Abstract
Background
High resolution colour Doppler ultrasound shows intratendinous Doppler activity in patients with chronic Achilles tendinopathy. Treatment of this neovascularisation with sclerosing therapy seems to relieve the pain. However, the procedure often has to be repeated.
Objective
To investigate the effect of electrocoagulation of the neovessels on tendon pain and tendon vascularity in patients with chronic Achilles tendinopathy.
Methods
Colour Doppler ultrasound guided electrocoagulation was used on vessels in the ventral portion of the Achilles tendon in 11 patients (seven men, four women, mean age 41 years) with painful chronic mid‐portion Achilles tendinosis. A unipolar coagulation device was used.
Results
One patient dropped out after two months (dissatisfied with the results). The remaining 10 patients (91%) were satisfied. These 10 patients were still satisfied at six months of follow up and had returned to their previous level of activity. All 10 patients were “cured” after one treatment. The patient who dropped out received two treatments because of lack of progress. There was significantly reduced pain (Likert pain scale, 0–10) during activity, from a median of 7 (range 4 to 10) at baseline to 0 (0 to 8) at six months' follow up (p<0.005); and at rest, from 1.5 (1 to 5) to 0 (0 to 8) (p = 0.005). In all patients, vascularisation was unchanged at the six months follow up, with no significant change in semiquantitative or quantitative colour scoring.
Conclusions
Coagulation in the area with vessels entering the tendon appears to be effective treatment for painful chronic mid‐tendinous Achilles tendinopathy. No effect on the intratendinous Doppler activity could be detected, suggesting that the effect is independent of changes in blood flow. Localisation of hyperaemia appears to be the key to the pathology and for targeting the treatment. One explanation could be that the effect is obtained by destruction of nerves accompanying the vessels.
Keywords: Achilles tendinopathy, colour Doppler, coagulation therapy
The pathogenesis of Achilles tendinopathy has not fully been clarified although most evidence supports the notion of a degenerative disease and thereby classifies the tendinopathy as a tendinosis.1 Although some investigators have abandoned the tendinitis myth completely,2 the terms “tendinitis” and “tendinosis” are used interchangeably. Achilles tendinopathy is a relatively common painful condition which is difficult to treat. High end ultrasound equipment with a very sensitive Doppler has the ability to detect flow in normal muscles and subcutaneous tissues, and occasionally in normal joints,3,4 while normal, asymptomatic Achilles tendons in many reports shows no sign of Doppler activity.5,6,7,8
Some investigators interpret the presence of intratendinous colour Doppler activity in tendinosis of the Achilles tendon as a sign of abnormality5,9 and possible new vessels (neovessels).5 Furthermore, in microdialysis studies and immunohistochemical and gene expression analysis, some of these workers have found not only higher levels of glutamate (a potent modulator of pain in the central nervous system) and the presence of glutamate receptors in close vicinity of nerves,10 but also an upregulation of vascular endothelial growth factor (VEGF) in patients with painful Achilles tendinopathy.11,12 These findings indicate that inside the Achilles tendinosis, nociceptive nerve fibres accompany the neovessels. This hypothesis was followed by reports of successful treatment of the neovessels with sclerosing therapy in both the mid‐portion of the tendon and at its insertion.13,14 The mean number of treatments necessary to obtain not only pain relief but also the disappearance of neovessels has been reported to be two to four, which might reflect insufficient closure of the vessels or recanalisation after successful initial closure. When patients are cured there are reportedly no intratendinous neovessels remaining.13,14
Sclerosing thermal therapy has recently been reported in the treatment of lateral epicondylitis using a radiofrequency probe to carry out microtenotomies.15 Furthermore, in the treatment of patients with shoulder instability, unipolar thermal capsular shrinkage, based on similar procedures, has in some cases proved effective in the short term.16 In surgery, perioperative haemostasis can be obtained by using either monopolar or bipolar coagulation.17 Thus in theory thermal energy applied to the site where neovessels enter the tendon might be an effective way of destroying these vessels in patients with chronic tendinopathies.
Our aim in this study was to develop an efficient technique to cure the pain in chronic Achilles tendinopathy and to examine its effect on tendon vascularity.
Methods
Eleven patients participated (five women and six men). The patients were recruited from private and public orthopaedic and rheumatological units and general practitioners. All had failed previous treatment regimens (table 1).
Table 1 Different treatments regimens that the patients had undertaken before inclusion.
| Treatment | Number of patients |
|---|---|
| Rest | 11 |
| Physiotherapy | 11 |
| Orthoses in shoes | 11 |
| NSAID | 9 |
| Corticoid injections | 8* |
| Eccentric exercise | 9 |
*Injections in close proximity to the tendon (range 1–5): the most recent injection was six months prior to the study.
NSAID, non‐steroidal anti‐inflammatory drug.
Mean age was 41 years (range 25 to 58) and the mean weight was 71 kg (62 to 92). The mean duration of symptoms was 31 months (6 to 120). In all patients, the pain was localised to the part of the Achilles tendon corresponding to the clinical non‐insertional area, >2 cm above the calcaneus, as defined by Clain and Baxter.18 On ultrasound the painful area corresponded to an area in the free part of the tendon with localised swelling and widening of the tendon, hypoechoic and irregular fibre structure, and in some patients also a poorly defined anterior border. All patients had neovessels.
All patients had constant pain or soreness from the Achilles tendons and in all, walking was associated with some pain and running was impossible. Two patients were semiprofessional athletes (a handball player and a triathlete), eight were recreational runners, and one had walking as her main physical activity.
Ultrasound guided coagulation therapy
Ultrasound was dome using a Siemens Sequoia™ (Mountainview, California, USA) equipped with a 14 MHz linear array transducer. The colour Doppler was optimised for low flow and the Nyquist limit was ±0.014 m s−1. The Doppler frequency was 7 MHz and the gain was set just below the level that produced random noise. The Doppler settings were the same for all examinations. We used colour Doppler and not power Doppler, as the sensitivity of the two are equal on the Sequoia. The patients were placed prone with a pillow under the distal tibia, and with the foot hanging freely in an approximately 90° position of the ankle. The Achilles tendon was scanned in longitudinal and transverse planes.19 The tendon and peritendinous tissues were evaluated with colour Doppler and the presence of intratendinous flow was recorded.20 A longitudinal image with maximum flow on colour Doppler was obtained to make a semiquantitative grading of disease activity (see below). The digitally stored images were exported to a computer as a DICOM file. The contralateral non‐painful tendon was also examined. When intratendinous Doppler activity was observed, the entrance of the vessels into the tendon was recorded and used during the coagulation.
Coagulation
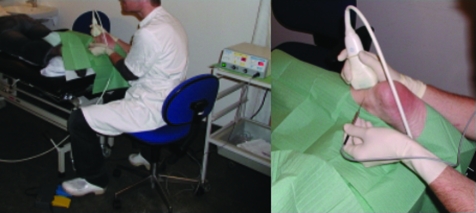
A unipolar 16 G (1.6 mm) coagulation needle connected to an ICC 80 electrosurgical Workstation for Minor Procedures (ERBE®, Tübingen, Germany) was used. The shaft of the needle was electrically isolated and electrocoagulation was only possible at the tip of the needle. The neutral electrode was placed in the patient's hand. The workstation was equipped with a foot switch to activate the coagulation (fig 1). The coagulation was carried out at 20–25 W. By activating the foot switch the monopolar current was transferred through the isolated needle into the tissue and back again via the neutral electrode. The power was hereby delivered at the unisolated tip.
Figure 1 Coagulation procedure. Left: The patient is lying prone and the right Achilles tendon is being treated from the medial side. The coagulation device is seen behind the physician. The device is activated by the foot switch seen at the bottom of the image. Right: Close up of the coagulation needle during insertion. The physician is wearing gloves and the ultrasound transducer is covered by a condom. The needle is connected to the coagulation device by the cable. Informed consent was obtained for publication of this figure.
Before treatment, the skin was washed with a solution of chlorhexidine and alcohol. The skin was then covered with sterile paper with a hole cut to reveal only the free part of the Achilles tendon. The skin and subcutaneous tissue were then anaesthetised locally with 5–10 ml of lignocaine (lidocaine) 2%.
The transducer was placed on the dorsal side of the Achilles tendon parallel or transverse to the fibres depending on the best visualisation of the vessels.
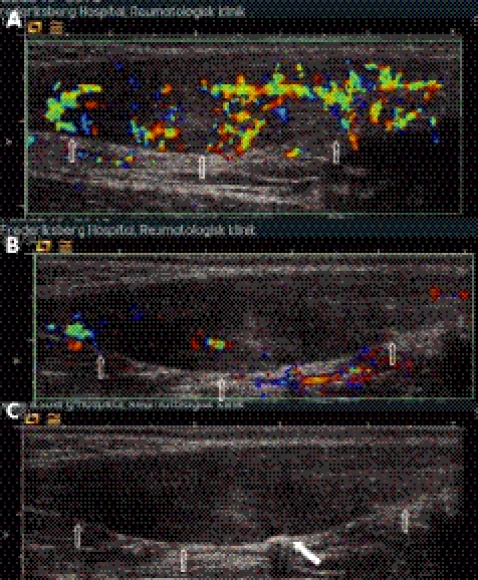
The needle was inserted from the medial side and placed against the vessels entering the Achilles tendon from the ventral side of the tendon (the site is identical to the site used during sclerosing therapy)13 (figs 2 and 3). When the tip of the needle was positioned correctly, coagulation was activated by the footswitch. Coagulation was carried out dynamically (on average for one to two seconds at each site) until the Doppler signal had disappeared (fig 2). By applying this procedure to all vessels entering the pathological area it was possible remove the baseline intratendinous flow visible on ultrasound Doppler. During the procedure, we observed transitory minor hyperaemia in the surrounding region both intratendinously and extratendinously.
Figure 2 Colour Doppler activity at baseline and during treatment. Longitudinal images of an Achilles tendon with proximal oriented left. The anterior borders of the tendons are indicated with arrows. In (A) the Doppler activity was graded as 2 and had a colour fraction of 33%. In (B) and (C) the same tendon is being treated with coagulation therapy. The distal part of the tendon is “shut down” and the proximal part remains to be treated (B); (C) is the corresponding grey scale image of (B). The white filled arrow marks the ongoing coagulation.
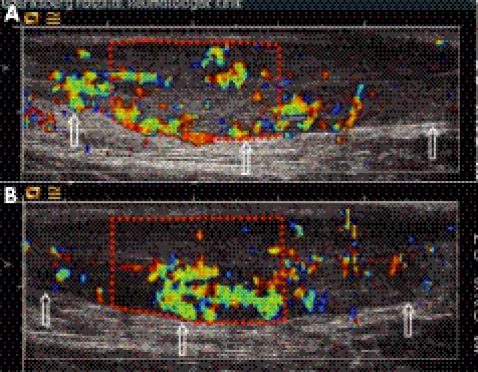
Figure 3 Treatment procedure. Transverse image of an Achilles tendon with the medial portion oriented to the left. The anterior border of the tendon is indicated with a white arrow. In (A) the tendon is seen to have intratendinous Doppler activity; in (B) the same tendon is seen in a grey scale image, with the coagulation needle approaching from the medial side (filled white arrow).
After the procedure, all patients were pain‐free because of the local anaesthesia. After the coagulation ice compression was applied for 10 to 20 minutes to reduce possible reactive hyperaemia after the invasive procedure. Because of the effect of the local anaesthetic there was no pain during the procedure, but in most patients pain returned the same evening.
The patients were allowed to walk, cycle, or swim on the day after the treatment but no jogging until two weeks later, depending on the follow up examinations which took place 14 days, one month, three months, four months, and six months after the treatment. All coagulations were undertaken by the same experienced radiologist (STP).
Outcome measures
At study entry and at the follow up examinations, the same investigator did the ultrasound examination and assessed the vascularisation, using both a semiquantitative and a quantitative scoring system according to the appearance of vessels inside the tendons.
Semiquantitative grading system
The tendons were graded according to the amount of intratendinous Doppler activity inside the region of interest (ROI). When Doppler activity was present, the centre with the most pronounced Doppler activity was defined and 1 cm of the tendon in both proximal and distal directions was used as the ROI.
The semiquantitative grading system has five grades (0–4): 0, no Doppler activity; 1, one or two tiny colour foci; 2, up to 50% colour inside the ROI; 3, 50–90% colour inside the ROI; 4, 90–100% colour inside the ROI (fig 4). This grading is similar to a grading system defined by Ohberg et al with respect to grades 0 and 1.13 Our grades 2, 3, and 4 were defined on the basis of ROI covered with colour, which has not been defined in previous studies.
Figure 4 Colour Doppler grading before treatment and after six months of follow up. Longitudinal images of an Achilles tendon with proximal oriented left. The anterior borders of the tendons are indicated with arrows. For illustration, the region of interest has been added (red dotted line) and gives a value of 33% in (A) (before treatment) and 45% in (B) (at follow up). The centre of the maximum Doppler activity defines the centre of the ROI, which extends 1 cm proximally and distally.
Quantitative scoring system
A longitudinal image with maximum flow on colour Doppler was obtained to calculate the percentage of colour pixels. The digitally stored image was exported as a DICOM file, and the colour fraction calculated with a β version of DataPro (Noesis, Courtaboeuf, France). The ROI of the Achilles tendon was traced inside the colour box, whereupon the software reported the number of colour pixels and the total number of pixels inside the trace. We then calculated the colour fraction as colour pixels/total pixels.21
Pain score
Achilles tendon pain was recorded using a Likert box scale (from 0 to 10) for pain during rest and activity at baseline and on follow up examination. Each patient's satisfaction with the result of treatment was also recorded as satisfying or not satisfying.
Treatment strategy
If there was any remaining intratendinous hyperaemia and if the patient's symptoms had not subsided, the treatment was offered again. In case of remaining hyperaemia at follow up but reduced pain, no further treatment was given.
Statistics
All analyses were based on 11 patients as the intention‐to‐treat population, although only 10 of the participating patients completed the follow up. The drop‐out was included as last observation carried forward.
All tests are based on the Wilcoxon matched pairs signed rank sum test with a two sided significance level at 5%; these are presented as median (range).
The investigation was approved by the ethics committee of Copenhagen (KF 11 284314).
Results
Before treatment
All patients had intratendinous Doppler activity. The median pain score before treatment for the 11 patients was 7 (range 4 to 10) during activity and 1.5 (range 1 to 8) during rest. Colour Doppler activity was grade 2 in most tendons (table 2). The mean (SD) colour fraction was 24 (18)%.
Table 2 Semiquantitative and quantitative scoring of the Achilles tendons before treatment and after six months of follow up.
| Patient | Semiquantitative grading* | Colour fraction† | ||
|---|---|---|---|---|
| Before | After | Before | After | |
| 1 | 2 | 0 | 7% | 0% |
| 2 | 2 | 2 | 4% | 35% |
| 3 | 2 | 2 | 12% | 11% |
| 4 | 2 | 2 | 10% | 14% |
| 5 | 2 | 3 | 33% | 45% |
| 6 | 2 | 2 | 19% | 23% |
| 7 | 2 | 2 | 12% | 9% |
| 8 | 3 | 2 | 51% | 44% |
| 9 | 3 | 2 | 44% | 33% |
| 10 | 3 | 3 | 45% | 52% |
*See the text for detailed description of the grading system.
†See the text for detailed description of the colour fraction.
All the contralateral tendons were slim with a regular tendon fibre structure and no intratendinous hyperaemia.
After treatment
One of the patients dropped out after the two months follow up because of disappointment with the results. She had received two treatments (at baseline and at the one month follow up) but declined an offer of further treatment.
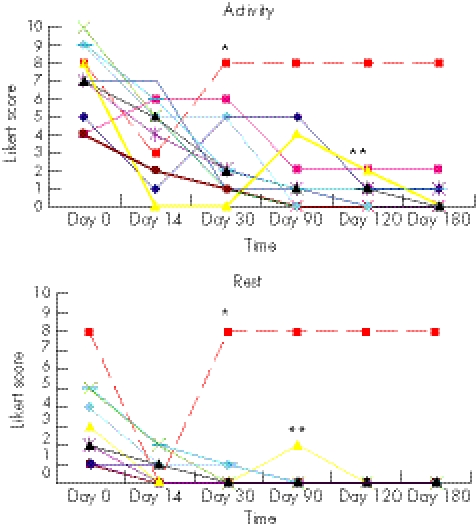
At the six months follow up, all the remaining 10 patients were satisfied with the treatment and had resumed their preinjury activities. The median pain score after treatment was 0 (range 0 to 8, n = 11) during activity and 0 (0 to 8, n = 11) during rest (fig 5, panels A and B).
Figure 5 Pain during follow up shown on a Likert box score in the 11 patients. The graphs illustrates the outcome on the Likert box scale for each patient (coloured line) during activity (A) and at rest (B). *The red dotted line indicates the patient who dropped out at the 30 day follow up. The thick black line shows development in median Likert score. **The yellow line indicates a patient who experienced relapse of his symptoms because he returned too fast to full training load and ran 20 km three days in a row. He recovered on conservative treatment.
There was a highly significant reduction in Likert pain score compared with baseline. The median overall pain score reduction (score at baseline minus score at day 180) was 7 during activity (p<0.005) and 1 during rest (p = 0.005). The reduction in pain was significant at all follow up examinations—baseline to day 14: activity, p = 0.009; rest, p = 0.011; baseline to day 30: activity, p = 0.011; rest, p = 0.007; baseline to day 90: activity, p = 0.008; rest, p = 0.004; baseline to day 120: activity, p = 0.005, rest, p = 0.004; baseline to day 180: activity, p = 0.005; rest, p = 0.004.
These differences corresponded to a self reported success rate of 10/10 (100%); the corresponding intention‐to‐treat success rate was: 10/11 (91%).
At the six months follow up, no change was observed in colour fraction or colour grading compared with baseline (table 2). The mean (SD) change in colour fraction (intratendinous hyperaemia) was an increase of 3 (12)% (p = 0.47). Likewise, on semiquantitative scoring there was no significant change in grading compared with baseline (p = 0.26).
On grey scale ultrasound there was no change in the diameter (thickening) or appearance (structure) of the tendons (data not shown).
No side effects were observed.
Discussion
In this study we found that electrocoagulation of the neovessels in patients with chronic Achilles tendinopathy significantly reduced tendon pain during activity and rest at a six month follow up in 10 of 11 patients. The treatment in the present study was applied to the pathological area with intratendinous hyperaemia seen on Doppler, and the immediate response was a disappearance of the intratendinous hyperaemia. In all cases, however, the hyperaemia reappeared in the two week interval between treatment and follow up (fig 4, panels A and B). In the same period a proportion of the patients experienced some degree of relapse or had even more pain than at baseline. Nevertheless, at the six month follow up, the treatment proved very effective with regard to pain relief. To our knowledge, we are the first to show that patients with Achilles tendinopathy and residual neovessels after perioperative electrocoagulation and termination of the intratendinous hyperaemia can be pain‐free. Our treatment was based on the same theory as sclerosing therapy13,22; however, electrocoagulation differs from the former in the way in which the vessels are obliterated and in being potentially more locally destructive.
Previous studies using ultrasound colour,5 power,23 and laser Doppler24 have shown that painful chronic Achilles tendinopathy is associated with neovascularisation within the tendon which may be accompanied by pain. However, the mere presence of intratendinous hyperaemia cannot be regarded as abnormal in all cases, because some visible perfusion may be physiological.25 In the reports of sclerosing therapy, it was concluded that clinical improvement corresponded to the elimination of the colour Doppler appearances of neovascularisation.22 Our results with respect to electrocoagulation directed against the area with Doppler activity (neovessels) gave a similar pain relief as found in the study by the Umeå group using sclerosering agents. However, in contrast to the sclerosing therapy we did not observe any significant effect on the presence of neovascularisation at the end of the follow up period. We do not know if more aggressive treatment of the hyperaemia could have resulted in the total disappearance of the neovessels and in better pain relief in some patients at the first follow up examination. An explanation of the remaining hyperaemia could be that coagulation therapy introduces local tissue damage which generates an inflammatory response. In our opinion this inflammatory response is healthy for the patient and may be the same we saw at follow up.
No significant change was found in the Doppler activity in the 10 patients at the six month follow up compared with baseline. As a result there was no correlation between Doppler and pain reduction in the present study. The improvement in pain was significant at all follow up examinations. However, based on our clinical impression, most patients felt the best pain relief after the second follow up (fig 5). We have no good explanation for this.
Our findings differ from the previously published results of a prospective double blind trial, in which an absence of neovascularisation was reported in the pain‐free tendons.22 The same investigators have, however, recently reported that hypervascularity persists for at least three weeks after sclerosing therapy. The hyperaemia found in our study persists throughout the follow up period of six months, indicating a difference between sclerosing therapy and coagulation therapy, even though they have the same objective—namely, the obliteration of visible vessels entering the Achilles tendon.
Our results indicate that the effect of the treatment may be a result of mechanisms other than the immediate change in vascular supply. One possibility may be a destruction of the nerves accompanying the vessels.12
The present treatment with electrocoagulation of the vessels was introduced as a supplement to sclerosing therapy, which have shown to be efficient in both patellar tendinopathy and Achilles tendinopathy.22,26
Besides being very efficient for pain relief, coagulation treatment has the advantage compared with the use of sclerosing treatments that a single therapist can undertake the procedure. The therapist may be a surgeon (senior/junior), radiologist, or sports physician with a licence to treat and experience with ultrasound and ultrasound guided injection/treatment.
What is already known on this topic
Refractory Achilles tendon pain may be reduced by sclerosing the neovascularised area.
Pain relief has been reported to be associated with disappearance of the neovessels.
What this study adds
Refractory Achilles tendon pain may be reduced by coagulating the area affected by neovascularisation, possibly because of denervation.
No effect on neovascularisation was seen at follow up.
Controlled trials are needed to test this new treatment further.
The heat effect of the treatment is delivered exactly at the tip of the coagulation needle. In contrast, sclerosing agents may diffuse into the adjacent areas after injection. A disadvantage with coagulation is that both ultrasound and coagulation equipment are highly equipment dependent, and that it has to be done under local anaesthesia. Full treatment takes about 45 minutes, and if the number of treatments can be reduced in the future then both patients and physicians may benefit from this new approach.
Potential complications include infection, especially at the insertion sites; nerve damage to the sural nerve, which may be avoided by always approaching from the medial side; and tendon rupture if the coagulation is done intratendinously by mistake.
Limitations
We are aware of some limitations to our study. The patients were not blinded to the treatment and there was no control group. The design of the treatment and the study did not allow this. However, the clear reduction in pain at the six month follow up shows that coagulation is a very effective treatment for patients with chronic Achilles pain. We do not know whether a randomised controlled study would support our results, or whether the same findings will be observed over a longer follow up period. Moreover, we do not know if the patients will remain at their preinjury activity level without a relapse in their symptoms. In this pilot study a Likert pain score was used, which is a categorised pain measure. In future studies it may be more appropriate to use outcome assessment tools, such as the VISA‐A score,27 the visual analogue scale (VAS), or other validated measurement tools.28 These measures are already included in our future studies on electrocoagulation. RCT studies with electrocoagulation versus placebo and longer follow up are currently ongoing.
Conclusions
Coagulation in the area showing neovessels entering the tendon appears to be an effective treatment for painful chronic mid‐tendinous Achilles tendinopathy. The procedure is simple to carry out in combination with ultrasound.
No lasting effect on the intratendinous Doppler activity could be detected, suggesting that the positive effect of the coagulation on pain results from destruction of nerves accompanying the neovessels. We believe that the localisation of neovessels remains the key to the pathology of this condition and for targeting treatment.
The link between pain and neovascularisation remains to be demonstrated and would enable a better understanding of the nature of chronic Achilles tendon problems.
Acknowledgements
The study was founded by the OAK foundation and Aase og Ejnar Danielsens Fond and by Direktør Einar Hansen and Hustru Fru Vera Hansens Fond.
Footnotes
Competing interests: none declared
Informed consent was obtained for publication of figure 1
References
- 1.Khan K M, Cook J L, Bonar F.et al Histopathology of common tendinopathies. Update and implications for clinical management. Sports Med 199927393–408. [DOI] [PubMed] [Google Scholar]
- 2.Khan K M, Cook J L, Kannus P.et al Time to abandon the “tendonitis” myth. BMJ 2002324626–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Terslev L, Torp‐Pedersen S, Bang N.et al Doppler ultrasound findings in healthy wrists and finger joints before and after use of two different contrast agents. Ann Rheum Dis 200564824–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Terslev L, Torp‐Pedersen S, Qvistgaard E.et al Doppler ultrasound findings in healthy wrists and finger joints. Ann Rheum Dis 200463644–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ohberg L, Lorentzon R, Alfredson H. Neovascularisation in Achilles tendons with painful tendinosis but not in normal tendons: an ultrasonographic investigation. Knee Surg Sports Traumatol Arthrosc 20019233–238. [DOI] [PubMed] [Google Scholar]
- 6.Silvestri E, Biggi E, Molfetta L.et al Power Doppler analysis of tendon vascularization. Int J Tissue React 200325149–158. [PubMed] [Google Scholar]
- 7.Reiter M, Ulreich N, Dirisamer A.et al Colour and power Doppler sonography in symptomatic Achilles tendon disease. Int J Sports Med 200425301–305. [DOI] [PubMed] [Google Scholar]
- 8.Richards P J, Win T, Jones P W. The distribution of microvascular response in Achilles tendonopathy assessed by colour and power Doppler. Skel Radiol 200534336–342. [DOI] [PubMed] [Google Scholar]
- 9.Martinoli C, Pretolesi F, Crespi G.et al Power Doppler sonography: clinical applications. Eur J Radiol 199827(suppl 2)S133–S140. [DOI] [PubMed] [Google Scholar]
- 10.Alfredson H, Forsgren S, Thorsen K.et al Glutamate NMDAR1 receptors localised to nerves in human Achilles tendons. Implications for treatment? Knee Surg Sports Traumatol Arthrosc 20019123–126. [DOI] [PubMed] [Google Scholar]
- 11.Alfredson H, Lorentzon M, Backman S.et al cDNA‐arrays and real‐time quantitative PCR techniques in the investigation of chronic Achilles tendinosis. J Orthop Res 200321970–975. [DOI] [PubMed] [Google Scholar]
- 12.Alfredson H, Ohberg L, Forsgren S. Is vasculo‐neural ingrowth the cause of pain in chronic Achilles tendinosis? An investigation using ultrasonography and colour Doppler, immunohistochemistry, and diagnostic injections. Knee Surg Sports Traumatol Arthrosc 200311334–338. [DOI] [PubMed] [Google Scholar]
- 13.Ohberg L, Alfredson H. Ultrasound guided sclerosis of neovessels in painful chronic Achilles tendinosis: pilot study of a new treatment. Br J Sports Med 200236173–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ohberg L, Alfredson H. Sclerosing therapy in chronic Achilles tendon insertional pain‐results of a pilot study. Knee Surg Sports Traumatol Arthrosc 200311339–343. [DOI] [PubMed] [Google Scholar]
- 15.Tasto J P, Cummings J, Medlock V.et al Microtenotomy using a radiofrequency probe to treat lateral epicondylitis. Arthroscopy 200521851–860. [DOI] [PubMed] [Google Scholar]
- 16.Savoie F H, Field L D. Thermal versus suture treatment of symptomatic capsular laxity. Clin Sports Med 20001963–75. [DOI] [PubMed] [Google Scholar]
- 17.Barret E, Guillonneau B, Cathelineau X.et al Laparoscopic partial nephrectomy in the pig: comparison of three hemostasis techniques. J Endourol 200115307–312. [DOI] [PubMed] [Google Scholar]
- 18.Clain M R, Baxter D E. Achilles tendinitis. Foot Ankle 199213482–487. [DOI] [PubMed] [Google Scholar]
- 19.Rasmussen O S. Sonography of tendons. Scand J Med Sci Sports 200010360–364. [DOI] [PubMed] [Google Scholar]
- 20.Weinberg E P, Adams M J, Hollenberg G M. Color Doppler sonography of patellar tendinosis. Am J Roentgenol 1998171743–744. [DOI] [PubMed] [Google Scholar]
- 21.Terslev L, Torp‐Pedersen S, Qvistgaard E.et al Spectral Doppler and resistive index. A promising tool in ultrasonographic evaluation of inflammation in rheumatoid arthritis. Acta Radiol 200344645–652. [DOI] [PubMed] [Google Scholar]
- 22.Alfredson H, Ohberg L. Sclerosing injections to areas of neo‐vascularisation reduce pain in chronic Achilles tendinopathy: a double‐blind randomised controlled trial. Knee Surg Sports Traumatol Arthrosc 200513338–344. [DOI] [PubMed] [Google Scholar]
- 23.Zanetti M, Metzdorf A, Kundert H P.et al Achilles tendons: clinical relevance of neovascularization diagnosed with power Doppler US. Radiology 2003227556–560. [DOI] [PubMed] [Google Scholar]
- 24.Knobloch K, Kraemer R, Lichtenberg A.et al Achilles tendon and paratendon microcirculation in midportion and insertional tendinopathy in athletes. Am J Sports Med 20063492–97. [DOI] [PubMed] [Google Scholar]
- 25.Boesen M I, Koenig M J, Torp‐Pedersen S.et al Tendinopathy and Doppler activity: The vascular response of the Achilles tendon to exercise. Scand J Med Sci Sports 2006 [DOI] [PubMed]
- 26.Alfredson H, Ohberg L. Neovascularisation in chronic painful patellar tendinosis‐promising results after sclerosing neovessels outside the tendon challenge the need for surgery. Knee Surg Sports Traumatol Arthrosc. (in press) [DOI] [PubMed]
- 27.Robinson J M, Cook J L, Purdam C.et al The VISA‐A questionnaire: a valid and reliable index of the clinical severity of Achilles tendinopathy. Br J Sports Med 200135335–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Paoloni J A, Appleyard R C, Murrell G A. The Orthopaedic Research Institute‐Ankle Strength Testing System: inter‐rater and intra‐rater reliability testing. Foot Ankle Int 200223112–117. [DOI] [PubMed] [Google Scholar]