Abstract
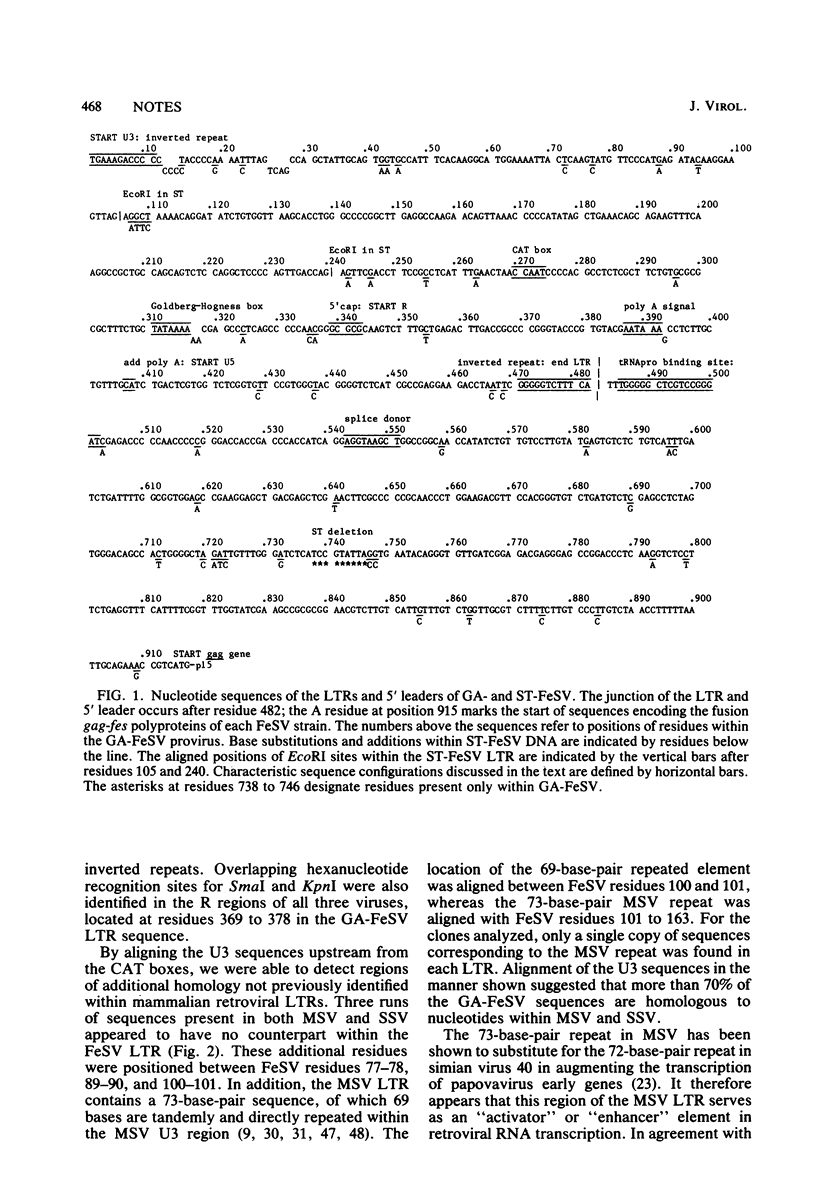
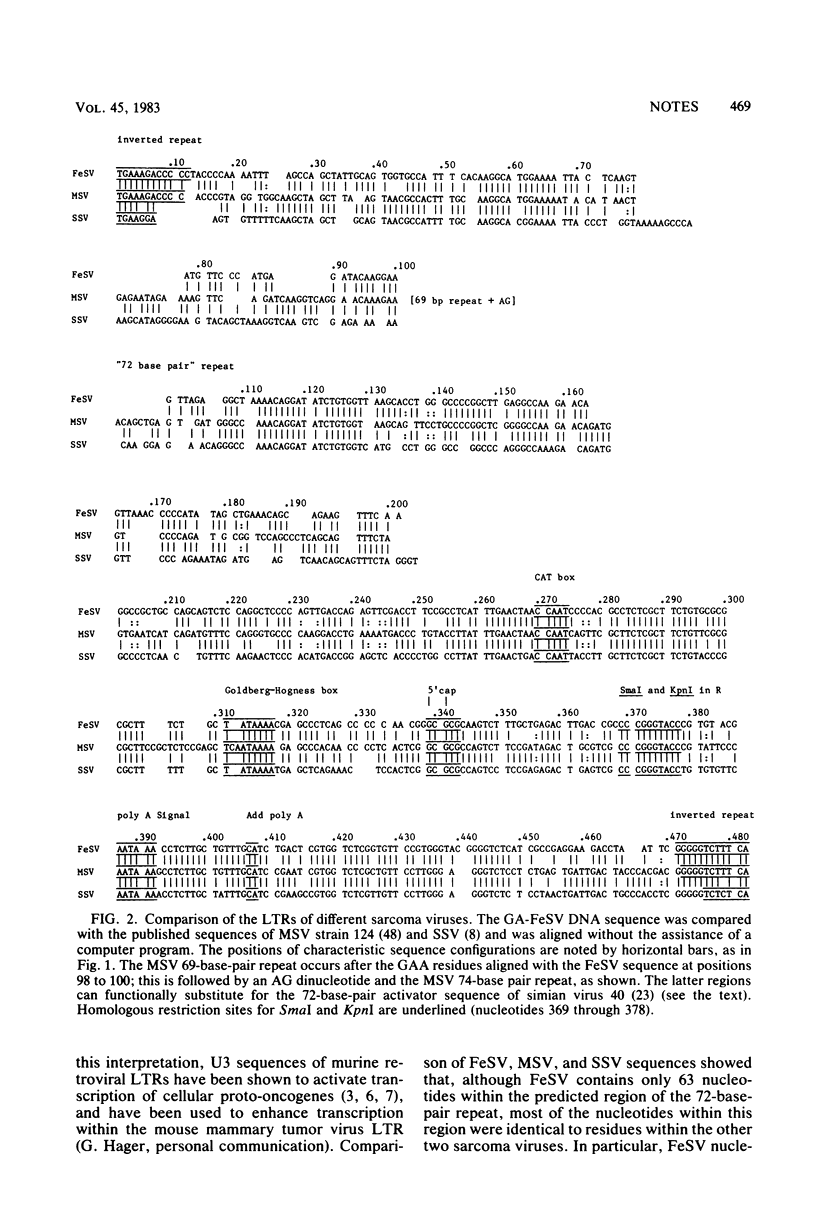
The nucleotide sequences of the Gardner-Arnstein feline sarcoma virus (FeSV) long terminal repeat and the adjacent leader sequences 5' to the viral gag gene were determined. These were compared with homologous portions of Synder-Theilen FeSV and with previously published sequences for Moloney murine sarcoma virus and simian sarcoma virus proviral DNA. More than 75% of the residues in the FeSV R and U5 regions were homologous to sequences within the same regions of the other viral long terminal repeats. Unexpectedly, alignment of the FeSV sequences with those of the Moloney murine sarcoma and simian sarcoma viruses showed similar extents of homology within U3. The homologous U3 regions included the inverted repeats, a single set of putative enhancer sequences, corresponding to a "72-base-pair" repeat, and sequences, including the CAT and TATA boxes, characteristic of eucaryotic promotors. The 5' leader sequences of both FeSV strains included a binding site for prolyl tRNA and a putative splice donor sequence. In addition, the FeSV leader contained a long open reading frame which was adjacent to and in phase with the ATG codon at the 5' end of the FeSV gag gene. The open reading frame could code for a signal peptide of about 7.4 kilodaltons. Our results support the concept that the virogenic portions of both FeSV and simian sarcoma virus were ancestrally derived from viruses of rodent origin, with conservation of regulatory sequences as well as the viral structural genes.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benveniste R. E., Callahan R., Sherr C. J., Chapman V., Todaro G. J. Two distinct endogenous type C viruses isolated from the asian rodent Mus cervicolor: conservation of virogene sequences in related rodent species. J Virol. 1977 Mar;21(3):849–862. doi: 10.1128/jvi.21.3.849-862.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benveniste R. E., Sherr C. J., Todaro G. J. Evolution of type C viral genes: origin of feline leukemia virus. Science. 1975 Nov 28;190(4217):886–888. doi: 10.1126/science.52892. [DOI] [PubMed] [Google Scholar]
- Blair D. G., McClements W. L., Oskarsson M. K., Fischinger P. J., Vande Woude G. F. Biological activity of cloned Moloney sarcoma virus DNA: Terminally redundant sequences may enhance transformation efficiency. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3504–3508. doi: 10.1073/pnas.77.6.3504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blobel G., Dobberstein B. Transfer of proteins across membranes. I. Presence of proteolytically processed and unprocessed nascent immunoglobulin light chains on membrane-bound ribosomes of murine myeloma. J Cell Biol. 1975 Dec;67(3):835–851. doi: 10.1083/jcb.67.3.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blobel G. Intracellular protein topogenesis. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1496–1500. doi: 10.1073/pnas.77.3.1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang E. H., Ellis R. W., Scolnick E. M., Lowy D. R. Transformation by cloned Harvey murine sarcoma virus DNA: efficiency increased by long terminal repeat DNA. Science. 1980 Dec 12;210(4475):1249–1251. doi: 10.1126/science.6254153. [DOI] [PubMed] [Google Scholar]
- Chang E. H., Furth M. E., Scolnick E. M., Lowy D. R. Tumorigenic transformation of mammalian cells induced by a normal human gene homologous to the oncogene of Harvey murine sarcoma virus. Nature. 1982 Jun 10;297(5866):479–483. doi: 10.1038/297479a0. [DOI] [PubMed] [Google Scholar]
- Devare S. G., Reddy E. P., Law J. D., Aaronson S. A. Nucleotide sequence analysis of the long terminal repeat of integrated simian sarcoma virus: evolutionary relationship with other mammalian retroviral long terminal repeats. J Virol. 1982 Jun;42(3):1108–1113. doi: 10.1128/jvi.42.3.1108-1113.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhar R., McClements W. L., Enquist L. W., Vande Woude G. F. Nucleotide sequences of integrated Moloney sarcoma provirus long terminal repeats and their host and viral junctions. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3937–3941. doi: 10.1073/pnas.77.7.3937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donehower L. A., Huang A. L., Hager G. L. Regulatory and coding potential of the mouse mammary tumor virus long terminal redundancy. J Virol. 1981 Jan;37(1):226–238. doi: 10.1128/jvi.37.1.226-238.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards S. A., Fan H. gag-Related polyproteins of Moloney murine leukemia virus: evidence for independent synthesis of glycosylated and unglycosylated forms. J Virol. 1979 May;30(2):551–563. doi: 10.1128/jvi.30.2.551-563.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efstratiadis A., Posakony J. W., Maniatis T., Lawn R. M., O'Connell C., Spritz R. A., DeRiel J. K., Forget B. G., Weissman S. M., Slightom J. L. The structure and evolution of the human beta-globin gene family. Cell. 1980 Oct;21(3):653–668. doi: 10.1016/0092-8674(80)90429-8. [DOI] [PubMed] [Google Scholar]
- Evans L. H., Dresler S., Kabat D. Synthesis and glycosylation of polyprotein precursors to the internal core proteins of Friend murine leukemia virus. J Virol. 1977 Dec;24(3):865–874. doi: 10.1128/jvi.24.3.865-874.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedele L. A., Even J., Garon C. F., Donner L., Sherr C. J. Recombinant bacteriophages containing the integrated transforming provirus of Gardner--Arnstein feline sarcoma virus. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4036–4040. doi: 10.1073/pnas.78.7.4036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner M. B., Rongey R. W., Arnstein P., Estes J. D., Sarma P., Huebner R. J., Rickard C. G. Experimental transmission of feline fibrosarcoma to cats and dogs. Nature. 1970 May 30;226(5248):807–809. doi: 10.1038/226807a0. [DOI] [PubMed] [Google Scholar]
- Gilboa E., Mitra S. W., Goff S., Baltimore D. A detailed model of reverse transcription and tests of crucial aspects. Cell. 1979 Sep;18(1):93–100. doi: 10.1016/0092-8674(79)90357-x. [DOI] [PubMed] [Google Scholar]
- Hampe A., Laprevotte I., Galibert F., Fedele L. A., Sherr C. J. Nucleotide sequences of feline retroviral oncogenes (v-fes) provide evidence for a family of tyrosine-specific protein kinase genes. Cell. 1982 Oct;30(3):775–785. doi: 10.1016/0092-8674(82)90282-3. [DOI] [PubMed] [Google Scholar]
- Hartley J. L., Donelson J. E. Nucleotide sequence of the yeast plasmid. Nature. 1980 Aug 28;286(5776):860–865. doi: 10.1038/286860a0. [DOI] [PubMed] [Google Scholar]
- Hayward W. S. Size and genetic content of viral RNAs in avian oncovirus-infected cells. J Virol. 1977 Oct;24(1):47–63. doi: 10.1128/jvi.24.1.47-63.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu T. W., Sabran J. L., Mark G. E., Guntaka R. V., Taylor J. M. Analysis of unintegrated avian RNA tumor virus double-stranded DNA intermediates. J Virol. 1978 Dec;28(3):810–818. doi: 10.1128/jvi.28.3.810-818.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hérissé J., Courtois G., Galibert F. Nucleotide sequence of the EcoRI D fragment of adenovirus 2 genome. Nucleic Acids Res. 1980 May 24;8(10):2173–2192. doi: 10.1093/nar/8.10.2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju G., Skalka A. M. Nucleotide sequence analysis of the long terminal repeat (LTR) of avian retroviruses: structural similarities with transposable elements. Cell. 1980 Nov;22(2 Pt 2):379–386. doi: 10.1016/0092-8674(80)90348-7. [DOI] [PubMed] [Google Scholar]
- Ledbetter J., Nowinski R. C. Identification of the Gross cell surface antigen associated with murine leukemia virus-infected cells. J Virol. 1977 Aug;23(2):315–322. doi: 10.1128/jvi.23.2.315-322.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levinson B., Khoury G., Vande Woude G., Gruss P. Activation of SV40 genome by 72-base pair tandem repeats of Moloney sarcoma virus. Nature. 1982 Feb 18;295(5850):568–572. doi: 10.1038/295568a0. [DOI] [PubMed] [Google Scholar]
- Lieber M. M., Sherr C. J., Todaro G. J., Benveniste R. E., Callahan R., Coon H. G. Isolation from the asian mouse Mus caroli of an endogenous type C virus related to infectious primate type C viruses. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2315–2319. doi: 10.1073/pnas.72.6.2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodish H. F., Braell W. A., Schwartz A. L., Strous G. J., Zilberstein A. Synthesis and assembly of membrane and organelle proteins. Int Rev Cytol Suppl. 1981;12:247–307. doi: 10.1016/b978-0-12-364373-5.50016-0. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Milstein C., Brownlee G. G., Harrison T. M., Mathews M. B. A possible precursor of immunoglobulin light chains. Nat New Biol. 1972 Sep 27;239(91):117–120. doi: 10.1038/newbio239117a0. [DOI] [PubMed] [Google Scholar]
- Ostrowski M. C., Berard D., Hager G. L. Specific transcriptional initiation in vitro on murine type C retrovirus promoters. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4485–4489. doi: 10.1073/pnas.78.7.4485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proudfoot N. J., Brownlee G. G. Sequence at the 3' end of globin mRNA shows homology with immunoglobulin light chain mRNA. Nature. 1974 Nov 29;252(5482):359–362. doi: 10.1038/252359a0. [DOI] [PubMed] [Google Scholar]
- Reddy E. P., Smith M. J., Aaronson S. A. Complete nucleotide sequence and organization of the Moloney murine sarcoma virus genome. Science. 1981 Oct 23;214(4519):445–450. doi: 10.1126/science.6170110. [DOI] [PubMed] [Google Scholar]
- Reddy E. P., Smith M. J., Canaani E., Robbins K. C., Tronick S. R., Zain S., Aaronson S. A. Nucleotide sequence analysis of the transforming region and large terminal redundancies of Moloney murine sarcoma virus. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5234–5238. doi: 10.1073/pnas.77.9.5234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothenberg E., Donoghue D. J., Baltimore D. Analysis of a 5' leader sequence on murine leukemia virus 21S RNA: heteroduplex mapping with long reverse transcriptase products. Cell. 1978 Mar;13(3):435–451. doi: 10.1016/0092-8674(78)90318-5. [DOI] [PubMed] [Google Scholar]
- Rothman J. E., Lenard J. Membrane asymmetry. Science. 1977 Feb 25;195(4280):743–753. doi: 10.1126/science.402030. [DOI] [PubMed] [Google Scholar]
- Schultz A. M., Lockhart S. M., Rabin E. M., Oroszlan S. Structure of glycosylated and unglycosylated gag polyproteins of Rauscher murine leukemia virus: carbohydrate attachment sites. J Virol. 1981 May;38(2):581–592. doi: 10.1128/jvi.38.2.581-592.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz A. M., Rabin E. H., Oroszlan S. Post-translational modification of Rauscher leukemia virus precursor polyproteins encoded by the gag gene. J Virol. 1979 Apr;30(1):255–266. doi: 10.1128/jvi.30.1.255-266.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segrest J. P., Feldmann R. J. Membrane proteins: amino acid sequence and membrane penetration. J Mol Biol. 1974 Aug 25;87(4):853–858. doi: 10.1016/0022-2836(74)90090-4. [DOI] [PubMed] [Google Scholar]
- Shank P. R., Hughes S. H., Kung H. J., Majors J. E., Quintrell N., Guntaka R. V., Bishop J. M., Varmus H. E. Mapping unintegrated avian sarcoma virus DNA: termini of linear DNA bear 300 nucleotides present once or twice in two species of circular DNA. Cell. 1978 Dec;15(4):1383–1395. doi: 10.1016/0092-8674(78)90063-6. [DOI] [PubMed] [Google Scholar]
- Shank P. R., Linial M. Avian oncovirus mutant (SE21Q1b) deficient in genomic RNA: characterization of a deletion in the provirus. J Virol. 1980 Nov;36(2):450–456. doi: 10.1128/jvi.36.2.450-456.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherr C. J., Fedele L. A., Oskarsson M., Maizel J., Vande Woude G. Molecular cloning of Snyder-Theilen feline leukemia and sarcoma viruses: comparative studies of feline sarcoma virus with its natural helper virus and with Moloney murine sarcoma virus. J Virol. 1980 Apr;34(1):200–212. doi: 10.1128/jvi.34.1.200-212.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimotohno K., Mizutani S., Temin H. M. Sequence of retrovirus provirus resembles that of bacterial transposable elements. Nature. 1980 Jun 19;285(5766):550–554. doi: 10.1038/285550a0. [DOI] [PubMed] [Google Scholar]
- Shoemaker C., Goff S., Gilboa E., Paskind M., Mitra S. W., Baltimore D. Structure of a cloned circular Moloney murine leukemia virus DNA molecule containing an inverted segment: implications for retrovirus integration. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3932–3936. doi: 10.1073/pnas.77.7.3932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder H. W., Jr, Stockert E., Fleissner E. Characterization of molecular species carrying gross cell surface antigen. J Virol. 1977 Aug;23(2):302–314. doi: 10.1128/jvi.23.2.302-314.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder S. P., Theilen G. H. Transmissible feline fibrosarcoma. Nature. 1969 Mar 15;221(5185):1074–1075. doi: 10.1038/2211074a0. [DOI] [PubMed] [Google Scholar]
- Swanstrom R., DeLorbe W. J., Bishop J. M., Varmus H. E. Nucleotide sequence of cloned unintegrated avian sarcoma virus DNA: viral DNA contains direct and inverted repeats similar to those in transposable elements. Proc Natl Acad Sci U S A. 1981 Jan;78(1):124–128. doi: 10.1073/pnas.78.1.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temin H. M. Function of the retrovirus long terminal repeat. Cell. 1982 Jan;28(1):3–5. doi: 10.1016/0092-8674(82)90367-1. [DOI] [PubMed] [Google Scholar]
- Van Beveren C., Goddard J. G., Berns A., Verma I. M. Structure of Moloney murine leukemia viral DNA: nucleotide sequence of the 5' long terminal repeat and adjacent cellular sequences. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3307–3311. doi: 10.1073/pnas.77.6.3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Beveren C., van Straaten F., Galleshaw J. A., Verma I. M. Nucleotide sequence of the genome of a murine sarcoma virus. Cell. 1981 Nov;27(1 Pt 2):97–108. doi: 10.1016/0092-8674(81)90364-0. [DOI] [PubMed] [Google Scholar]
- Weiss S. R., Varmus H. E., Bishop J. M. The size and genetic composition of virus-specific RNAs in the cytoplasm of cells producing avian sarcoma-leukosis viruses. Cell. 1977 Dec;12(4):983–992. doi: 10.1016/0092-8674(77)90163-5. [DOI] [PubMed] [Google Scholar]


