Abstract
Spectrin isoforms are often segregated within specialized plasma membrane subdomains where they are thought to contribute to the development of cell surface polarity. It was previously shown that ankyrin and β spectrin are recruited to sites of cell–cell contact in Drosophila S2 cells expressing the homophilic adhesion molecule neuroglian. Here, we show that neuroglian has no apparent effect on a second spectrin isoform (αβH), which is constitutively associated with the plasma membrane in S2 cells. Another membrane marker, the Na,K-ATPase, codistributes with ankyrin and αβ spectrin at sites of neuroglian-mediated contact. The distributions of these markers in epithelial cells in vivo are consistent with the order of events observed in S2 cells. Neuroglian, ankyrin, αβ spectrin, and the Na,K-ATPase colocalize at the lateral domain of salivary gland cells. In contrast, αβH spectrin is sorted to the apical domain of salivary gland and somatic follicle cells. Thus, the two spectrin isoforms respond independently to positional cues at the cell surface: in one case an apically sorted receptor and in the other case a locally activated cell–cell adhesion molecule. The results support a model in which the membrane skeleton behaves as a transducer of positional information within cells.
INTRODUCTION
The spectrin-based membrane skeleton is a nearly ubiquitous structural component of the plasma membrane in eukaryotic cells. It forms a proteinaceous network at the cytoplasmic face of the membrane where it interacts with integral membrane proteins and other molecules of the cytoskeleton (reviewed by Bennett and Gilligan, 1993; Lux and Palek, 1995). Genetic studies have established essential roles for spectrin and related proteins in cell shape and tissue integrity. Defects in human erythrocyte spectrin cause abnormal cell shape, altered membrane deformability, and consequent anemia (reviewed by Lux and Palek, 1995). Defects in a nonerythroid spectrin from Drosophila are lethal and result in abnormal cell shape and aberrant epithelial organization (Lee et al., 1993). Defects in the spectrin-like molecule dystrophin are the cause of Duchenne muscular dystrophy (Anderson and Kunkel, 1992; Campbell, 1995). In each case, the membrane skeleton defect is thought to disrupt the mechanical properties of the plasma membrane.
Most of our knowledge of the structure and biochemistry of the membrane skeleton has come from studies of erythrocyte spectrin. Spectrin is a rod-shaped ∼200-nm-long heterotetramer composed of α and β subunits (Bennett and Gilligan, 1993; Lux and Palek, 1995). An actin-binding site is found at each end of the spectrin tetramer, enabling spectrin and actin to associate in a long-range two-dimensional network beneath the plasma membrane. In the erythrocyte, ∼6 spectrin molecules converge at junctional complexes containing a single actin filament to yield a geodesic structure beneath the lipid bilayer (Byers and Branton, 1985; Liu et al., 1987). Direct structural evidence is lacking, but the conserved protein interactions of nonerythroid spectrins suggest that they are also arranged in a protein network beneath the plasma membrane.
Studies of the membrane skeleton in nonerythroid cells demonstrated that spectrin and ankyrin are often segregated within specialized domains of the plasma membrane (Lazarides and Nelson, 1983; Drenckhahn et al., 1985; Flucher and Daniels, 1989; reviewed by Bennett and Gilligan, 1993). In some cases there are multiple membrane skeleton domains composed of distinct spectrin and ankyrin isoforms within a single cell (Lazarides et al., 1984; Nelson and Lazarides, 1984; Goodman et al., 1995). Therefore, sorting mechanisms must exist to correctly target the membrane skeleton to a particular plasma membrane domain and to segregate spectrin and ankyrin isoforms in the plane of the membrane. However, it is difficult to identify the cues that target the membrane skeleton, because spectrin and ankyrin are already associated with the plasma membrane in most cells that have been examined and also because of the complexity of possible attachments between spectrin and the plasma membrane.
There are two major classes of interaction between spectrin and the plasma membrane. The first identified and best characterized link is mediated by ankyrin (Bennett, 1992). Ankyrin is a peripheral membrane protein that interacts with the β subunit of spectrin and with the cytoplasmic domains of several integral membrane proteins such as the erythrocyte anion exchanger, the Na,K-ATPase, sodium channels, and cell adhesion molecules (Bennett and Gilligan, 1993). A second class of interactions involves the ankyrin-independent sites on spectrin which are thought to directly bind integral membrane proteins (Steiner and Bennett, 1988; Lombardo et al., 1994). Spectrin is known to interact with epithelial sodium channels (Rotin et al., 1994) and CD45 (Iida et al., 1994) by this mechanism. Additional indirect connections between spectrin and the plasma membrane have been described in the human erythrocyte (e.g., via protein 4.1; reviewed by Lux and Palek, 1995), but the significance of these sites in nonerythroid cells is not known.
The interactions between the membrane and membrane skeleton can also be grouped according to their downstream effects. In some cases, membrane proteins appear to target the assembly of the membrane skeleton. For example, spectrin and ankyrin are recruited to sites of cell–cell contact by a direct interaction between ankyrin and the cytoplasmic domains of Drosophila neuroglian and human L1 (Dubreuil et al., 1996; Hortsch et al., 1997). Spectrin and ankyrin are also recruited to sites of E-cadherin-mediated adhesion (McNeill et al., 1990), perhaps via an association between spectrin and α catenin (Devarajan and Morrow, 1996). In other cases, the membrane skeleton appears to influence the composition of a plasma membrane domain by stabilizing interacting membrane proteins. One example is the Na,K-ATPase, which codistributes with spectrin and ankyrin at sites of cell contact in E-cadherin-expressing cells (McNeill et al., 1990). Na,K-ATPase molecules are short-lived at the cell surface in the absence of spectrin and ankyrin (Hammerton et al., 1991). Another example is the loss of dystrophin-associated glycoproteins in Duchenne muscular dystrophy patients. A complement of sarcolemmal glycoproteins copurifies with dystrophin from normal muscle, but these proteins are virtually absent from the sarcolemma of dystrophic muscle (Ervasti et al., 1990; Matsumura and Campbell, 1994).
Drosophila S2 tissue culture cells provide a unique model system in which to study the cues for membrane skeleton assembly and its contribution to plasma membrane domain organization. Ankyrin and the αβ isoform of spectrin are not detectably associated with the plasma membrane in the absence of a suitable membrane anchor. However, spectrin and ankyrin are selectively recruited to sites of cell–cell contact in transfected S2 cells that express the cell adhesion molecule neuroglian (Dubreuil et al., 1996). Neuroglian is the Drosophila homologue of the L1-neurofascin-NrCam family of vertebrate cell adhesion molecules which are known to interact with ankyrin in vitro (Davis and Bennett, 1994). By manipulating neuroglian expression, it is possible to study the steps of membrane skeleton assembly under controlled conditions in S2 cells. Here, we examine three parameters of membrane skeleton function and organization. First, we describe an isoform of spectrin in S2 cells (αβH) that behaves independently of αβ spectrin and ankyrin. Second, we examine the distribution of the Na,K-ATPase as a marker for the effect of the membrane skeleton on interacting membrane proteins. Third, we describe the distribution of all of these proteins in dissected Drosophila tissues and we propose that mechanisms identified in S2 cells also contribute to the segregation of membrane skeleton domains during the development of polarized cells in vivo.
MATERIALS AND METHODS
Antibodies
The specificities of affinity-purified rabbit anti-Drosophila ankyrin antibody ( 5 μg/ml; Dubreuil and Yu, 1994; Dubreuil et al., 1996), mouse monoclonal α spectrin antibody (1:10 culture supernatant; Dubreuil et al., 1987), and rabbit anti-Drosophila β spectrin antibody (1:250–1:500 serum; Byers et al., 1989) were described previously. The mouse antichicken Na,K-ATPase antibody α5 was previously shown to cross-react with the α subunit of the Drosophila Na,K-ATPase (1:500 ascites fluid; Lebovitz et al. 1989; Schubiger et al., 1994). The monoclonal 1B7 antibody was previously shown to react specifically with Drosophila neuroglian (1:500 ascites fluid; Bieber et al., 1989). Affinity-purified fluorescein isothiocyanate- (FITC) conjugated goat anti-rabbit antibody (Sigma Chemical Co., St. Louis, MO) and affinity-purified Texas Red-conjugated goat anti-mouse antibody (Jackson Immunoresearch Laboratories, West Grove, PA) were used at 1:250–1:500. Alkaline phosphatase-conjugated secondary goat anti-rabbit antibody (Zymed Laboratories, South San Francisco, CA) was used at 1:1000.
A βH spectrin-specific antibody was produced against a recombinant βH spectrin fragment expressed in bacteria. A 1.8-kb fragment (BamHI-KpnI) of βH spectrin cDNA from the amino terminal coding region (Dubreuil et al., 1990) was expressed in pBluescript (Stratagene, La Jolla, CA), and the protein product was purified by its insolubility after cell lysis in the presence of neutral detergent. The protein was electroeluted from polyacrylamide gels and injected into a rabbit, as previously described for production of ankyrin antibody (Dubreuil and Yu, 1994). Immune serum was affinity purified using standard methods, and the antibody was diluted to 0.48 μg/ml-0.96 μg/ml for immunolocalizations and 0.24 μg/ml for Western blots.
Cell Lines and Immunofluorescent Staining
Drosophila S2 tissue culture cells were grown under standard conditions in Schneiders Drosophila medium with 10% fetal calf serum (both from Life Technologies, Gaithersburg, MD) and 50 U/ml penicillin-50 μg/ml streptomycin (Sigma) at 25°C. Stably transfected cells were induced to express a neuroglian transgene (180-kDa isoform) under control of the metallothionein promoter (Hortsch et al., 1995) by addition of 0.7 mM copper chloride to the growth medium. Cells were incubated for 18 to 24 h to allow neuroglian expression and formation of cell aggregates before attaching them to Alcian blue-coated microscope slides. Cells were fixed with 2% freshly prepared formaldehyde in phosphate-buffered saline and then permeabilized with 0.1% Triton X-100 in buffered saline before staining as previously described (Dubreuil et al., 1996).
Cells were viewed and photographed with an ausJena microscope (Jena, Germany) using a 50× or 25× Plan Apo objective. Photographs were taken on either TMax400 or Ektachrome 400 film. Transparencies were digitized with a Polaroid SprintScan scanner.
Isolation and Staining of Drosophila Tissues
Salivary glands were dissected from late third instar larvae in fresh 2% formaldehyde in phosphate-buffered saline, pH 7.1. Glands were fixed for an additional 10 min at room temperature, rinsed for 10 min in Drosophila Ringer’s solution, and then permeabilized for 30 min in Tris-buffered saline (pH 7.5) containing 0.1% Tween 20 and 1% Triton X-100. Staining of glands was carried out as previously described for S2 cells (Dubreuil et al., 1996), except that tissues were kept in suspension, primary antibody incubations were carried out overnight at 4°C, and secondary antibody incubations were carried out for 3 h at room temperature. Ovaries were dissected from adult female flies and processed as above except that formaldehyde fixation was carried out on ice rather than at room temperature.
Western Blots
Gel electrophoresis, electrophoretic transfer to nitrocellulose, and reaction with antibodies were carried out as described previously (Dubreuil et al., 1987).
RESULTS
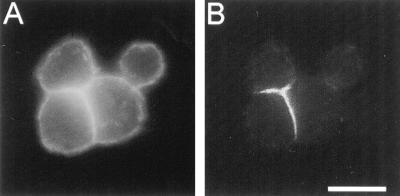
We previously used ankyrin and β spectrin as markers of membrane skeleton assembly in neuroglian-expressing S2 cells (Dubreuil et al., 1996). Upon expression of neuroglian and formation of cell aggregates, ankyrin (Figure 1B) and β spectrin (Figure 3B) were selectively recruited to sites of cell–cell contact. In Figure 1, the distribution of the α subunit of spectrin was compared with ankyrin using a previously characterized monoclonal antibody (Dubreuil et al., 1987; Figure 2, lane 1). Unlike ankyrin, α spectrin was constitutively associated with the plasma membrane of single S2 cells and neuroglian-expressing S2 cell aggregates (Figure 1A). The lack of ankyrin colocalization with α spectrin at nonadherent regions of the plasma membrane suggested that there is a population of spectrin in S2 cells that associates with the plasma membrane independently of ankyrin.
Figure 1.
Spectrin and ankyrin have distinct, partially overlapping distributions in neuroglian-expressing Drosophila tissue culture cells. Cell aggregates, formed upon expression of neuroglian, were double labeled with mouse anti-α spectrin (A) and rabbit antiankyrin (B) primary antibodies, followed by Texas red- and FITC-conjugated secondary antibodies, respectively. Bar, 10 μm.
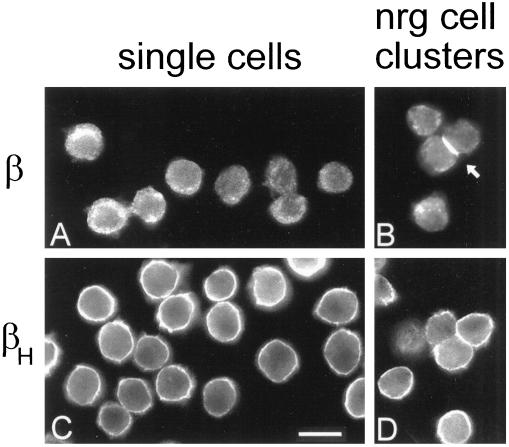
Figure 3.
Distinct distributions of two β spectrin isoforms in Drosophila tissue culture cells before and after expression of neuroglian and formation of cell–cell contacts. Control S2 cells (A and C) and neuroglian-expressing S2 cell aggregates (B and D) were attached to microscope slides and stained with antibodies against β spectrin (A and B; 1:500 serum) or βH spectrin (C and D; 5 μg/ml) followed by FITC-conjugated secondary antibody. Arrowhead marks the accumulation of β spectrin at a cell–cell contact. Bar, 10 μm.
Figure 2.

Western blot analysis of α spectrin and isoform-specific β spectrin antibodies. Total S2 cell proteins were reacted with monoclonal anti-α spectrin antibody (lane 1), polyclonal anti-β spectrin (lane 2), or affinity-purified anti-βH spectrin antibodies (lane 3) and stained with alkaline phosphatase-conjugated secondary antibody.
Two isoforms of spectrin have been described in Drosophila tissue culture cells: αβ and αβH (Dubreuil et al., 1990). There is a single known α spectrin gene in Drosophila (Byers et al., 1987), and its product associates with either the β or the βH subunit (products of distinct genes; Byers et al., 1989; Dubreuil et al., 1990) to form spectrin heterotetramers. The specificity of polyclonal antibodies against the two β spectrin isoforms was demonstrated in Western blots of total S2 cell proteins (Figure 2). The anti-β spectrin antibody (Byers et al., 1989) reacted with its 265-kDa antigen (Figure 2, lane 2) and the anti-βH spectrin antibody reacted with its 430-kDa antigen (Figure 2, lane 3) with no detectable cross-reactions. Despite its unusually large size, Drosophila βH spectrin is a bona fide β subunit that forms spectrin tetramers resembling conventional spectrins (Dubreuil, 1996; see DISCUSSION).
The isoform-specific β spectrin antibodies revealed two distinct spectrin distributions in control S2 cells and neuroglian-expressing S2 cell clusters. The sum of their staining patterns corresponded to the broad distribution of α spectrin (Figure 1A). β spectrin was not detectably associated with the plasma membrane of control cells (Figure 3A) but it was recruited to the plasma membrane at sites of cell–cell contact in neuroglian-expressing cells (Figure 3B). In contrast, the βH-specific antibody stained the plasma membrane of all cells (Figure 3C). βH spectrin appeared to be uniformly distributed along the plasma membrane, although some variations in staining intensity were apparent (presumably because of the topology of the cell surface). Neuroglian-expressing S2 cell clusters exhibited the same uniform distribution of βH spectrin at the plasma membrane, with no apparent concentration of this isoform at sites of neuroglian-mediated cell-cell contact (Figure 3D).
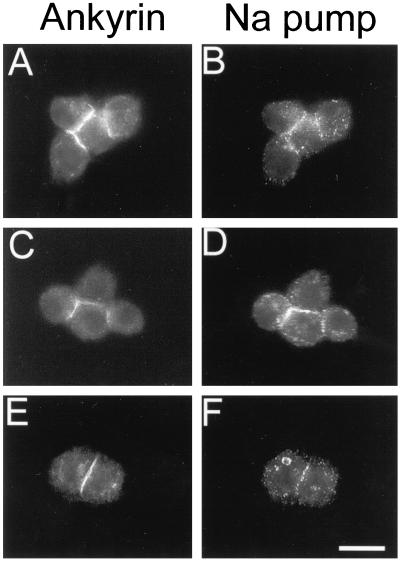
Previous studies demonstrated that the Na,K-ATPase interacts directly with ankyrin (Nelson and Veshnock, 1987) and that it colocalizes with spectrin and ankyrin at sites of E-cadherin-mediated cell–cell contact in mammalian cells (McNeill et al., 1990). We examined the distribution of the Na,K-ATPase in neuroglian-expressing S2 cells using a monoclonal antibody against the α subunit of the chicken Na,K-ATPase that was previously shown to react with the Drosophila Na,K-ATPase (Lebovitz et al., 1989; Schubiger et al., 1994). Antibody staining revealed colocalization of the Na,K-ATPase with ankyrin at sites of cell–cell contact (Figure 4). Of the cell contacts that stained detectably with ankyrin antibody, 67% also stained with the Na,K-ATPase antibody (n = 610). The Na,K-ATPase was weakly stained in a punctate pattern at the plasma membrane of nonadherent cells or at noncontact sites of adherent cells, presumably because of its low abundance in S2 cells relative to other Drosophila cell types that have been studied (e.g., salivary gland, Figure 5).
Figure 4.
The Na,K-ATPase codistributes with ankyrin at sites of neuroglian-mediated cell–cell contact. Neuroglian-expressing S2 cells were double labeled with mouse monoclonal anti-Na,K-ATPase antibody and rabbit antiankyrin antibody and secondary antibodies as in Figure 1. Bar, 10 μm.
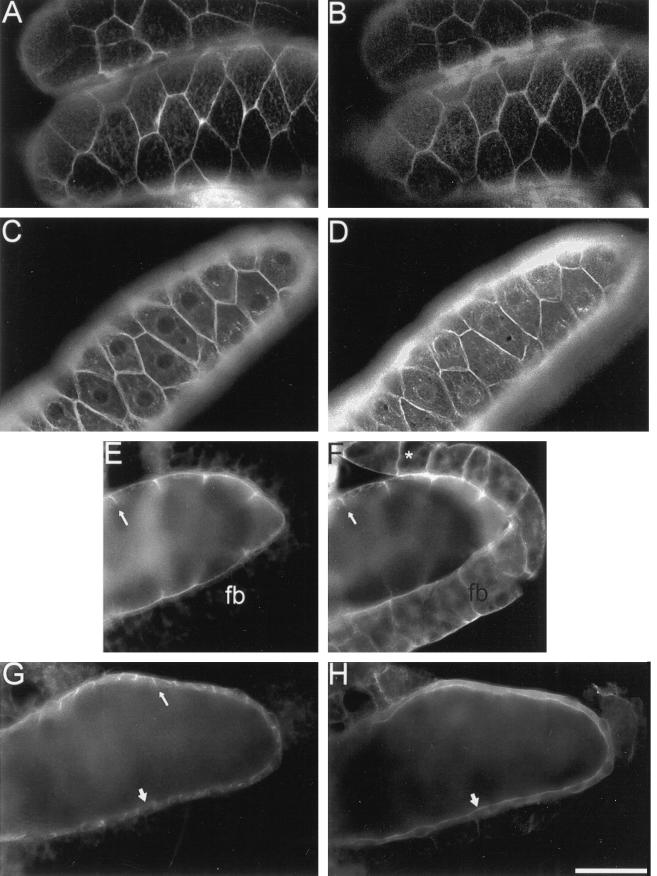
Figure 5.
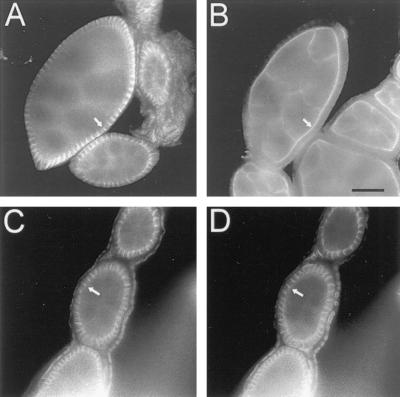
Distribution of neuroglian, ankyrin, Na,K-ATPase, and βH spectrin in the larval salivary gland epithelium. Salivary glands were dissected from late third instar wild-type larvae, fixed, permeabilized and double labeled with mouse antineuroglian (A), rabbit anti-Drosophila ankyrin (B, D, and F), mouse anti-Na,K-ATPase (C, E, and G); and rabbit anti-Drosophila βH spectrin (H). Mouse antibodies were detected with Texas Red-conjugated secondary antibodies (A, C, E, and G) and rabbit antibodies were detected with FITC-conjugated secondary antibodies (B, D, F, and H). Neuroglian, ankyrin, and Na,K-ATPase colocalize at lateral sites of cell–cell contact (A–D). Ankyrin and the Na,K-ATPase colocalize at the lateral margin of salivary gland cells (arrow, E and F). Ankyrin is also concentrated at sites of cell adhesion (*, F) in the fat body (fb). Although the Na,K-ATPase was concentrated at lateral sites of cell contact in the salivary gland (thin arrow, G), the βH subunit of spectrin was concentrated at the apical cell surface (wide arrow, H). Bars: A–D and G–H, 100 μM; E and F, 67 μm.
We next examined the distribution of α, β, and βH spectrin, ankyrin, neuroglian, and the Na,K-ATPase in vivo. The epithelial cells of the larval salivary gland abundantly expressed neuroglian at lateral regions of cell–cell contact (Figure 5A). Ankyrin colocalized with neuroglian at these sites (Figure 5B), as observed in S2 cells. A similar staining pattern was detected with antibodies against α and β spectrin (our unpublished results). The Na,K-ATPase (Figure 5C) codistributed with ankyrin (Figure 5D) at lateral sites of cell–cell contact. Ankyrin and the Na,K-ATPase were also detected at the basal surface of cells (Figure 5, E and F), but neither protein was detected at the apical surface, facing the gland lumen. Whereas βH spectrin was uniformly distributed at the surface of cultured S2 cells, it was found almost exclusively at the apical domain of the salivary gland epithelium (Figure 5H, thick arrow). In the same field, the Na,K-ATPase was concentrated at lateral sites of cell–cell contact (Figure 5G, thin arrow). Thus, the two spectrin isoforms are segregated in a nonoverlapping distribution in the salivary gland epithelium, in contrast to their overlapping distribution in neuroglian-expressing S2 cells.
Salivary glands from third instar larvae typically exhibited a thin epithelial cell layer surrounding a large gland lumen (Figure 5). The βH spectrin staining pattern in glands from first and second instar larvae (our unpublished results) and from embryos (Thomas and Kiehart, 1994) defined a relatively small lumen and a proportionately thicker epithelium. However, regardless of the stage examined, the distributions of αβH spectrin at the apical surface and αβ spectrin/ankyrin at the lateral surface remained segregated.
Ankyrin was detected at sites of cell–cell contact in a segment of the fat body that usually remains attached to the salivary glands after dissection (Figure 5F). However, the Na,K-ATPase (Figure 5E) and neuroglian (our unpublished results) were not detectable in the fat body.
Lee et al. (1997) recently described the polarized distributions of αβ and αβH spectrins in the somatic follicle epithelium of the adult ovary. Here, we examined the distribution of neuroglian in relation to the follicle cell membrane skeleton. Neuroglian staining was concentrated along the lateral membranes of the epithelium (Figure 6D) along with ankyrin (Figure 6, A and C) and β spectrin (our unpublished results). In contrast, βH spectrin was restricted to the apical surface facing the enclosed nurse cells and oocyte (Figure 6B). Thus, in follicle cells as in the salivary gland, the two spectrin isoforms appear to respond independently to their respective positional cues at the cell surface.
Figure 6.
Distribution of ankyrin (A and C), βH spectrin (B), and neuroglian (D) in the somatic follicle epithelium of the adult ovary. Ovaries were dissected from adult Drosophila females, fixed, permeabilized, and stained as in Figure 5. Arrows mark staining of ankyrin at lateral cell contacts (A and C), neuroglian at cell contacts (D), and βH spectrin at the apical surface (B). Bar, 20 μm.
DISCUSSION
The membrane skeleton forms a protein scaffold that contributes to the shape and stability of the plasma membrane. Because of its polarized distribution in many cell types, the membrane skeleton is also thought to contribute to the development and maintenance of specialized membrane domains (reviewed by Beck and Nelson, 1996; Devarajan and Morrow, 1996; Lambert and Bennett, 1996). Moreover, individual cells can segregate multiple spectrin isoforms to divide the plasma membrane into multiple unique domains. Here, we have used Drosophila S2 cells to analyze the mechanisms behind spectrin isoform segregation and the response of an interacting membrane marker, the Na,K-ATPase.
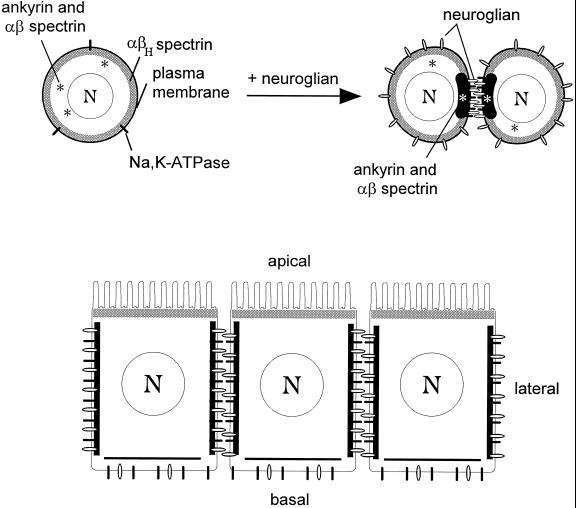
The distribution of segregated membrane skeleton domains in S2 cells is summarized schematically in Figure 7A. One spectrin isoform, αβH, is uniformly associated with the plasma membrane (submembrane shading), whether or not neuroglian is present. In contrast, ankyrin and αβ spectrin are recruited from the cytoplasm to sites of cell–cell adhesion upon expression of neuroglian. Interestingly, the recruitment of αβ spectrin and ankyrin coincides with sites of adhesion, and not with the distribution of total neuroglian, implying that neuroglian is somehow activated to recruit membrane skeleton assembly (Dubreuil et al., 1996). The Na,K-ATPase also becomes concentrated at sites of neuroglian-mediated cell adhesion, presumably through a direct interaction with ankyrin (Nelson and Veshnock, 1987).
Figure 7.
Segregation of spectrin isoforms in response to positional cues at the cell surface. (A) The uniform distribution of αβH spectrin (submembrane shading) at the plasma membrane of S2 cells (left) implies the presence of a constitutive βH-specific receptor that is uniformly distributed at the cell surface. In contrast, ankyrin and αβ spectrin are found in the cytoplasm of control S2 cells (*, left), but they are recruited to sites of cell–cell contact in response to neuroglian-mediated cell adhesion (right). The Na,K-ATPase also becomes concentrated at cell contacts in response to neuroglian expression and ankyrin/αβ spectrin assembly. (B) Neuroglian, ankyrin, αβ spectrin, and Na,K-ATPase are concentrated at sites of cell–cell contact in the larval salivary gland epithelium, in agreement with the order of events observed in S2 cells. However, the putative αβH spectrin receptor is sorted apically in these polarized cells. Despite their common features (including an identical α subunit), the two spectrin isoforms respond independently to their respective membrane receptors to form segregated membrane skeleton domains.
The segregated distribution of membrane markers in salivary glands from third instar Drosophila larvae is consistent with the mechanism of segregation found in S2 cells (Figure 7B). Neuroglian and the Na,K-ATPase are concentrated at lateral cell–cell contacts. Spectrin and ankyrin are also concentrated at lateral sites of cell contact, as expected if they serve as a bridge between sites of cell adhesion and the Na,K-ATPase. Since neuroglian is likely to be activated by cell–cell adhesion in the lateral domain of follicle cells and salivary gland cells, it is a likely candidate to provide the signal that recruits ankyrin and αβ spectrin in these epithelia. Although αβH spectrin is uniformly associated with the plasma membrane in S2 cells, it is restricted to the apical domains of salivary gland cells, somatic follicle cells of the adult ovary (Lee et al., 1997 and present study), and a subset of cells in the developing Drosophila embryo (Thomas and Kiehart, 1994). The results imply that a sorting mechanism acts on αβH spectrin to produce its apical distribution in polarized cells.
An ankyrin-independent membrane-binding site is likely to be responsible for targeting αβH spectrin to the plasma membrane. The present results reveal that in S2 cells, larval salivary glands, and adult ovaries, ankyrin, and βH spectrin have strikingly different distributions. There appears to be a single ankyrin gene in Drosophila with a single protein product (Dubreuil and Yu, 1994). Even though the evidence is negative, it is significant that degenerate polymerase chain reaction, low stringency hybridizations with evolutionarily conserved regions of the ankyrin gene, and the reactivities of three independent polyclonal antibodies have all failed to detect additional ankyrin products (our unpublished observations). If there is only one ankyrin, and that ankyrin codistributes with αβ spectrin, then that ankyrin cannot be the membrane attachment site for αβH spectrin. Membrane-targeting activity is not likely to reside in the α subunit of spectrin either, since the two isoforms described here share the same α subunit (Dubreuil et al., 1990) yet they acquire distinct subcellular distributions.
Drosophila βH spectrin resembles TW260, the avian terminal web-associated β spectrin. Based on their similarities, we suggest that Drosophila αβH spectrin and vertebrate terminal web spectrins constitute a distinct class of apically directed spectrin isoforms. Both proteins are unusually large compared with other known β spectrin subunits and both utilize an α spectrin subunit found in other spectrin isoforms (Coleman et al., 1989; Dubreuil et al., 1990). In addition, both proteins are found in the apical region of epithelial cells. A terminal web spectrin has been identified in mammals, although its exact subunit composition is not known (Hirokawa et al., 1983). The apical receptor for the vertebrate terminal web spectrins is not known, but it does not appear to be ankyrin (Howe et al., 1985). The nature of the binding site on the spectrin molecule that responds to an ankyrin-independent, apical targeting cue is also unknown. Based on its association with the plasma membrane of S2 cells, where there is no terminal web, we suggest that targeting of αβH spectrin occurs via its direct or indirect association with an integral membrane receptor.
Differential use of ankyrin-dependent and ankyrin-independent membrane-binding sites by two spectrin isoforms provides a sorting mechanism that segregates spectrin isoforms in the plane of the membrane. Just as αβH spectrin appears to lack an ankyrin-binding site, αβ spectrin lacks a binding site for the αβH spectrin receptor. As a result, the two spectrin isoforms are able to respond independently to positional information specified by their respective membrane anchors. The segregated pattern of membrane skeleton domains found in a cell is thus likely to depend on which receptor proteins that cell expresses and on the positional cues received by the cell during development.
The codistribution of the Na,K-ATPase with αβ spectrin and ankyrin at sites of neuroglian-mediated cell–cell adhesion suggests that the link between cell adhesion and Na,K-ATPase polarity is a general one. The α subunit of the Drosophila Na,K-ATPase is ∼80% identical to the vertebrate Na,K-ATPase (Lebovitz et al., 1989) and is 84% identical within a region implicated in the Na,K-ATPase interaction with ankyrin (Jordan et al., 1995). The membrane-binding domain of Drosophila ankyrin is also conserved in amino acid sequence (Dubreuil and Yu, 1994) and function. For example, Drosophila ankyrin is recruited to sites of cell–cell contact by L1, the human homologue of neuroglian (Hortsch et al., 1997). The striking sequence conservation between these molecules in mammals and Drosophila suggests that the interaction between ankyrin and the Na,K-ATPase is conserved as well. It appears that, regardless of the source of positional information (E-cadherin or neuroglian), polarized assembly of ankyrin and the membrane skeleton can produce a codistribution of the Na,K-ATPase within the same membrane domain. The lack of an effect of an α spectrin mutation on the distribution of Na,K-ATPase molecules in vivo (Lee et al., 1993; Lee et al., 1997) is puzzling. However, there is evidence to suggest that residual ankyrin and β spectrin function in these mutants (Dubreuil and Yu, 1994) could explain the maintenance of Na,K-ATPase polarity in salivary glands and midgut epithelium. An important next step will be to examine the effects of ankyrin and β spectrin mutations on Na,K-ATPase polarity in these cells.
The results reported here illustrate mechanisms that govern the two-way flow of information between the plasma membrane and membrane skeleton. There are positional cues that direct polarized membrane skeleton assembly (neuroglian and the βH spectrin receptor), which in turn confers polarity on an interacting membrane marker (the Na,K-ATPase). An important future goal will be to classify other interacting membrane proteins as either inducers (that provide positional information for the membrane skeleton) or responders (that conform to membrane skeleton polarity). Many of the proteins that associate with the membrane skeleton are physiologically important factors whose position within the cell is likely to be critical to their function [e.g., sodium channels (Srinivasan et al., 1988; Rotin et al., 1994), IP3 receptor (Joseph and Samanta, 1993), H,K-ATPase (Mercier et al., 1989), and anion exchangers (Morgans and Kopito, 1993)]. Their interactions with the membrane skeleton may have evolved as a mechanism that provides access to positional information within the cell.
ACKNOWLEDGMENTS
We thank Stacey Asem, Jason Frankel, and Runna Moussa for technical assistance, Chris Schonbaum for help with ovary dissections, and Jerry Lorenz for computer assistance. We thank Nava Segev and Ted Steck for comments on the manuscript. We also thank Dr. Michael Hortsch for providing neuroglian antibody and Dr. Doug Fambrough for providing α5 Na,K-ATPase antibody. Supported by National Institutes of Health grant GM-49301 and the American Heart Association Chicago Affiliate.
REFERENCES
- Anderson MS, Kunkel LM. The molecular and biochemical basis of Duchenne muscular dystrophy. Trends Biol Sci. 1992;17:289–292. doi: 10.1016/0968-0004(92)90437-e. [DOI] [PubMed] [Google Scholar]
- Beck KA, Nelson WJ. Once there, making the decision to stay or leave. In: Nelson WJ, editor. Current Topics in Membranes. Vol. 43. San Diego: Academic Press; 1996. pp. 15–25. [Google Scholar]
- Bennett V. Ankyrins. J Biol Chem. 1992;267:8703–8706. [PubMed] [Google Scholar]
- Bennett V, Gilligan DM. The spectrin-based membrane skeleton and micron-scale organization of the plasma membrane. Annu Rev Cell Biol. 1993;9:27–66. doi: 10.1146/annurev.cb.09.110193.000331. [DOI] [PubMed] [Google Scholar]
- Bieber AJ, Snow PM, Hortsch M, Patel NH, Jacobs JR, Traquina ZR, Schilling J, Goodman CS. Drosophila neuroglian: a member of the immunoglobulin superfamily with extensive homology to the vertebrate neural adhesion molecule L1. Cell. 1989;59:447–460. doi: 10.1016/0092-8674(89)90029-9. [DOI] [PubMed] [Google Scholar]
- Byers TJ, Branton D. Visualization of the protein associations in the erythrocyte membrane skeleton. Proc Natl Acad Sci USA. 1985;82:6153–6157. doi: 10.1073/pnas.82.18.6153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byers TJ, Dubreuil RR, Branton D, Kiehart DP, Goldstein LSB. Drosophila spectrin II. Conserved features of the alpha subunit are revealed by analysis of cDNA clones and fusion proteins. J Cell Biol. 1987;105:2103–2110. doi: 10.1083/jcb.105.5.2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byers TJ, Husain-chishti A, Dubreuil RR, Branton D, Goldstein LS, B. Drosophila beta-spectrin: sequence similarity to the amino-terminal domain of alpha-actinin and dystrophin. J Cell Biol. 1989;109:1633–1641. doi: 10.1083/jcb.109.4.1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell KP. Three muscular dystrophies: loss of cytoskeleton-extracellular matrix linkage. Cell. 1995;80:675–679. doi: 10.1016/0092-8674(95)90344-5. [DOI] [PubMed] [Google Scholar]
- Coleman TR, Fishkind DJ, Mooseker MS, Morrow JS. Functional diversity among spectrin isoforms. Cell Motil Cytoskeleton. 1989;12:225–247. doi: 10.1002/cm.970120405. [DOI] [PubMed] [Google Scholar]
- Davis JQ, Bennett V. Ankyrin binding activity shared by the neurofascin/L1/NrCAM family of nervous system cell adhesion molecules. J Biol Chem. 1994;269:27163–27166. [PubMed] [Google Scholar]
- Devarajan P, Morrow JS. The spectrin cytoskeleton and organization of polarized epithelial cell membranes. In: Nelson WJ, editor. Current Topics in Membranes. Vol. 43. San Diego: Academic Press; 1996. pp. 97–128. [Google Scholar]
- Drenckhahn D, Schluter K, Allen DP, Bennett V. Colocalization of band 3 with ankyrin and spectrin at the basal membrane of intercalated cells in the rat kidney. Science. 1985;230:1287–1289. doi: 10.1126/science.2933809. [DOI] [PubMed] [Google Scholar]
- Dubreuil R, Byers TJ, Branton D, Goldstein LS, B, Kiehart DP. Drosophila spectrin I. Characterization of the purified protein. J Cell Biol. 1987;105:2095–2102. doi: 10.1083/jcb.105.5.2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubreuil RR. Molecular and genetic dissection of the membrane skeleton in Drosophila. In: Nelson WJ, editor. Current Topics in Membranes. Vol. 43. San Diego: Academic Press; 1996. pp. 147–167. [Google Scholar]
- Dubreuil RR, Byers TJ, Stewart CT, Kiehart DP. A beta spectrin isoform from Drosophila (βH) is similar in size to vertebrate dystrophin. J Cell Biol. 1990;111:1849–1858. doi: 10.1083/jcb.111.5.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubreuil RR, McVicker G, Dissanayake S, Liu C, Homer D, Hortsch M. Neuroglian-mediated adhesion induces assembly of the membrane skeleton at cell contact sites. J Cell Biol. 1996;133:647–655. doi: 10.1083/jcb.133.3.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubreuil RR, Yu J. Ankyrin and beta spectrin accumulate independently of alpha spectrin in Drosophila. Proc Natl Acad Sci USA. 1994;91:10285–10289. doi: 10.1073/pnas.91.22.10285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ervasti JM, Ohlendieck K, Kahl SD, Gaver MG, Campbell KP. Deficiency of a glycoprotein component of the dystrophin complex in dystrophic muscle. Nature. 1990;345:315–319. doi: 10.1038/345315a0. [DOI] [PubMed] [Google Scholar]
- Flucher BE, Daniels MP. Distribution of Na channels and ankyrin in neuromuscular junctions is complementary to that of acetylcholine receptors and the 43 kd protein. Neuron. 1989;3:163–175. doi: 10.1016/0896-6273(89)90029-9. [DOI] [PubMed] [Google Scholar]
- Goodman SR, Zimmer WE, Clark MB, Zagon IS, Barker JE, Bloom ML. Brain spectrin: of mice and men. Brain Res Bull. 1995;36:593–606. doi: 10.1016/0361-9230(94)00264-2. [DOI] [PubMed] [Google Scholar]
- Hammerton RW, Krzeminski KA, Mays RW, Ryan TA, Wollner DA, Nelson WJ. Mechanism for regulating cell surface distribution of Na+,K+-ATPase in polarized epithelial cells. Science. 1991;254:847–850. doi: 10.1126/science.1658934. [DOI] [PubMed] [Google Scholar]
- Hirokawa N, Cheney RE, Willard M. Location of a protein of the fodrin-spectrin-TW-260/240 family in the mouse intestinal brush border. Cell. 1983;32:953–965. doi: 10.1016/0092-8674(83)90080-6. [DOI] [PubMed] [Google Scholar]
- Hortsch M, Wang Y-ME, Marikar Y, Bieber AJ. The cytoplasmic domain of the Drosophila cell adhesion molecule neuroglian is not essential for its homophilic adhesive properties. J Biol Chem. 1995;270:18809–18817. doi: 10.1074/jbc.270.32.18809. [DOI] [PubMed] [Google Scholar]
- Hortsch, M., O’Shea, K.S., Zhao, G., Kim, F., Vallejo, Y., and Dubreuil, R.R. (1997). Functional conservation in the L1 family of neural cell adhesion molecules. Cell Adhesion & Communication (in press).
- Howe CL, Sacramone LM, Mooseker MS, Morrow JS. Mechanisms of cytoskeletal regulation: modulation of membrane affinity in avian brush border and erythrocyte spectrins. J Cell Biol. 1985;101:1379–1385. doi: 10.1083/jcb.101.4.1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iida N, Lokeshwar VB, Bourguignon LY, W. Mapping the fodrin binding domain of CD45, a leukocyte membrane-associated tyrosine phosphatase. J Biol Chem. 1994;269:29576–28583. [PubMed] [Google Scholar]
- Jordan C, Puschel B, Koob R, Drenckhahn D. Identification of a binding motif for ankyrin on the alpha-subunit of Na+,K+-ATPase. J Biol Chem. 1995;270:29971–29975. doi: 10.1074/jbc.270.50.29971. [DOI] [PubMed] [Google Scholar]
- Joseph SK, Samanta S. Detergent solubility of the inositol trisphosphate receptor in rat brain membranes. J Biol Chem. 1993;268:6477–6486. [PubMed] [Google Scholar]
- Lambert S, Bennett V. Axonal ankyrins and ankyrin-binding proteins: Potential participants in lateral membrane domains and transcellular connections at the node of Ranvier. In: Nelson WJ, editor. Current Topics in Membranes. Vol. 43. San Diego, CA: Academic Press; 1996. pp. 129–145. [Google Scholar]
- Lazarides E, Nelson WJ. Erythrocyte and brain forms of spectrin in cerebellum: Distinct membrane-cytoskeleton domains in neurons. Science. 1983;220:1295–1297. doi: 10.1126/science.6190228. [DOI] [PubMed] [Google Scholar]
- Lazarides E, Nelson WJ, Kasamatsu T. Segregation of two spectrin forms in the chicken optic system: A mechanism for establishing restricted membrane-cytoskeleton domains in neurons. Cell. 1984;36:269–278. doi: 10.1016/0092-8674(84)90220-4. [DOI] [PubMed] [Google Scholar]
- Lebovitz RM, Takeyasu K, Fambrough DM. Molecular characterization and expression of the (Na+ + K+)-ATPase alpha-subunit in Drosophila melanogaster. EMBO J. 1989;8:193–202. doi: 10.1002/j.1460-2075.1989.tb03364.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Coyne R, Dubreuil RR, Goldstein LS, B, Branton D. Cell shape and interaction defects in alpha-spectrin mutants of Drosophila melanogaster. J Cell Biol. 1993;123:1797–1809. doi: 10.1083/jcb.123.6.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JK, Brandin E, Branton D, Goldstein LS, B. alpha-spectrin is required for ovarian follicle monolayer integrity in Drosophila melanogaster. Development. 1997;124:353–362. doi: 10.1242/dev.124.2.353. [DOI] [PubMed] [Google Scholar]
- Liu S-C, Derick LH, Palek J. Visualization of the hexagonal lattice in the erythrocyte membrane skeleton. J Cell Biol. 1987;104:527–536. doi: 10.1083/jcb.104.3.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombardo CR, Weed SA, Kennedy SP, Forget BG, Morrow JS. BetaII-spectrin (fodrin) and Beta IE2-Spectrin (muscle) contain NH2- and COOH-terminal membrane association domains (MAD-1 and MAD2) J Biol Chem. 1994;269:29212–29219. [PubMed] [Google Scholar]
- Lux SE, Palek J. Disorders of the red cell membrane. In: Handin RI, Lux SE, Stossel TP, editors. Blood: Principles and Practice of Hematology. Philadelphia: J.B. Lippincott Co.; 1995. pp. 1701–1818. [Google Scholar]
- Matsumura K, Campbell KP. Dystrophin-glycoprotein complex: its role in the molecular pathogenesis of muscular dystrophies. Muscle & Nerve, 1994;17:2–15. doi: 10.1002/mus.880170103. [DOI] [PubMed] [Google Scholar]
- McNeill H, Ozawa M, Kemler R, Nelson WJ. Novel function of the cell adhesion molecule uvomorulin as an inducer of cell surface polarity. Cell. 1990;62:309–316. doi: 10.1016/0092-8674(90)90368-o. [DOI] [PubMed] [Google Scholar]
- Mercier F, Teggio H, Deviliers G, Bataille D, Mangeat P. Membrane-cytoskeleton dynamics in rat parietal cells: mobilization of actin and spectrin upon stimulation of gastric acid secretion. J Cell Biol. 1989;108:441–453. doi: 10.1083/jcb.108.2.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgans CW, Kopito RR. Association of the brain anion exchanger, AE3, with the repeat domain of ankyrin. J Cell Sci. 1993;105:1137–1142. doi: 10.1242/jcs.105.4.1137. [DOI] [PubMed] [Google Scholar]
- Nelson WJ, Lazarides E. The patterns of expression of two ankyrin isoforms demonstrate distinct steps in the assembly of the membrane skeleton in neuronal morphogenesis. Cell. 1984;39:309–320. doi: 10.1016/0092-8674(84)90009-6. [DOI] [PubMed] [Google Scholar]
- Nelson WJ, Veshnock PJ. Ankyrin binding to (Na+ & K+) ATPase and implications for the organization of membrane domains in polarized cells. Nature. 1987;328:533–536. doi: 10.1038/328533a0. [DOI] [PubMed] [Google Scholar]
- Rotin D, Bar-Sagi D, O’Brodovich H, Merilainen J, Lehto VP, Canessa CM, Rossier BC, Downey GP. An SH3 binding region in the epithelial Na+ channel (alpharENaC) mediates its localization at the apical membrane. EMBO J. 1994;13:4440–4450. doi: 10.1002/j.1460-2075.1994.tb06766.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubiger M, Feng Y, Fambrough DM, Palka J. A mutation of the Drosophila sodium pump alpha subunit gene results in bang sensitive paralysis. Neuron. 1994;12:373–381. doi: 10.1016/0896-6273(94)90278-x. [DOI] [PubMed] [Google Scholar]
- Srinivasan Y, Elmer L, Davis J, Bennett V, Angelides K. Ankyrin and spectrin associate with voltage-dependent sodium channels in brain. Nature. 1988;333:177–180. doi: 10.1038/333177a0. [DOI] [PubMed] [Google Scholar]
- Steiner JP, Bennett V. Ankyrin-independent membrane protein-binding sites for brain and erythrocyte spectrin. J Biol Chem. 1988;263:14417–14425. [PubMed] [Google Scholar]
- Thomas GH, Kiehart DP. Beta-heavy spectrin has a restricted tissue and subcellular distribution during Drosophila embryogenesis. Development. 1994;120:2039–2050. doi: 10.1242/dev.120.7.2039. [DOI] [PubMed] [Google Scholar]