Abstract
Alcoholic ketoacidosis (AKA) is a common reason for investigation and admission of alcohol dependent patients in UK emergency departments. Although well described in international emergency medicine literature, UK emergency physicians rarely make the diagnosis of AKA. There is increasing evidence that rather than being benign and self limiting, AKA may be a significant cause of mortality in patients with alcohol dependence. This literature review discusses the history, characterisation, pathophysiology, diagnosis, and management of AKA.
Keywords: emergency department, alcoholic ketoacidosis, alcohol
Alcoholic ketoacidosis (AKA), also termed alcoholic ketosis or alcoholic acidosis, is rarely diagnosed in UK emergency departments (EDs). The clinical features (nausea, intractable vomiting, and abdominal pain) overlap with a number of conditions that manifest as acute crises in alcohol dependent patients. When treated, AKA resolves rapidly and completely with no apparent sequelae. However, recent forensic studies suggest that the untreated metabolic disturbance may be associated with sudden death in patients with severe alcoholism. This review will examine the characterisation, pathophysiology, diagnosis and management of AKA within the ED.
METHODS
A literature search was carried out on Medline (via PubMed and OVID 1966‐2004), EMBASE (1980‐2004), and CINAHL (1980‐2004) using the search strategy: {Alcohol$ OR Ethanol$} AND {Keto‐acido$ OR Ketoacido$ OR Ketosis OR Ketotic} LIMIT TO HUMAN.
The majority of papers detected by this search focus primarily on diabetes mellitus and its complications, and were excluded. General literature reviews, single case reports, and letters were also excluded. All remaining papers were retrieved and the reference lists hand searched for any additional information sources.
CHARACTERISATION
In 1940, Dillon et al1 described a series of nine patients who had episodes of severe ketoacidosis in the absence of diabetes mellitus, all of whom had evidence of prolonged excessive alcohol consumption. It was not until 1970 that Jenkins et al2 described a further three non‐diabetic patients with a history of chronic heavy alcohol misuse and recurrent episodes of ketoacidosis. This group also proposed a possible underlying mechanism for this metabolic disturbance, naming it alcoholic ketoacidosis.
Further case series by Levy et al, Cooperman et al, and Fulop et al were subsequently reported, with remarkably consistent features.3,4,5 All patients presented with a history of prolonged heavy alcohol misuse, preceding a bout of particularly excessive intake, which had been terminated several days earlier by nausea, severe vomiting, and abdominal pain.
Clinical signs included tachypnoea, tachycardia, and hypotension. In 1974, Cooperman's series of seven ketoacidotic alcoholic patients all displayed diffuse epigastric tenderness on palpation.4 In contrast to patients with diabetic ketoacidosis, the patients were usually alert and lucid despite the severity of the acidosis and marked ketonaemia. When altered mental status occurred, this was clearly attributable to other causes. Laboratory results included absent blood alcohol with normal or low blood glucose level, no glycosuria, and a variably severe metabolic acidosis with a raised anion gap. This acidosis appeared to result from the accumulation in plasma of lactate and ketone bodies including beta‐hydroxybutyrate (BOHB) and acetoacetate (AcAc).3
Cooperman et al found that near patient testing for ketone bodies using nitroprusside test (Acetest, Ketostix) produced a low to moderate result in these patients.4 The nitroprusside reaction is most sensitive to acetoacetate, less so to acetone, and not at all to BOHB.5 BOHB may therefore be considerably elevated without significant ketonaemia being detected in this standard way. This study also confirmed the elevated ketone levels and an association with an increased BOHB:AcAc previously noted by Levy et al.3,4 This increased ratio was inversely related to the serum pH.4
Many patients with AKA were found to have extremely elevated concentrations of plasma free fatty acids, with mean levels much higher than reported in patients with diabetic ketoacidosis.3,4 Patients also had markedly raised cortisol and growth hormone, and relatively low plasma insulin levels. Liver function tests were often mildly abnormal. Patients improved rapidly (within 12 hours) with intravenous glucose and large amounts of intravenous saline, usually without insulin (although small amounts of bicarbonate were sometimes used).
Larger studies by Fulop and Hoberman5 and Wrenn et al6 (24 and 74 patients, respectively) clarified the underlying acid base disturbance. Although many patients had a significant ketosis with high plasma BOHB levels (5.2–14.2 mmol/l), severe acidaemia was uncommon. In the series from Fulop and Hoberman, seven patients were alkalaemic. It appeared that concurrent disease processes, including extracellular fluid depletion, alcohol withdrawal, pain, sepsis, and severe liver disease, resulted in mixed acid base disturbances in individual patients.6 The resultant blood pH was dependent on the final balance of these factors (table 1).
Table 1 Metabolic factors affecting acid base balance in AKA .
| Acidosis | Alkalosis | |||
|---|---|---|---|---|
| Metabolic | Ketoacidosis | Prolonged vomiting with significant extracellular fluid contraction | ||
| Lactic acidosis | ||||
| Acetic acidosis | ||||
| Hyperchloraemic acidosis | ||||
| Respiratory | Hyperventilation | |||
| Primary (delirium tremens) |
After Fulop and Hoberman.5
Wrenn et al6 studied the clinical presentation in detail. Nausea, vomiting, and abdominal pain were by far the most commonly observed complaints. Despite the frequency of abdominal symptoms, objective findings other than tenderness were infrequent. Abdominal distension, decreased bowel sounds, ascites, or rebound tenderness occurred rarely and only in the presence of other demonstrable intra‐abdominal pathology such as pancreatitis, severe hepatitis, and sepsis or pneumonia. Almost all had hepatomegaly. Both Wrenn et al6 and Fulop and Hoberman5 found evidence of alcoholic hepatitis to be common, with frequent elevations in serum transaminase activities and bilirubin.
Wrenn et al found altered mental status in 15% of patients, attributable in all but one case to hypoglycaemia, severe alcohol intoxication, or infection. Fever was seen in only two patients, both with other likely underlying causes.
In up to 10% of sudden unexpected deaths in patients with chronic alcoholism, the immediate cause and mechanism of death are unclear even after scene investigation, necropsy, and standard toxicology screen.7 Alcohol is commonly low or absent, and fatty liver is frequently the only pathological abnormality detected. Severe derangements of potassium and/or magnesium may be implicated in the sudden deaths of some alcoholic patients, but not all. These unexplained cases often feature markedly elevated levels of BOHB.7,8,9,10 In the study from Kadis et al of 30 cases of sudden death in patients with chronic alcoholism,11 BOHB levels were 10 times higher than in alcoholic patients in whom another cause of death was found (p<0.05).
Denmark noted elevated BOHB levels in these unexplained deaths and hypothesised that alcohol ketosis may have caused fatal hypoglycaemia.7 Thomsen et al theorised that the acidosis itself caused metabolic disruption of vital functions and death, proposing the term ketoalcoholic death.8 Thomsen subsequently reported ketoalcoholic deaths in 7% of sudden deaths in alcoholic patients in a prospective series.9 Similarly, Pounder et al detected very high levels of total ketone bodies, suggesting profound AKA, in 10% of sudden unexplained deaths in alcoholic patients.12 AKA may itself be a cause of sudden death in alcoholic patients through a direct toxic effect or other unknown mechanism.
PATHOPHYSIOLOGY
Ethanol metabolism
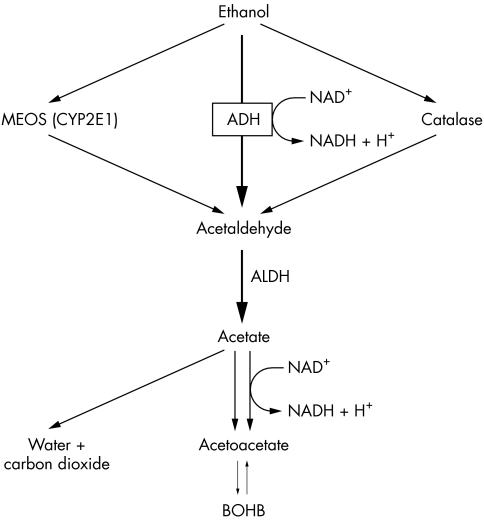
Ethanol is a toxin, the majority of which is oxidised in the liver to acetaldehyde. There are three oxidative pathways: the cytosolic alcohol dehydrogenase pathway, the microsomal ethanol oxidising system, and the peroxisomal catalase pathway. The alcohol dehydrogenase reaction is the principal oxidative pathway and involves the reduction of NAD to NADH (fig 1). Acetaldehyde is then further oxidised in hepatic mitochondria to acetic acid, which then forms acetyl coenzyme A (acetyl CoA). Mitochondrial NAD is reduced to NADH during this process.13 In liver tissue exposed to alcohol, this mitochondrial NADH accumulates, increasing the NADH/NAD ratio, and interfering with mitochondrial metabolic activity. This raised NADH/NAD ratio is thought to be pivotal in the development of ketoacidosis and lactic acidosis as described below.
Figure 1 Metabolism of ethanol. ADH, alcohol dehydrogenase; ALDH, acetaldehyde dehydrogenase; MEOS, microsomal ethanol oxidising; CYP2E1, cytochrome p450 isoform.
Jenkins et al2 suggested that alcohol induced mitochondrial damage might account for AKA. Alcohol produces structural changes in human liver mitochondria within days. Fulop and Hoberman5 argued that a functional abnormality is more likely to be responsible, as even severe AKA usually improves rapidly with treatment. They attributed this to the administration of therapy (intravenous dextrose) rather than the withdrawal of the toxin, ethanol.
Acetyl CoA may be metabolised to carbon dioxide and water, converted to fat, or combined with another acetyl CoA to form acetoacetate (fig 1).
Ketoacidosis
Chronic alcohol misusers have markedly depleted protein and carbohydrate stores. Although they may still receive some caloric intake from ethanol, other sources of dietary intake may be chronically reduced, resulting in starvation and depleted hepatic glycogen stores. The metabolism of ethanol raises the NADH/NAD ratio, impairing hepatic gluconeogenesis from metabolism of lactate, glycerol, and amino acids. In acute alcohol intoxication, this can result in hypoglycaemia.15
Severe vomiting induced by alcohol or its complications may result in decreased extracellular fluid volume, which leads to hypotension and a sympathetic response. Both hypoglycaemia and catecholamine release result in a number of hormonal effects.14 Insulin production decreases, while cortisol, adrenaline, and glucagon levels all rise,3 along with an increased release of growth hormone. In addition, starvation increases levels of cortisol and growth hormone. These changes promote free fatty acid release from triglycerides stored in adipose tissue. Ethanol may also directly stimulate lipolysis, further increasing the supply of fatty acids to the liver.16
Raised glucagon levels and an abundance of free fatty acids promote fatty acid oxidation to acetyl CoA, some of which is diverted to ketogenesis. Two molecules of acetyl CoA are bound together to form acetoacetate plus a hydrogen ion. At high NADH/NAD ratios, acetoacetate is predominantly reduced to form BOHB, which can be decarboxylated to acetone. Acetone and its metabolites are raised in AKA, raising the patient's osmolal gap.17,18 The ratio of BOHB to acetoacetate is significantly higher in patients with AKA than in those with diabetic ketoacidosis who have similar presenting levels of BOHB (19:1 v 11:1).19
Lactic acidosis
Lactic acidosis occurs when ethanol metabolism results in a high hepatic NADH/NAD ratio, diverting pyruvate metabolism towards lactate and inhibiting gluconeogenesis. The excess lactate is consequently exported from the liver. In peripheral tissues, where NADH levels are lower, this lactate may be converted to pyruvate for metabolic needs. Pyruvate and lactate are then maintained in steady state at much higher levels than normal. Severe lactic acidosis is uncommon, in the absence of coexistent sepsis, seizures, thiamine deficiency, or impaired liver function, in alcoholic patients who continue to ingest alcohol.20 Once ethanol ingestion ceases, hepatic NADH/NAD ratio returns toward normal, allowing gluconeogenesis to return to normal. This results in a decrease in circulating lactic acid and an increase in acetoacetate.
DIAGNOSIS
The clinical and biochemical features of AKA are summarised in boxes 1 and 2. The classical presentation is of an alcoholic patient with abdominal pain and intractable vomiting following a significant period of increased alcohol intake and starvation. There may be a history of previous episodes requiring brief admissions with labels of “query pancreatitis” or “alcoholic gastritis”.
BOX 1 PRESENTING FEATURES OF AKA
Characteristic history
Chronic alcohol abuse, plus recent binge
Binge terminated by severe nausea, vomiting, and abdominal pain
History of recurrent episodes
Clinical findings
Tachycardia, hypotension, and increased respiratory rate
Abdominal tenderness with no other specific abdominal findings
Minimal alteration of conscious level despite marked metabolic acidosis
BOX 2 BIOCHEMICAL FEATURES OF AKA
Raised anion gap metabolic acidosis
Normal or low blood glucose
Normal or moderately elevated urea and creatinine
Lactate insufficiently high to explain extent of acidosis
Low or absent blood alcohol level
Urinary ketones usually present on Ketostix testing, but absence does not exclude AKA
Examination should reveal a clear level of consciousness, generalised abdominal tenderness (without peritoneal signs), and tachypnoea. There may be concomitant features of dehydration or early acute alcohol withdrawal. Bedside testing reveals a low or absent breath alcohol, normal blood sugar, metabolic acidosis, and the presence of urinary ketones, although these may sometimes be low or absent. An altered level of consciousness should prompt consideration of alternative diagnoses such as hypoglycaemia, seizures, sepsis, thiamine deficiency, or head injury. Arterial blood gas and biochemistry studies reveal a raised anion gap metabolic acidosis without evidence of lactic or diabetic ketoacidosis.
Toxicity from methanol or ethylene glycol is an important differential diagnosis. Toxic metabolites of both substances result in severe metabolic acidosis with wide anion gap and wide osmolal gap.18 Neither, however, causes ketosis. In addition, there are important clinical differences. Both cause abdominal pain, with marked central nervous system depression, but methanol toxicity results in visual impairment, while ethylene glycol toxicity results in crystalluria, oliguria, and renal failure.
MANAGEMENT
The greatest threats to patients with alcoholic ketoacidosis are marked contraction in extracellular fluid volume (resulting in shock), hypokalaemia, hypoglycaemia, and acidosis. The management of AKA is summarised in box 3.
BOX 3 MANAGEMENT OF AKA
Intravenous rehydration with 5% dextrose (intravenous saline may paradoxically worsen acidosis)
Intravenous thiamine as Pabrinex infusion
Potassium supplementation (may be low on presentation, or fall rapidly on rehydration)
Magnesium and phosphate supplementation if indicated
-
Exclude other serious underlying pathology
-
-
Sepsis
-
-
Involve addictions/alcohol support services once patient is clinically well
Halperin et al used rapid isotonic saline infusion augmented, where required, with potassium supplements.14 This group also recommended glucose to prevent hypoglycaemia, but not insulin or bicarbonate. Miller showed that treatment with 5% dextrose corrected the acidosis and returned the BOHB:AcAc ratio to normal more rapidly than did saline alone.21 In patients with AKA treated with saline alone, lactate levels declined but BOHB levels increased, and the acidaemia in these subjects was significantly worse at 12–24 hours. It is possible that chloride overload contributed to hyperchloraemic metabolic acidosis in these patients. Treatment with 5% dextrose results in progressively increased pH with diminishing lactate and BOHB levels, and a corresponding return to normal BOHB:AcAc ratio. This is also associated with declining plasma free fatty acid, growth hormone, and cortisol levels, and an increased insulin level. Mean time to resolution of acidosis in patients treated for AKA is significantly shorter than that in patients treated for diabetic ketoacidosis (5–7 hours v 14–18 hours).
Current treatment should therefore consist of infusion of dextrose plus rapid rehydration with saline, adding potassium supplementation as necessary. Magnesium and phosphate levels should be monitored during treatment and replaced if required. Marked phosphate drops are noted with glucose treatment, and although there is little evidence to support phosphate supplementation, it should be considered in severe hypophosphataemia (below 0.33 mmol/l).22
All chronic alcohol misusers attending the ED should receive intravenous B vitamins as recommended by The Royal College of Physicians.23 Strenuous efforts must be made to exclude concomitant pathology.
All alcoholic patients presenting with acute illness should be offered contact with addiction services prior to or following discharge wherever possible.
CONCLUSIONS
Alcoholic ketoacidosis is a recognised acute complication in alcohol dependent patients. Reporting of cases is uncommon in the UK. Given the frequency with which the condition is seen in other countries, the possibility exists that many cases may be unrecognised and misdiagnosed in UK EDs. AKA should be included in the differential diagnosis of alcohol dependent patients presenting with acute illness. Management is based around exclusion of serious pathology and specific treatment for AKA where it is present. A possible link between AKA and sudden death in chronic alcoholism has been proposed but remains unconfirmed.
Abbreviations
AcAc - acetoacetate
AKA - alcoholic ketoacidosis
BOHB - beta‐hydroxybutyrate
ED - emergency department
Footnotes
Competing interests: there are no competing interests
References
- 1.Dillon E S, Dyer W W, Smelo L S. Ketone acidosis of non‐diabetic adults. Med Clin N Am 1940241813–1822. [Google Scholar]
- 2.Jenkins D W, Eckle R E, Craig J W. Alcoholic ketoacidosis. JAMA 1971217177–183. [PubMed] [Google Scholar]
- 3.Levy L J, Duga J, Girgis M.et al Ketoacidosis associated with alcoholism in non‐diabetic subjects. Ann Int Med 197378213–219. [DOI] [PubMed] [Google Scholar]
- 4.Cooperman M T, Davidoff F, Spark R.et al Clinical studies of alcoholic ketoacidosis. Diabetes 197423433–439. [DOI] [PubMed] [Google Scholar]
- 5.Fulop M, Hoberman H D. Alcoholic ketosis. Diabetes 197524785–790. [DOI] [PubMed] [Google Scholar]
- 6.Wrenn K D, Slovis C M, Minion G E.et al The syndrome of alcoholic ketoacidosis. Am J Med 199191119–128. [DOI] [PubMed] [Google Scholar]
- 7.Denmark L N. The investigation of beta‐hydroxybutyrate as a marker for sudden death due to hypoglycaemia in alcoholics. Forensic Sci Int 199362225–232. [DOI] [PubMed] [Google Scholar]
- 8.Thomsen J L, Simonsen K W, Felby S.et al A prospective toxicology analysis in alcoholics. Forensic Sci Int 19979033–40. [DOI] [PubMed] [Google Scholar]
- 9.Thomsen J L, Felby S, Theilade P.et al Alcoholic ketoacidosis as a cause of death in forensic cases. Forensic Sci Int 199575163–170. [DOI] [PubMed] [Google Scholar]
- 10.Iten P X, Meier M. β‐Hydroxybutyric acid—an indicator for an alcoholic ketoacidosis as cause of death in deceased alcohol abusers. J Forensic Sci 200045624–632. [PubMed] [Google Scholar]
- 11.Kadis P, Balazic J, Ferlan‐Marolt V. Alcoholic ketoacidosis: a cause of sudden death in chronic alcoholics Forensic Sci Int1999103S53–S59. [Google Scholar]
- 12.Pounder D J, Stevenson R J, Taylor K K. Alcoholic Ketoacidosis at Autopsy. J Forensic Sci 199843812–816. [PubMed] [Google Scholar]
- 13.Palmer J P. Alcoholic ketoacidosis: clinical and laboratory presentation, pathophysiology and treatment. Clin Endocrin Metab 198312381–389. [DOI] [PubMed] [Google Scholar]
- 14.Halperin M L, Hammeke M, Josse R G.et al Metabolic acidosis in the alcoholic: a pathophysiologic approach. Metab Clin Exp 198332308–315. [DOI] [PubMed] [Google Scholar]
- 15.Adler R A. Clinically important effects of alcohol on endocrine function. J Clin Endocrin Metab 199274957–960. [DOI] [PubMed] [Google Scholar]
- 16.Lefevre A, Adler H, Lieber C S. Effect of ethanol on ketone metabolism. J Clin Invest 1970491775–1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schelling J R, Howard R L, Winter S D.et al Increased osmolal gap in alcoholic ketoacidosis and lactic acidosis. Ann Intern Med 1990113580–582. [DOI] [PubMed] [Google Scholar]
- 18.Braden G L, Strayhorn C H, Germain M J.et al Increased osmolal gap in alcoholic acidosis. Arch Int Med 19931532377380. [PubMed] [Google Scholar]
- 19.Umpierrez G E, Digirolamo M, Tuvlin J A.et al Differences in metabolic and hormonal milieu in diabetic and alcohol‐induced ketoacidosis. J Crit Care 20001552–59. [DOI] [PubMed] [Google Scholar]
- 20.Fulop M, Bock J, Ben‐Ezra J.et al Plasma lactate and 3‐hydroxybutyrate levels in patients with acute ethanol intoxication. Am J Med 198680191–194. [DOI] [PubMed] [Google Scholar]
- 21.Miller P D, Heinrig R E, Waterhouse C. Treatment of alcoholic acidosis: the role of dextrose and phosphorus. Arch Int Med 197813867–72. [PubMed] [Google Scholar]
- 22.Miller D W. Hypophosphataemia in emergency department therapeutics. Am J Emerg Med 200018457–461. [DOI] [PubMed] [Google Scholar]
- 23.Royal College of Physicians Alcohol—can the NHS afford it? Recommendations for a coherent alcohol strategy for hospitals. London: RCP, 2001