Mol Biol Cell. 1997;8:1481–1499. doi: 10.1091/mbc.8.8.1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols, B.J., Holthuis, J.C.M., and Pelham, H.R.B. (1998). The Sec1p homologue Vps45p binds to the syntaxin Tlg2p. Eur. J. Cell Biol. (in press). [DOI] [PubMed]
- Nichols BJ, Pelham HRB. SNAREs and membrane fusion in the Golgi apparatus. Biochim Biophys Acta. 1998;1404:9–31. doi: 10.1016/s0167-4889(98)00044-5. [DOI] [PubMed] [Google Scholar]
- Nichols BJ, Ungermann C, Pelham HRB, Wickner WT, Haas A. Homotypic vacuolar fusion mediated by t- and v-SNAREs. Nature. 1997;387:199–202. doi: 10.1038/387199a0. [DOI] [PubMed] [Google Scholar]
- Pelham HRB. Getting through the Golgi complex. Trends Cell Biol. 1998;8:45–49. doi: 10.1016/s0962-8924(97)01185-9. [DOI] [PubMed] [Google Scholar]
- Piper RC, Bryant NJ, Stevens TH. The membrane protein alkaline phosphatase is delivered to the vacuole by a route that is distinct from the VPS-dependent pathway. J Cell Biol. 1997;138:531–545. doi: 10.1083/jcb.138.3.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prescianotto-Baschong C, Riezman H. Morphology of the yeast endocytic pathway. Mol Biol Cell. 1998;9:173–189. doi: 10.1091/mbc.9.1.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Protopopov V, Govindan B, Novick P, Gerst JE. Homologs of the synaptobrevin/VAMP family of synaptic vesicle proteins function on the late secretory pathway in S. cerevisiae. Cell. 1993;74:855–861. doi: 10.1016/0092-8674(93)90465-3. [DOI] [PubMed] [Google Scholar]
- Raths S, Rohrer J, Crausaz F, Riezman H. end3 and end4: two mutants defective in receptor-mediated and fluid-phase endocytosis in Saccharomyces cerevisiae. J Cell Biol. 1993;120:55–65. doi: 10.1083/jcb.120.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman JE. Mechanisms of intracellular protein transport. Nature. 1994;372:55–63. doi: 10.1038/372055a0. [DOI] [PubMed] [Google Scholar]
- Santos B, Snyder M. Targeting of chitin synthase 3 to polarized growth sites in yeast requires Chs5p and Myo2p. J Cell Biol. 1997;136:95–110. doi: 10.1083/jcb.136.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauer B. Functional expression of the cre-lox site-specific recombination system in the yeast Saccharomyces cerevisiae. Mol Cell Biol. 1987;7:2087–2096. doi: 10.1128/mcb.7.6.2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schandel KA, Jenness DD. Direct evidence for ligand-induced internalization of the yeast α-factor pheromone receptor. Mol Cell Biol. 1994;14:7245–7255. doi: 10.1128/mcb.14.11.7245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw JA, Mol PC, Bowers B, Silverman SJ, Valdivieso MH, Duran A, Cabib E. The function of chitin synthases 2 and 3 in the Saccharomyces cerevisiae cell cycle. J Cell Biol. 1991;114:111–123. doi: 10.1083/jcb.114.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikorski RS, Hieter P. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics. 1989;122:19–27. doi: 10.1093/genetics/122.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonsen A, Bremmes B, Ronning E, Aasland R, Stenmark H. Syntaxin 16, a putative Golgi t-SNARE. Eur J Cell Biol. 1998;75:223–231. doi: 10.1016/S0171-9335(98)80116-7. [DOI] [PubMed] [Google Scholar]
- Singer-Kruger B, Frank R, Crausaz F, Riezman H. Partial purification and characterization of early and late endosomes from yeast. Identification of four novel proteins. J Biol Chem. 1993;268:14376–14386. [PubMed] [Google Scholar]
- Stack JH, Horazdovsky BF, Emr SD. Receptor-mediated protein sorting to the vacuole in yeast: roles for a protein kinase, a lipid kinase and GTP-binding proteins. Annu Rev Cell Dev Biol. 1995;11:1–33. doi: 10.1146/annurev.cb.11.110195.000245. [DOI] [PubMed] [Google Scholar]
- Stepp JD, Huang K, Lemmon SK. The yeast adaptor protein complex, AP-3, is essential for the efficient delivery of alkaline phosphatase by the alternate pathway to the vacuole. J Cell Biol. 1997;139:1761–1774. doi: 10.1083/jcb.139.7.1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vida TA, Emr SD. A new vital stain for visualizing vacuolar membrane dynamics and endocytosis in yeast. J Cell Biol. 1995;128:779–792. doi: 10.1083/jcb.128.5.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada Y, Nakamura N, Ohsumi Y, Hirata A. Vam3p, a new member of syntaxin related protein, is required for vacuolar assembly in the yeast Saccharomyces cerevisiae. J Cell Sci. 1997;110:1299–1306. doi: 10.1242/jcs.110.11.1299. [DOI] [PubMed] [Google Scholar]
- Walch-Solimena C, Collins RN, Novick PJ. Sec2p mediates nucleotide exchange on Sec4p and is involved in polarized delivery of post-Golgi vesicles. J Cell Biol. 1997;137:1495–1509. doi: 10.1083/jcb.137.7.1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weimbs T, Low SH, Chapin SJ, Mostov KE, Bucher P, Hofmann K. A conserved domain is present in different families of vesicular fusion proteins: a new superfamily. Proc Natl Acad Sci USA. 1997;94:3046–3051. doi: 10.1073/pnas.94.7.3046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wooding S, Pelham HRB. The dynamics of Golgi protein traffic visualized in living yeast cells. Mol Biol Cell. 1998;9:2667–2680. doi: 10.1091/mbc.9.9.2667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziman M, Chuang JS, Schekman RW. Chs1p and Chs3p, two proteins involved in chitin synthesis, populate a compartment of the Saccharomyces cerevisiae endocytic pathway. Mol Biol Cell. 1996;7:1909–1919. doi: 10.1091/mbc.7.12.1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziman M, Chuang JS, Tsung M, Hamamoto S, Schekman R. Chs6p-dependent anterograde transport of Chs3p from the chitosome to the plasma membrane in Saccharomyces cerevisiae. Mol Biol Cell. 1998;9:1565–1576. doi: 10.1091/mbc.9.6.1565. [DOI] [PMC free article] [PubMed] [Google Scholar]