Abstract
Background
Previous studies have demonstrated that in patients with coronary artery disease (CAD) upward deflection of the heart rate (HR) performance curve can be observed and that this upward deflection and the degree of the deflection are correlated with a diminished stress dependent left ventricular function. Magnesium supplementation improves endothelial function, exercise tolerance, and exercise induced chest pain in patients with CAD.
Purpose
We studied the effects of oral magnesium therapy on exercise dependent HR as related to exercise tolerance and resting myocardial function in patients with CAD.
Methods
In a double blind controlled trial, 53 male patients with stable CAD were randomised to either oral magnesium 15 mmol twice daily (n = 28, age 61±9 years, height 171±7 cm, body weight 79±10 kg, previous myocardial infarction, n = 7) or placebo (n = 25, age 58±10 years, height 172±6 cm, body weight 79±10 kg, previous myocardial infarction, n = 6) for 6 months. Maximal oxygen uptake (VO2max), the degree and direction of the deflection of the HR performance curve described as factor k<0 (upward deflection), and the left ventricular ejection fraction (LVEF) were the outcomes measured.
Results
Magnesium therapy for 6 months significantly increased intracellular magnesium levels (32.7±2.5 v 35.6±2.1 mEq/l, p<0.001) compared to placebo (33.1±3.1.9 v 33.8±2.0 mEq/l, NS), VO2max (28.3±6.2 v 30.6±7.1 ml/kg/min, p<0.001; 29.3±5.4 v 29.6±5.2 ml/kg/min, NS), factor k (−0.298±0.242 v −0.208±0.260, p<0.05; −0.269±0.336 v −0.272±0.335, NS), and LVEF (58±11 v 67±10%, p<0.001; 55±11 v 54±12%, NS).
Conclusion
The present study supports the intake of oral magnesium and its favourable effects on exercise tolerance and left ventricular function during rest and exercise in stable CAD patients.
Keywords: echocardiography, exercise, heart rate performance curve, magnesium
Recent studies have reported that oral magnesium supplementation has favourable effects on left ventricular ejection fraction (LVEF)1 and improves endothelial function as measured by brachial artery flow mediated dilation in patients with coronary artery disease (CAD).2 The favourable effect of oral magnesium on endothelial function was shown to be similar to that observed following lipid lowering therapy.3 In addition, it was shown that functional capacity is greater in stable CAD patients with higher intracellular levels of magnesium ([Mg]i), suggesting that magnesium plays a role in CAD pathophysiology, possible via ventricular unloading.4,5 Additionally, 6 month oral magnesium supplementation in CAD patients resulted in a significant improvement in exercise induced chest pain and quality of life, suggesting a potential mechanism whereby magnesium could beneficially alter outcomes in patients with CAD.5 We have recently reported that an upward deflection of the heart rate performance curve (HRPC) can be observed in patients after myocardial infarction and that this upward deflection and the degree of the deflection are correlated with a diminished stress dependent LVEF.6
The aim of the current study was to compare the effects of oral magnesium supplementation with placebo on exercise dependent heart rate behaviour as a non‐invasive indicator of exercise dependent myocardial function in patients with CAD. We hypothesised that magnesium supplementation would improve both resting and exercise induced myocardial function as indicated by exercise induced heart rate behaviour.6
methods
Patients were recruited consecutively into a randomised, prospective, double blind, placebo controlled trial. Inclusion criteria included CAD patients >40 years old documented by a previous myocardial infarction, coronary artery bypass surgery, or coronary angiography or angioplasty. Exclusion criteria were unstable angina, chronic heart failure, New York Heart Association functional class III, chronic diarrhoea, renal failure (serum creatinine >3 mg/dl), acute myocardial infarction within the preceding 6 months, hyper/hypothyroidism, diabetes mellitus, peripheral vascular disease, history of drug or alcohol abuse, chronic liver disease, or chronic obstructive pulmonary disease. The study protocol was approved by the Institutional Ethics Committee of the University of Vienna and signed written informed consent was obtained from each participant.
Patients were randomised to either oral Magnosolv‐Granulat (15 mmol magnesium ion, total magnesium of 365 mg provided as a citrate, and 5.4 mmol potassium as a hydrogen carbonate; Viatris Arzneimittel, Vienna, Austria) or placebo twice daily for 6 months, and instructed to continue their regular medications and diet during the study. Medications, diet, and exercise patterns were assessed via questionnaire before and at the end of the study. None of the patients participated in an organised or structured resistance or endurance exercise program before or during the study and daily physical activity level remained unchanged. At entry and after 6 months, patients underwent a physical examination, exercise testing (cycle ergometer), and sublingual [Mg]i measurement.
The study population consisted of 70 male patients. All patients had documented CAD as determined by a previously conducted coronary angiography. Seven patients in the magnesium group and 10 patients in the placebo group discontinued the study prematurely for the following reasons: four withdrew due to patient decision, but not because of any adverse events (one in the magnesium group and three in the placebo group); elective surgery (one in the magnesium group and one in the placebo group); pacemaker implantation (one in the placebo group); atrial fibrillation (two in the placebo group); poor study drug compliance (one in the magnesium group and two in the placebo group); and adverse events – diarrhoea (four in the magnesium group and one in the placebo group).
Exercise testing
Protocol
Remaining participants performed an incremental exercise test on a cycle ergometer in an upright position to the limit of tolerance. The exercise test started at an initial workload of 20 W followed by 10 W increments every minute until exhaustion.
Measures
For safety reasons, the ECG was monitored continuously via a 12 lead ECG, recorded at rest, during the last 10 s of each increment, at the end of the exercise test, and during recovery. Blood pressure was monitored throughout the test and during recovery by the auscultation method.
Capillary blood samples for the analysis of blood lactate concentration (LA) were collected from the hyperaemic ear lobe at rest, during the last 10 s of each increment, at the end of the exercise test, and every minute of a 6 min recovery period. LA was measured by a fully enzymatic amperometric method in whole blood using the Eppendorf automatic analyser (EBIO 6666, Eppendorf, Germany).
As in previous studies, LA performance curves7,8 were used to determine three phases of energy supply according to Skinner and McLellan.9 The first (LTP1) and second lactate turn points (LTP2) were determined by means of linear regression break point analysis as presented earlier.7,8 LTP1 was defined as the point where the LA level began to increase systematically above resting values, which is comparable to the lactate threshold and the anaerobic threshold according to Wassermann et al.10 LTP2 was defined as the second abrupt increase in LA, termed lactate turn point according to Davis et al.11
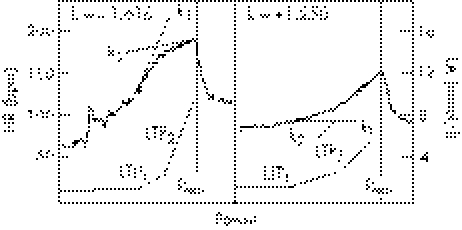
Heart rate (HR) was recorded continuously at 5 s intervals using Polar Vantage NV telemetry (Polar Electro, Kempele, Finland). The direction and degree of HRPC deflection were calculated by a second degree polynomial equation of the HRPC between LTP1 and maximum power (Pmax), satisfying the conditions of least error squares. From the function thus found, the slopes of the tangents k1HR and k2HR at the points of LTP1 and Pmax were calculated, as well as the differences of angles represented by the following equation: kHR = (k1HR−k2HR)⋅(1+k1HR⋅k2HR)−1. The degree and the direction of the deflection of HRPC were described as factor kHR (<0: downward deflection indicates negatively accelerated increase in HR; >0: upward deflection indicates positively accelerated increase in HR)6,7,12,13,14 (fig 1).
Figure 1 Principle of the determination of the inflection of the heart rate work performance curve by means of second degree polynomial fitting of the curve and calculation of slopes of the tangents at the first lactate turn point (LTP1) and maximal power output (Pmax). The differences of angles are given for a single subject with a negatively accelerated heart rate (left; kHR<0: downward deflection) and a positively accelerated heart rate (right; kHR>0: upward deflection).
Two dimensional (2D) echocardiograms were performed on all subjects before and after 6 months of treatment using a commercially available instrument (Ultrasound AS CVFM 800 and 3.5 MHz transducer; GE Vingmed, Horten, Norway). 2D images in the parasternal long axis were obtained at rest. We used Vingmed's anatomical M‐mode equipped with a special mode feature that allows extraction of M‐mode sweeps from stored 2D loops and performs the M‐mode measurements just distal to the tips of the mitral valve. Subjects were instructed to lie in the left lateral decubitus position so that left ventricular internal diameter during diastole (LVDD) and systole (LVSD) could be measured. Left ventricular systolic function was evaluated by calculating the LVEF using established formulae. All measures were taken in accordance with the guidelines of the American Society of Echocardiography (ACC/AHA).15 The investigator analysed echoes online in a blinded manner.
Respiratory gas exchange measures were analysed continuously utilising an open air spirometry system for all tests in breath‐by‐breath mode (Oxycon‐Alpha; Jäger, Würzburg, Germany). The fraction of expired oxygen (O2) and carbon dioxide (CO2) concentrations (FEO2/FECO2) were measured using rapid gas analysers, while a turbine analysed the ventilatory flow and breathing frequency to calculate pulmonary ventilation. O2 uptake was calculated from these measures. All instruments were calibrated before each test and the necessary environmental adjustments were made according to the manufacturer's guidelines.
Intracellular magnesium assessment
Intracellular magnesium ([Mg2+]i) was measured by x ray dispersive microanalysis utilising a specially configured electron microscope which images and irradiates cells with a focused electron beam. Sublingual epithelial cells were chosen as markers since they are easily accessible, are non‐cornified, are aerobic, have a high cytoplasm to nucleus ratio, turn over in less than 3 days, have a long shelf life, and exhibit 99% viability. Excitation of cellular atoms displaces inner orbital electrons which are replaced by electrons from higher energy cells releasing fluoresced x rays which allow quantitation of elements. EXA tm units are converted to mEq/l by a conversion constant for each element derived from a reference standard of a highly stable matrix synthetic glass containing known amounts of the elements to be analysed (normal mean±standard deviation (SD) values: 37.9±4.0 mEq/l).16
Statistics
The results are expressed as means±SD. Paired and unpaired Student's t tests were used to evaluate differences of the selected variable. Furthermore, analysis of variance (ANOVA) with repeated measures was used to evaluate differences in the time course of power output before and after treatment. Differences between LTP1, LTP2, and Pmax were obtained by a post hoc LSD (least significant differences) analysis test.
Results
At 6 months, 28 patients who had received magnesium and 25 patients who had received placebo completed the study and were included in the efficacy analysis. No significant group differences in baseline characteristics were seen (table 1). There were no significant changes in the use of concomitant medications throughout the course of the study.
Table 1 Baseline characteristics of the study population.
| Variable | Magnesium (n = 28) | Placebo (n = 25) |
|---|---|---|
| Age (years) | 61±9 | 58±10 |
| Height (cm) | 171±7 | 172±6 |
| Body weight (kg) before treatment | 79±10 | 79±10 |
| Body weight (kg) after treatment | 80±10 | 79±10 |
| Systemic hypertension | 15 | 15 |
| Previous myocardial infarction | 7 | 6 |
| Previous coronary angiography | 28 | 25 |
| Previous coronary angioplasty | 17 | 18 |
| Previous coronary bypass | 7 | 3 |
| β Receptor antagonists | 22 | 21 |
| Calcium antagonists | 6 | 7 |
| Digitalis | 0 | 0 |
| Diuretics | 0 | 0 |
| Acetylsalicylic acids | 26 | 27 |
| Long acting nitrates | 5 | 3 |
| Angiotensin converting enzyme | 17 | 17 |
| inhibitors | ||
| Lipid lowering agents | 19 | 20 |
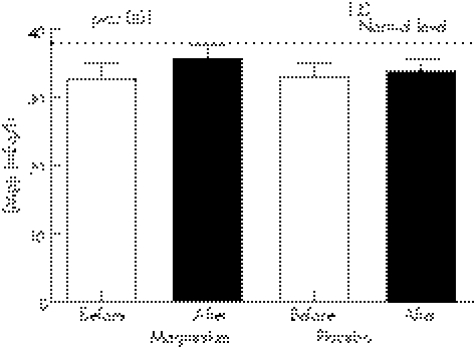
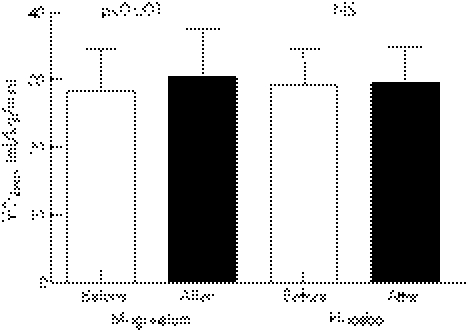
Baseline [Mg]i was similar between the magnesium (32.7±2.5 mEq/l) and the placebo (33.1±1.9 mEq/l) groups. Magnesium therapy significantly increased [Mg]i versus placebo (35.7±2.1 v 33.8±2.0 mEq/l, p<0.001) (fig 2).
Figure 2 Intracellular magnesium levels ([Mg]i) before and after treatment.
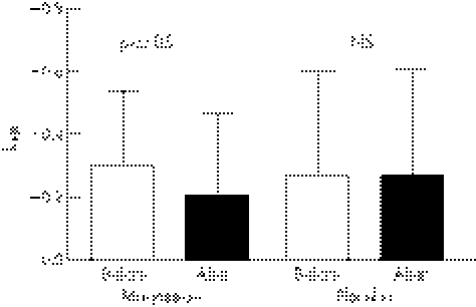
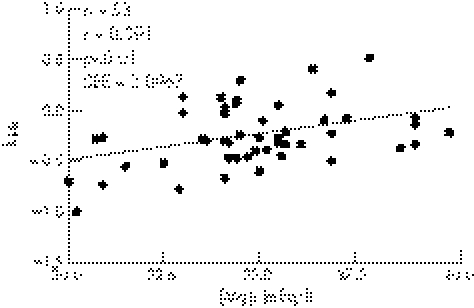
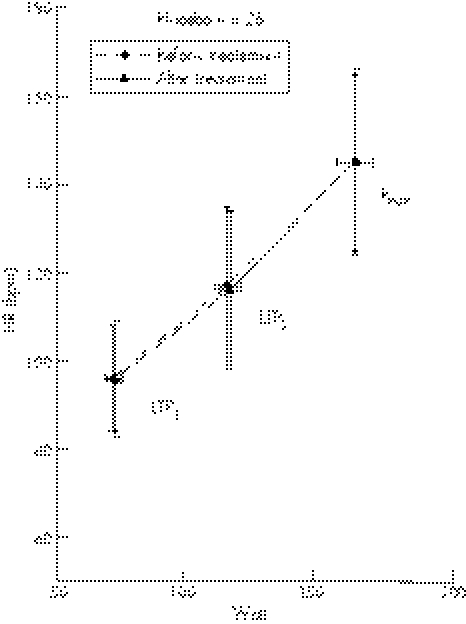
Baseline kHR was similar between the magnesium (−0.297±0.242) and placebo (−0.269±0.335) groups. Magnesium therapy significantly increased kHR compared to placebo (−0.208±0.260 v −0.272±0.335, p<0.05) (fig 3). There was a significant correlation in the total population between exit [Mg]i and kHR (r = 0.391, p⩽0.01) (fig 4).
Figure 3 Degree and direction of the deflection of the heart rate performance curve described as factor kHR (>0 indicates upward deflection) before and after treatment.
Figure 4 Correlation of the intracellular magnesium levels ([Mg]i) and the degree and direction of the deflection of the heart rate performance curve described as factor kHR (>0 indicates upward deflection).
Before treatment, VO2max was similar (p⩾0.05) between the magnesium (28.3±6.2 ml/kg/min) and placebo (29.3±5.4 ml/kg/min) groups. However, after magnesium treatment, VO2max was significantly increased in the magnesium but not in the placebo group (30.5±7.1 v 29.6±5.2 ml/kg/min, p<0.001) (fig 5).
Figure 5 Maximal oxygen uptake (VO2max) before and after treatment.
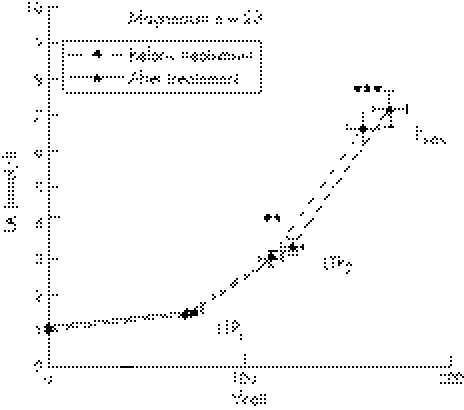
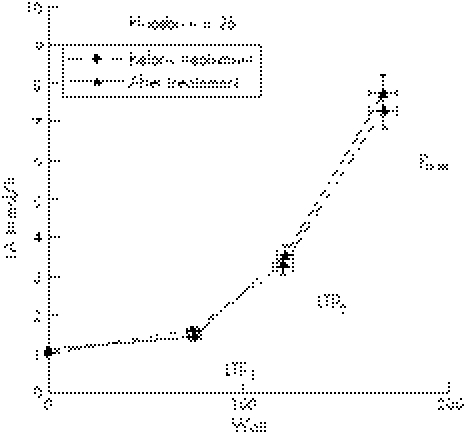
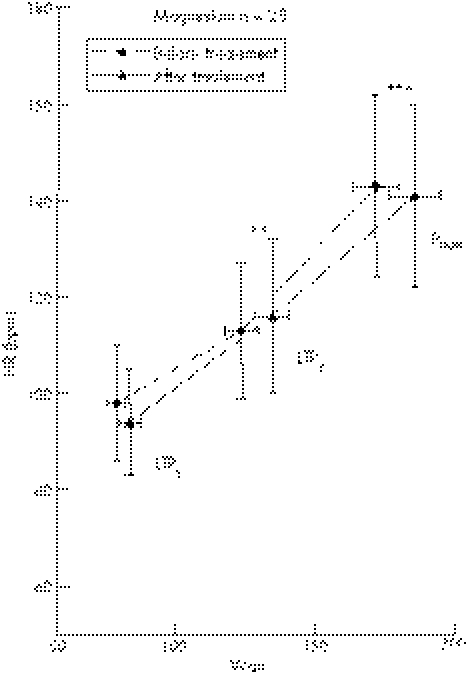
Blood LA concentrations measured at rest and during the incremental cycle ergometer test are shown in fig 6 for the magnesium group and in fig 7 for the placebo group. There were no significant differences in LA between both groups before and after treatment at LTP1, LTP2, and Pmax.
Figure 6 Time course of mean values (±SD) for blood lactate concentration (LA) for patients before and after treatment. The first lactate turn point (LTP1), the second lactate turn point (LTP2), maximal power output (Pmax), and significant differences in power output at LTP2 and Pmax are shown (**p⩽0.01; ***p⩽0.001).
Figure 7 Time course of mean values (±SD) for blood lactate concentration (LA) for patients before and after treatment. The first lactate turn point (LTP1), the second lactate turn point (LTP2), and maximal power output (Pmax) are shown.
Oral magnesium supplementation significantly increased power output compared to baseline at LTP2 (117±6 v 128±7 W, p⩽0.01) and Pmax (165±8 v 180±9 W, p⩽0.001), but not at LTP1 (72±4 v 77±5 W) (fig 6). In the placebo group, however, no significant difference in power output at LTP1 (73±3 v 73±4 W, NS), LTP2 (118±5 v 118±5 W, NS), and Pmax (168±7 v 167±8 W, NS) was found (fig 7). HR response was significantly altered by magnesium supplementation in the magnesium but not in placebo group as depicted in figs 8 and 9.
Figure 8 Time course of mean values (±SD) for heart rate (HR) for patients before and after treatment. First lactate turn point (LTP1), second lactate turn point (LTP2), and maximal power output (Pmax) are shown.
Figure 9 Time course of mean values (±SD) for heart rate (HR) for patients before and after treatment. First lactate turn point (LTP1), second lactate turn point (LTP2), and maximal power output (Pmax) are shown.
The impact of magnesium therapy on echocardiography can be seen in table 2. The two groups were similar at baseline for echocardiographic data. However, the magnesium intervention resulted in a significant decrease in LVDD and LVSD and a significant increase in LVEF, a finding which was not seen in the placebo group. Changes over time in treated patients versus the placebo group were expressed as Δ. ΔLVDD: magnesium 2.5±4.5 mm, placebo 0.1±1.1 mm (p<0.05); ΔLVSD: magnesium 4.1±5.8 mm, placebo 0.1±0.6 mm (p<0.001); ΔLVEF: magnesium 8.5±8.4%, placebo 1.0±4.32% (p<0.001).
Table 2 Left ventricular end diastolic diameter (LVDD), left ventricular end systolic diameter (LVSD), and ejection fraction (LVEF) as measured using echocardiography.
| LVDD (mm) | LVSD (mm) | LVEF (%) | ||||
|---|---|---|---|---|---|---|
| Magnesium (n = 28) | Placebo (n = 25) | Magnesium (n = 28) | Placebo (n = 25) | Magnesium (n = 28) | Placebo (n = 25) | |
| Before treatment | 49.0±0.6 | 47.5±0.5 | 36.1±0.5 | 35.8±0.4 | 58±11 | 56±11 |
| After treatment | 46.4**±0.6 | 47.4±0.5 | 31.5***±0.4 | 35. 9±0.4 | 67***±10 | 55±12 |
Asterisks indicate significance differences between before and after treatment (**p<0.01, ***p<0.001). Values are mean±SD.
Discussion
The major finding of our current study is the significant inverse correlation between [Mg]i and upward deflection of the HRPC observed in CAD patients. In addition, our study results support previous studies1,12 demonstrating magnesium therapy induced improvement in aerobic power. The strong association between magnesium therapy, increase in tissue [Mg]i, and clinical outcomes further supports our hypothesis that the beneficial effects are attributed to magnesium supplementation.
The mechanisms responsible for this are most likely multifactorial.2 Most of our patients had a baseline [Mg]i below normal levels, reflecting a magnesium deficient state (fig 2). After 6 months of oral magnesium supplementation we observed a significant increase in [Mg]i. Previous studies have demonstrated that 6 month oral magnesium intervention in patients with CAD and lower [Mg]i resulted in a significant improvement in brachial artery endothelial function.2 Furthermore, higher [Mg]i levels may improve intracellular adenosine triphosphate production and glucose use because magnesium is a cofactor of adenosine triphosphate.4 Magnesium is considered nature's physiologic calcium blocker,4,17,18 reducing the release of calcium from and into the sarcoplasmic reticulum while protecting the cells against calcium overload under conditions of ischaemia. Magnesium reduces systemic and pulmonary vascular resistance with a concomitant decrease in blood pressure and a slight increase in the cardiac index.4,17,19,20 Elevation of extracellular magnesium levels reduces arteriolar tone and tension in a wide variety of arteries,21 and potentiates the dilating effects of some endogenous (adenosine, potassium, and some prostaglandins) vasodilators.21 As a result, magnesium can mildly reduce systolic blood pressure,22 thereby assisting in unloading of the ischaemic ventricle.4,18,21
An augmented HR increase near maximal exercise was described to protect against a loss of myocardial function in CAD patients.23 Radionuclide studies demonstrated that the degree and the direction of the HRPC were related to the LVEF during incremental exercise in healthy young subjects6,12,24,25 and in CAD patients.6,25 In patients with CAD, a significant decrease in LVEF demonstrated above LTP2 was accompanied by an augmented HR response (upward deflection).6,25 Foster et al25 recently reported that a significant deterioration in left ventricular function during heavy exercise was normalised after percutaneous transluminal coronary angioplasty and that upward deflection of the HRPC was reduced. Standard treatment in CAD patients with abnormal exertional ventricular function consists of invasive therapy to improve myocardial perfusion or reduce pharmacological afterload. In patients with magnesium deficiency, magnesium supplementation should be given to improve endothelial2,19,20 and myocardial function. In our current study, both groups taken together demonstrated a significant correlation between [Mg]i and kHR after supplementation. Furthermore, resting myocardial function and kHR improved significantly following oral magnesium citrate therapy. As kHR was shown to be a significant predictor of exercise dependent myocardial function,6,12,25 we suggest that these effects may be caused by increased exercise induced myocardial perfusion due to improved vascular endothelial function2 in magnesium deficient CAD patients.
Study limitations
The limitation of the study was that exercise dependent myocardial function was not measured. However, in an earlier study in patients after myocardial infarction we could demonstrate this relationship between heart rate and left ventricular function when performing radionuclide ventriculography.6 As these study only provides preliminary evidence, future research should directly measure exercise dependent myocardial function.
Conclusion
As shown previously, oral magnesium supplementation improved resting myocardial function and exercise performance. From the improvement in kHR we may also expect increased exercise dependent myocardial function although this was not measured directly.
What is already known about this topic
Magnesium is an essential element in the body
Decreased levels of magnesium may adversely affect myocardial cells and cause myocardial cell death
Epidemiologic evidence suggests that magnesium plays an important role in regulating blood pressure
What this study adds
Oral magnesium supplementation improved resting myocardial function and exercise performance
Oral magnesium supplementation improved both resting and exercise induced myocardial function as indicated by the exercise induced heart rate response
Abbreviations
ANOVA - analysis of variance
CAD - coronary artery disease
2D - two dimensional
HR - heart rate
HRPC - heart rate performance curve
LA - blood lactate concentration
LSD - least significant differences
LTP - lactate turn point
LVDD - left ventricular internal diameter during diastole
LVEF - left ventricular ejection fraction
LVSD - left ventricular internal diameter during systole
[Mg]i - intracellular magnesium level
Pmax - maximum power
SD - standard deviation
Footnotes
Competing interests: none declared
References
- 1.Geiss K R, Stergiou Jester N, Neuenfeld H U.et al Effects of magnesium orotate on exercise tolerance in patients with coronary heart disease. Cardiovasc Drugs Ther 199812153–156. [DOI] [PubMed] [Google Scholar]
- 2.Shechter M, Sharir M, Paul‐Labrador M.et al Oral magnesium therapy improves endothelial function in patients with coronary artery disease. Circulation 20001022353–2358. [DOI] [PubMed] [Google Scholar]
- 3.Vogel R A. Cholesterol lowering and endothelial function. Am J Med 1999107479–487. [DOI] [PubMed] [Google Scholar]
- 4.Shechter M, Paul‐Labrador M J, Rude R K.et al Intracellular magnesium predicts functional capacity in patients with coronary artery disease. Cardiology 199890168–172. [DOI] [PubMed] [Google Scholar]
- 5.Shechter M, Bairey Merz N, Stuehlinger H G.et al Effects of oral magnesium therapy on exercise tolerance, exercise‐induced chest pain, and quality of life in patients with coronary artery disease. Am J Cardiol 200391517–521. [DOI] [PubMed] [Google Scholar]
- 6.Pokan R, Hofmann P, von Duvillard S P.et al The heart rate performance curve and left ventricular function during exercise in patients after myocardial infarction. Med Sci Sports Exerc 1998301475–1480. [DOI] [PubMed] [Google Scholar]
- 7.Hofmann P, Pokan R, von Duvillard S P.et al Heart rate performance curve during incremental cycle ergometer exercise in healthy young male subjects. Med Sci Sports Exer 199729762–768. [DOI] [PubMed] [Google Scholar]
- 8.Pokan R, Hofmann P, von Duvillard S P.et al Left ventricular function in response to the transition from aerobic to anaerobic metabolism. Med Sci Sports Exerc 1997291040–1047. [DOI] [PubMed] [Google Scholar]
- 9.Skinner J S, McLellan T. The transition from aerobic to anaerobic metabolism. Res Q Exerc Sport 198051234–248. [DOI] [PubMed] [Google Scholar]
- 10.Wasserman K, Hansen J E, Sue D Y.et alPrinciples of exercise testing and interpretation. 4th ed. Baltimore, MD: Lippincott Williams & Wilkins, 200586–87.
- 11.Davis H A, Bassett J, Hughes P.et al Anaerobic threshold and lactate turn point. Eur J Appl Physiol 198350383–392. [DOI] [PubMed] [Google Scholar]
- 12.Pokan R, Hofmann P, Preidler K.et al Correlation between inflection of heart rate/work performance curve and myocardial function in exhausting cycle ergometer exercise. Eur J Appl Physiol 199367385–388. [DOI] [PubMed] [Google Scholar]
- 13.Pokan R, Hofmann P, Lehmann M.et al Heart rate deflection related to lactate performance curve and plasma catecholamine response during incremental cycle ergometer exercise. Eur J Appl Physiol 199570175–179. [DOI] [PubMed] [Google Scholar]
- 14.Pokan R P, Hofmann S P, von Duvillard F.et al Parasympathetic receptor blockade and the heart rate performance curve. Med Sci Sports Exerc 199830229–233. [DOI] [PubMed] [Google Scholar]
- 15.Cheitlin M D, Alpert J S, Armstrong W F.et al ACC/AHA guidelines for clinical application of echocardiography: executive summary. A report of the American College of Cardiology/American Heart Association. Task force on practice guidelines (Committee on Clinical Application of Echocardiography). Developed in collaboration with the American Society of Echocardiography. J Am Coll Cardiol 199729862–879. [DOI] [PubMed] [Google Scholar]
- 16.Haigney M C, Silver B, Tanglao E.et al Noninvasive measurement of tissue magnesium and correlation with cardiac levels. Circulation 1995922190–2197. [DOI] [PubMed] [Google Scholar]
- 17.Iseri L T, French J H. Magnesium: nature's physiologic calcium blocker. Am Heart J 1984108188–193. [DOI] [PubMed] [Google Scholar]
- 18.Shechter M, Kaplinsky E, Rabinowitz B. The rationale of magnesium supplementation in acute myocardial infarction. A review of the literature. Arch Intern Med 19921522189–2196. [PubMed] [Google Scholar]
- 19.Mroczek W J, Lee W R, Davidov M E. Effect of magnesium sulfate on cardiovascular hemodynamics. Angiology . 1977;28720–724. [DOI] [PubMed]
- 20.Rasmussen H S, Meier K, Larsen O G.et al Hemodynamic effects of intravenously administered magnesium in patients with ischemic heart disease. Clin Cardiol 198811824–828. [DOI] [PubMed] [Google Scholar]
- 21.Shechter M, Kaplinsky E, Rabinowitz B. Review of clinical evidence ‐ is there a role for supplemental magnesium in acute myocardial infarction in high‐risk populations (patients ineligible for thrombolysis and the elderly)? Coron Artery Dis 19967352–358. [DOI] [PubMed] [Google Scholar]
- 22.Whelton P K, Klay M J. Magnesium and blood pressure: review of the epidemiologic and clinical trial experience. Am J Cardiol 19896326G–30G. [DOI] [PubMed] [Google Scholar]
- 23.Wasserman K, Hansen J E, Sue D Y.et alPrinciples of exercise testing and interpretation. 4th ed. Baltimore, MD: Lippincott Williams & Wilkins, 2005
- 24.Hofmann P, Pokan R, Preidler W.et al Relationship between heart rate threshold, lactate turn point and myocardial function. Int J Sports Med 199415232–237. [DOI] [PubMed] [Google Scholar]
- 25.Foster C, Porcari J P, Cadwell K, LeMura L M, von Duvillard S P.et al Ischemic cardiovascular disease. In: eds. Clinical exercise physiology. Lippincott Williams & Wilkins 200429–41.