Abstract
Background
There is concern about whether cardiac damage occurs as a result of prolonged strenuous exercise.
Objective
To investigate whether competing in a triathlon is associated with cardiac damage based on a sustained increase in cardiac troponin T (cTnT), and whether such an increase correlates with echocardiographic changes
Methods
cTnT and echocardiographic measurements were made in 38 participants in the 2001 Australian ironman triathlon. cTnT was measured the day before, immediately after, and the day following the race. Echocardiography was done the day before, immediately after, and two to six weeks later for measurement of ejection fraction, stroke volume, cardiac output, wall motion analysis, and global left ventricular function (LVF).
Results
No subject had detectable cTnT in the pre‐race sample. Following the race, 32 subjects (86.5%) had detectable levels of cTnT (>0.01 ng/ml), with six (16.2%) having >0.10 ng/ml. The day after the race, nine subjects (23.7%) still had detectable cTnT, with two recording a level >0.10 ng/ml. Previously described echocardiographic changes of “cardiac fatigue” were observed in the whole cohort. There was a modest but significant correlation between change in ejection fraction and peak cTnT level (p = 0.02, r = 0.39). Athletes with a post‐race cTnT >0.10 ng/ml had a greater decrease in global LVF (p = 0.02) and a trend toward a greater fall in ejection fraction and stroke volume than athletes with cTnT levels <0.10 ng/ml. Cardiac output fell in the group with cTnT >0.10 ng/ml (p>0.05).
Conclusions
Participation in ironman triathlon often resulted in persistently raised cTnT levels, and the troponin rise was associated with echocardiographic evidence of abnormal left ventricular function. The clinical significance and long term sequelae of such damage remains to be determined.
Keywords: cardiac troponin T, echocardiograph, exercise, triathlon
Triathlon is enjoying increasing popularity as a participation sport, attracting people with a wide range of ages and capabilities. Successful completion of ultra endurance events in the form of ironman triathlon is becoming the focus of annual training for large numbers of professional and recreational athletes. The ironman triathlon consists of a 3.8 km swim, a 180.2 km cycle, and a 42.2 km run. Common medical problems associated with ironman triathlons include dehydration, hyperthermia, hyponatraemia, hypothermia, and musculoskeletal damage.
There is increasing evidence that cardiac muscle may also be damaged.1,2,3,4,5,6,7,8 We have under our care several patients with ventricular tachycardia of multifocal origin requiring implantable defibrillators, who have in the past competed in numerous ironman triathlons or endurance cycling events. It has been hypothesised that permanent cardiac injury could develop in endurance athletes, in the absence of coronary atherosclerosis, resulting in focal fibrosis of the myocardium.9 Cardiac troponin T (cTnT) is a highly sensitive and specific indicator of cardiomyocyte necrosis, even in the presence of skeletal myocyte damage,10 and several studies have shown significant increases in plasma levels of cTnT or cardiac troponin I (cTnI) in the early post‐race period following endurance events.1,2,3,4,5,6,7,8,11 There are also data indicating that increases in plasma cTnT and echocardiographic changes both occur following ironman triathlons.1,2 However, whether such changes are correlated and sustained is unknown. Our aim in the current study was to investigate whether competing in the ironman triathlon is associated with cardiac damage based on a sustained rise in cTnT, and whether such an elevation correlates with echocardiographic changes.
Methods
Subjects
Athletes competing in the 2001 Australian Ironman Triathlon (held in Forster on the mid‐east coast of Australia), who had competed in at least one previous ironman triathlon, were invited to participate in this study. A questionnaire was completed to obtain demographic data, training history, cardiovascular risk factors, any history of cardiac symptoms, and competition history of previous triathlons, other ultra endurance events, and marathons. Exclusion criteria were significant cardiac history or pathological electrocardiograph at baseline examination. This study was approved by the Royal Prince Alfred Hospital ethics committee and written informed consent was obtained from each subject.
Data collection
The day before the event, each subject had blood collected for measurement of cTnT and creatine kinase (CK). At the same time cross sectional transthoracic echocardiography was carried out and recorded for later analysis. Participants were asked to avoid strenuous exercise for 24 hours before collection of pre‐race data. Echocardiography and repeat blood sampling were done within 20 minutes of race completion. Further blood samples were taken the next day (14 to 24 hours post‐race completion). Subjects were then invited for follow up echocardiography two to six weeks post‐race.
Laboratory analysis
Blood samples were collected in heparinised tubes, which were centrifuged at 2500 rpm for five minutes within 20 minutes of blood collection. The supernatant was removed and kept at 4°C during transport to Royal Prince Alfred Hospital for analysis of the biochemical variables. The Roche troponin T electrochemiluminescence assay for cTnT (lower detectable limit 0.01 ng/ml) and the Roche photometric rate reaction assay for CK (normal range being 0 to 175 U/l for women and 0 to 250 U/l for men) were used. Both these assays were undertaken on the Roche 917 autoanalyser.
Echocardiographic analysis
Echocardiographic examination was carried out by two experienced technicians, using one of two Acuson “Cypress” portable ultrasonograph machines. Two observers, blinded to the biochemical results, assessed left ventricular wall motion using semiquantitative analysis. Each examination was graded according to a 16 segment model, with a score of 1 = normal, 2 = hypokinetic, and 3 = akinetic allotted to each segment. A normal score would thus be 16, and higher values would indicate dysfunction. Cross sectional echocardiography using the apical four chamber view and automated border detection was employed to determine ejection fraction, which was calculated using a modified Simpson's rule method with five consecutive beats averaged. Global left ventricular function (LVF) was assessed compared with baseline pre‐race function. This was scaled to 0 (unchanged), −1 (mildly impaired), and −2 (severely impaired) before the statistical analysis.
Data analysis
Biochemical and echocardiographic data pre‐race, immediately post‐race, and the following day or after two to six weeks follow up were summarised and expressed as mean (SD). Using SAS version 8 (SAS Institute Inc, Cary, North Carolina, USA), within and between group differences were assessed by repeated measures analysis of variance with adjustments for multiple comparisons. The cohort was analysed overall and within subgroups defined by (a) cTnT >0.01 ng/ml (that is, detectable) post‐race; (b) cTnT >0.10 ng/ml (that is, greater than the clinical threshold accepted for myocardial injury) at any time; and (c) cTnT >0.01 ng/ml on the following day.
Results
Thirty eight subjects (36 men, two women) participated in the study. Demographics, training, and past competition histories of the participants are outlined in table 1. Thirty six subjects completed the race with a mean time of 694 minutes. The two subjects who did not finish completed 546 and 576 minutes, retiring midway during the run leg because of fatigue, and were included in the results. All subjects underwent post‐race and next day testing. Because of geographical constraints (and not individual test findings), only 21 subjects underwent follow up echocardiography two to six weeks post‐race.
Table 1 Characteristics of the 38 study participants.
| Characteristic | Mean (SD) |
|---|---|
| Age (years) | 38 (5.2) |
| Total years training for triathlon (n) | 9.7 (4.2) |
| No of olympic length races (n)* | 23.7 (41.4) |
| No of ironman length races (n)† | 4.0 (2.7) |
| No of marathons (n)‡ | 1.9 (2.0) |
| Average weekly distance past 6 months (km) | |
| Swimming | 9.3 (6.0) |
| Running | 54.2 (3.3) |
| Cycling | 247.2 (6.5) |
| Race time (min)§ | 686.4 (83.4) |
*1.5 km swim, 40 km cycle, 10 km run.
†3.8 km swim, 180.2 km cycle, 42.2 km run.
‡42 km run.
§Includes two participants who did not finish the race.
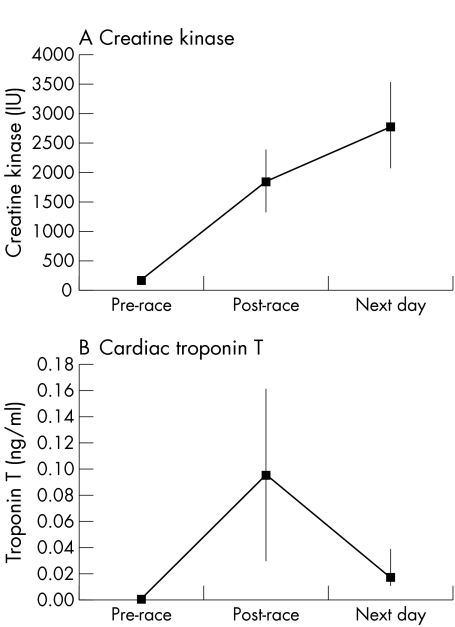
Mean values of CK and cTnT are shown in fig 1. In the overall cohort, the mean (SD) pre‐race value for CK was 162 (104) IU/l. CK values were markedly raised immediately post‐race, with a mean value of 1836 (1605) IU/l (p<0.001), and continued to rise the next day to 2782 (2201) IU/l. No subject had detectable cTnT in the pre‐race sample. There was an increase in the mean value of cTnT immediately post‐race to 0.095 (0.19) ng/ml (p<0.001), which fell the following day to 0.017 (0.04) ng/ml (p = 0.004). Thirty two subjects (86.5%) had detectable cTnT in the immediate post‐race sample (range 0.015 to 1.130 ng/ml), with six subjects (16.2%) having levels >0.10 ng/ml (range 0.127 to 1.130 ng/ml). Nine subjects (23.7%) had persisting detectable levels the following day (range 0.011 to 0.221 ng/ml), with two subjects still recording a level >0.10 ng/ml. Those with detectable cTnT (>0.01 ng/ml) post‐race had a significantly higher CK level than those without detectable cTnT (2045 (1664) v 720 (1048), p = 0.03).
Figure 1 Time course of changes in plasma creatine kinase and troponin T among study participants. Values are means (95% confidence intervals).
Table 2 summarises the echocardiographic data for the overall cohort. All participants except one had normal wall motion scores during pre‐race testing. In the overall cohort, both ejection fraction and stroke volume fell significantly immediately post‐race, with no change in cardiac output. There was a significant increase in the mean abnormal wall motion score from 16.2 to 17.6 (p = 0.004). Global LVF was also altered immediately post‐race with a mean score of −0.58 when compared with the pre‐race value (p = 0.004). All echocardiographic indices returned to baseline values in the follow up examination, which was done on 21 of the 38 subjects.
Table 2 Echocardiographic measures in the overall cohort.
| Pre‐race | Post‐race | 2–6 Weeks | |
|---|---|---|---|
| No of subjects | 38 | 38 | 21 |
| Ejection fraction (%) | 64.2 (7.9) | 58.6 (7.9)* | 64.2 (7.4)† |
| Stroke volume (ml) | 113.1 (25.6) | 89.5 (21.8)* | 113.6 (27.4)† |
| Cardiac output (l/min) | 6.66 (1.7) | 7.23 (1.8)† | 6.54 (1.8)† |
| Wall motion score | 16.2 (0.5) | 17.6 (1.9)* | 16.2 (0.4)† |
| Global LVF | 0 (0) | −0.58 (0.66)* | 0 (0.21)† |
Data expressed as mean (SD).
*p<0.01 compared with pre‐race values.
†p>0.05 compared with pre‐race values.
LVF, left ventricular function.
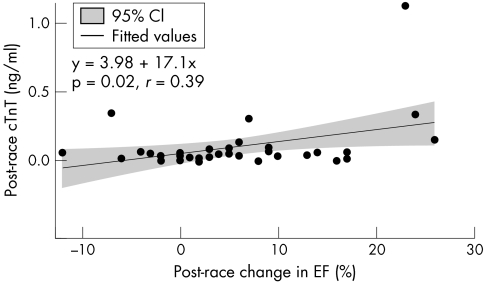
Overall, there was a modest, statistically significant correlation between change in ejection fraction immediately post‐race and peak cTnT level (fig 2). Both ejection fraction and stroke volume showed trends toward a greater reduction immediately post‐race among athletes with cTnT values above 0.10 ng/ml at the conclusion of the race (n = 6), compared with those without such an increase (n = 32); however, these differences were not statistically significant (table 3). Cardiac output also fell post‐race in the group with cTnT >0.10 ng/ml, although this failed to reach statistical significance. Segmental wall motion scores were also not statistically different between groups; however, global LVF fell significantly more in the athletes with cTnT >0.10 ng/ml post‐race (p = 0.02). Similarly, the nine athletes who had persistently detectable cTnT the following day showed a greater reduction in global LVF (p = 0.04) at the end of the race compared with the other athletes.
Figure 2 Correlation between absolute change in ejection fraction post‐race and cTnT level post‐race. The regression equation and fitted line (with 95% confidence intervals) are shown.
Table 3 Echocardiographic measures in participants grouped by troponin elevation.
| cTnT >0.10 ng/ml | cTnT <0.10 ng/ml | cTnT >0.01 ng/ml | cTnT <0.01 ng/ml | |
|---|---|---|---|---|
| post‐race | post‐race | next day | next day | |
| (n = 6)* | (n = 32)† | (n = 9)‡ | (n = 29)§ | |
| Stroke volume (ml) | ||||
| Pre‐race | 136 (27) | 109 (23) | 127 (33) | 109 (21) |
| Post‐race | 85 (23) | 90 (22) | 99 (25) | 87 (20) |
| 2–6 weeks | 133 (21) | 109 (27) | 130 (24) | 111 (28) |
| Ejection fraction (%) | ||||
| Pre‐race | 72 (5) | 63 (8) | 65 (9) | 64 (8) |
| Post‐race | 59 (9) | 59 (8) | 57 (8) | 59 (8) |
| 2–6 weeks | 70 (2) | 63 (7) | 69 (4) | 63 (8) |
| Cardiac output (l/min) | ||||
| Pre‐race | 7.9 (2.0) | 6.4 (1.5) | 7.6 (2.0) | 6.4 (1.5) |
| Post‐race | 6.9 (2.1) | 7.3 (1.8) | 8.1 (2.0) | 7.0 (1.7) |
| 2–6 weeks | 7.5 (1.8) | 6.3 (1.8) | 7.8 (2.0) | 6.3 (1.8) |
| Wall motion score | ||||
| Pre‐race | 16.0 (0) | 16.4 (1.8) | 16.0 (0) | 16.4 (1.9) |
| Post‐race | 16.8 (1.6) | 17.8 (2.1) | 17.4 (2.2) | 17.7 (2.0) |
| 2–6 weeks | 16.3 (0.6) | 16.1 (0.3) | 16.3 (0.6) | 16.1 (0.3) |
| Global LVF | ||||
| Pre‐race | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Post‐race | −1.1 (0.8) | −0.48 (0.60) | −0.83 (0.8) | −0.50 (0.62) |
| 2–6 weeks | 0 (0) | −0.06 (0.17) | 0 (0) | 0.06 (0.16) |
Data expressed as mean (SD).
*4 of these participants underwent echocardiography two to six weeks post‐race.
†17 of these participants underwent echocardiography two to six weeks post‐race.
‡3 of these participants underwent echocardiography two to six weeks post‐race.
§18 of these participants underwent echocardiography two to six weeks post‐race.
cTnT, cardiac troponin T; LVF, left ventricular function.
Discussion
In this study, we have confirmed that participation in an ironman triathlon is associated with in rise in plasma cTnT levels, and the development of abnormal echocardiographic indices of left ventricular function.1,2 The important new findings are first, that the increase in cTnT reaches “clinically significant” levels and is persistent in a substantial proportion of individuals, and second, that a raised cTnT is associated with echocardiographic evidence of abnormal left ventricular function.
Raised cTnT has become the standard for the diagnosis of myocardial injury and for the stratification of an individual's risk in the clinical setting of myocardial ischaemia.12,13 The European Society of Cardiology and the American College of Cardiology have defined acute myocardial infarction as an increase in plasma cTnT levels to at least 0.10 μg/l, with a slow decline in the appropriate clinical context. The significance of lower levels of troponin leak and those occurring outside an ischaemic clinical context remains unclear, but they probably represent cardiac injury.13 Ninety five per cent of cTnT is complexed to the contractile apparatus of the myocardial cell, with an additional small cytosolic store.13 In the event of myocardial injury, cTnT is released in a biphasic pattern, with the initial peak reflecting release from the cytoplasm after membrane destabilisation (reversible damage), and the subsequent peak reflecting disintegration of the entire contractile structure of the cell (irreversible damage).11 It seems likely there is a continuum from reversible to irreversible cardiomyocyte damage.
Several other studies have reported leaks of cTnT (some using less specific first generation assays) and cardiac troponin I in small groups of individuals after various types of prolonged strenuous exercise.1,2,3,4,5,6,7,8,14,15 Second generation assays of cTnT, which show less that 0.005% cross reactivity with skeletal TnT, are particularly useful in the study of cardiac damage among exercising individuals.10 Previous studies of athletes competing in endurance events have reported rises in plasma cTnT of >0.10 ng/ml (using second generation assays) in between 0 and 11% of subjects.1,2,5,7 To the best of our knowledge, only one study2 has measured plasma cTnT the day following prolonged strenuous exercise, and the values were not reported as elevated. The current study is consistent with previous data showing raised plasma cTnT levels following prolonged strenuous exercise, but also indicates that this elevation persists beyond the immediate post‐exercise period in a proportion of athletes.
In the current study, ejection fraction and stroke volume in the entire cohort both fell significantly at the end of the race but cardiac output was maintained. Furthermore, abnormal segmental wall motion scores rose significantly. All these variables returned to baseline at follow up. These findings are entirely consistent with previous data,1,2,16,17,18 and reflect the concept of cardiac “fatigue” or reduced myocardial contractility following prolonged strenuous exercise.16 However, there are few data showing correlation between increases in plasma troponin level and changes in left ventricular function.1,2 Rifai et al studied 23 athletes following the Hawaii Ironman Triathlon, 12 of whom had post‐race echocardiography. Cardiac troponin T was detectable in six athletes (>0.10 μg/l in two). They reported a significant correlation with increased number of abnormal wall motion segments, no correlation with changes in ejection fraction, and a negative correlation with race time.1 Whyte et al reported mean data on a group of 10 ironman triathletes, with significant changes in both cTnT and echocardiographic data but no comparisons were made between them.2 The changes observed returned to baseline levels at 48 hours.2 We observed that the six athletes with cTnT >0.10 ng/ml post‐race showed a fall in the mean value of cardiac output immediately post‐race, while those without such elevation did not. Although the difference in these changes between the groups did not achieve statistical significance, this may be a reflection of the small numbers studied. Furthermore, while the whole cohort showed a significant fall in global LVF at the end of the race (which recovered to baseline levels at follow up), there was a significantly greater fall in those subjects with cTnT >0.10 ng/ml. This was also true for individuals whose cTnT remained detectable the day after the race.
The groups showed baseline differences in echocardiographic parameters but speculation that these baseline differences may be explained by more profound changes of “athlete's heart” is not supported by significant differences in race time, age, training history, or the number of previous competition events (data not shown). These baseline differences are most probably explained by chance.
Although the echocardiographic changes resolved by the time of the two to six week examination, the presence of plasma cTnT elevations and the possible correlation of these with early changes in left ventricular function support the hypothesis of myocardial damage. The long term consequences of such damage remain undetermined. However, biochemical and histological research into exercise induced skeletal muscle injury has show the occurrence of multifocal necrosis.19,20,21,22,23,24 Furthermore, histopathological evidence of localised cardiomyocyte damage after strenuous prolonged exercise has been found in animal studies.18 In a report following the necropsy of a marathon runner (who had run 524 marathons), focal myocardial fibrosis was observed in the absence of coronary artery disease.25 We initiated the current study because we have treated several current or retired endurance athletes presenting with symptomatic ventricular tachycardia, which was shown on electrophysiological study to be of multifocal origin and not limited to the right ventricular outflow tract.26 These patients required a combination of drug treatment, ablation, and insertion of automatic implantable cardioverter‐defibrillators. It is unclear whether this is a chance observation or possibly relates to microscopic multifocal fibrosis resulting from myocardial cell injury during prolonged strenuous exercise.
What is already known on this subject
Research is consistent in showing that biochemical markers of cardiac damage (including troponin T) are raised following prolonged strenuous exercise.
Prolonged strenuous exercise is associated with diastolic and systolic dysfunction on echocardiography.
Most studies have reported an absence of correlation between cTnT and echocardiographic abnormalities. The significance of cTnT elevation is unknown.
What this study adds
As well as confirming previous data on cTnT post‐exercise, this study shows that cTnT can remain elevated on the day after exercise.
A correlation was found between a fall in ejection fraction and peak cTnT.
There were some limitations to the study. Even though this is probably the largest study to date addressing plasma cTnT and echocardiographic changes after exercise, the conclusions remain limited by small numbers. The logistical constraints resulting in fewer participants returning for the two to six week echocardiogram further reduced the power to show any longer term changes in left ventricular function. In addition, the global LVF assessment, which attempts to describe alterations in overall left ventricular systolic function not reflected in abnormal wall motion scores (which study regional effects of ischaemic changes in coronary artery disease), is an unvalidated measurement.
In conclusion, our study confirms that following ironman triathlon, cTnT is commonly increase to >0.10 ng/ml and can still be detected the day after the race in many individuals. In this study, raised cTnT was associated with a decrease in global left ventricular function and was modestly correlated with reduced ejection fraction. Demonstrating a causal relation between endurance competition and long term cardiac abnormalities, such as ventricular tachycardia, will require a national register of cases.
Acknowledgements
We acknowledge Accuson for supplying the echocardiographic equipment, Ken Baggs, Joe Ferris, and the organising committee for the 2001 Australian Ironman Triathon, Geoff Fuller, David Stacey, Sheila McInnes, Tom Prendergast, and Margaret Tulloh for their assistance.
Abbreviations
CK - creatine kinase
CTnT - cardiac troponin T
LVF - left ventricular function
Footnotes
Competing interests: none declared
References
- 1.Rifai N, Douglas P S, O'Toole M.et al Cardiac troponin T and I, electrocardiographic wall motion analyses, and ejection fractions in athletes participating in the Hawaii Ironman Triathlon. Am J Cardiol 1999831085–1089. [DOI] [PubMed] [Google Scholar]
- 2.Whyte G P, George K, Sharma S.et al Cardiac fatigue following prolonged endurance exercise of differing distances. Med Sci Sports Exerc 2000321067–1072. [DOI] [PubMed] [Google Scholar]
- 3.Laslett L, Eisenbud E, Lind R. Evidence of myocardial injury during prolonged strenuous exercise. Am J Cardiol 199678488–490. [DOI] [PubMed] [Google Scholar]
- 4.Neumayr G, Gaenzer H, Pfister R.et al Plasma levels of cardiac troponin I after prolonged strenuous endurance exercise. Am J Cardiol. 2001;87: 369–71, A10, [DOI] [PubMed]
- 5.Neumayr G, Pfister R, Mitterbauer G.et al Effect of the “Race Across The Alps” in elite cyclists on plasma cardiac troponins I and T. Am J Cardiol 200289484–486. [DOI] [PubMed] [Google Scholar]
- 6.Ohba H, Takada H, Musha H.et al Effects of prolonged strenuous exercise on plasma levels of atrial natriuretic peptide and brain natriuretic peptide in healthy men. Am Heart J 2001141751–758. [DOI] [PubMed] [Google Scholar]
- 7.Shave R E, Dawson E, Whyte G.et al Evidence of exercise‐induced cardiac dysfunction and elevated cTnT in separate cohorts competing in an ultra‐endurance mountain marathon race. Int J Sports Med 200223489–494. [DOI] [PubMed] [Google Scholar]
- 8.Denvir M A, Galloway P J, Meighan A S.et al Changes in skeletal and cardiac muscle enzymes during the Scottish Coast to Coast Triathlon. Scot Med J 19994449–51. [DOI] [PubMed] [Google Scholar]
- 9.Rowe W J. Extraordinary unremitting endurance exercise and permanent injury to normal heart. Lancet 1992340712–714. [DOI] [PubMed] [Google Scholar]
- 10.Muller‐Bardorff M, Hallermayer K, Schroder A.et al Improved troponin T ELISA specific for cardiac troponin T isoform: assay development and analytical and clinical validation. Clin Chem 199743458–466. [PubMed] [Google Scholar]
- 11.Bonetti A, Tirelli F, Albertini R.et al Serum cardiac troponin T after repeated endurance exercise events. Int J Sports Med 199617259–262. [DOI] [PubMed] [Google Scholar]
- 12.Ohman E M, Armstrong P W, Christenson R H.et al Cardiac troponin T levels for risk stratification in acute ischemia. N Engl J Med 19963351333–1341. [DOI] [PubMed] [Google Scholar]
- 13.Jaffe A S, Ravkilde J, Roberts R.et al It's time for a change to troponin standard. Circulation 20001021216. [DOI] [PubMed] [Google Scholar]
- 14.Lucia A, Serratosa L, Saborido A.et al Short‐term effects of marathon running: no evidence of cardiac dysfunction. Med Sci Sports Exerc 1999311414–1421. [DOI] [PubMed] [Google Scholar]
- 15.Koller A, Summer P, Moser H. Regular exercise and subclinical myocardial injury during prolonged aerobic activity. JAMA 19992821816. [DOI] [PubMed] [Google Scholar]
- 16.Douglas P S, O'Toole M L, Hiller W D.et al Cardiac fatigue after prolonged exercise. Circulation 1987761206–1213. [DOI] [PubMed] [Google Scholar]
- 17.McGavock J M, Warburton D E R, Taylor D.et al The effects of prolonged strenuous exercise on left ventricular function: a brief review. Heart Lung 200231279–292. [DOI] [PubMed] [Google Scholar]
- 18.Seals D R, Rogers M A, Hagberg J M.et al Left ventricular function after prolonged strenuous exercise in healthy subjects. Am J Cardiol 198861875–879. [DOI] [PubMed] [Google Scholar]
- 19.Hikida R S, Staron R S, Hagerman F C.et al Muscle fiber necrosis associated with human marathon runners. J Neurol Sci 198359185–203. [DOI] [PubMed] [Google Scholar]
- 20.Ebbeling C B, Clarkson P M. Exercise‐induced muscle damage and adaptation. Sports Med 19897207–234. [DOI] [PubMed] [Google Scholar]
- 21.Komulainen J, Vihko V. Exercise‐induced necrotic muscle damage and enzyme release in the four days following prolonged submaximal running in rats. Pflugers Arch 1994428346–351. [DOI] [PubMed] [Google Scholar]
- 22.Armstrong R B. Muscle damage and endurance events. Sports Med 19863370–381. [DOI] [PubMed] [Google Scholar]
- 23.Chen Y, Serfass R C, Mackey‐Bojack S M.et al Cardiac troponin T alterations in myocardium and serum of rats after stressful, prolonged intense exercise.[comment]. J Appl Physiol 2000881749–1755. [DOI] [PubMed] [Google Scholar]
- 24.Rowe W J. A world record marathon runner with silent ischaemia without coronary atherosclerosis. Chest 1991991306–1308. [DOI] [PubMed] [Google Scholar]
- 25.Carraro U, Franceschi C. Apoptosis of skeletal and cardiac muscles and physical exercise. Aging (Milan) 19979(1–2)19–34. [DOI] [PubMed] [Google Scholar]
- 26.Rossi L. Structural and non‐structural disease underlying high‐risk cardiac arrhythmias relevant to sports medicine. J Sports Med Phys Fitness 19953579–86. [PubMed] [Google Scholar]