Abstract
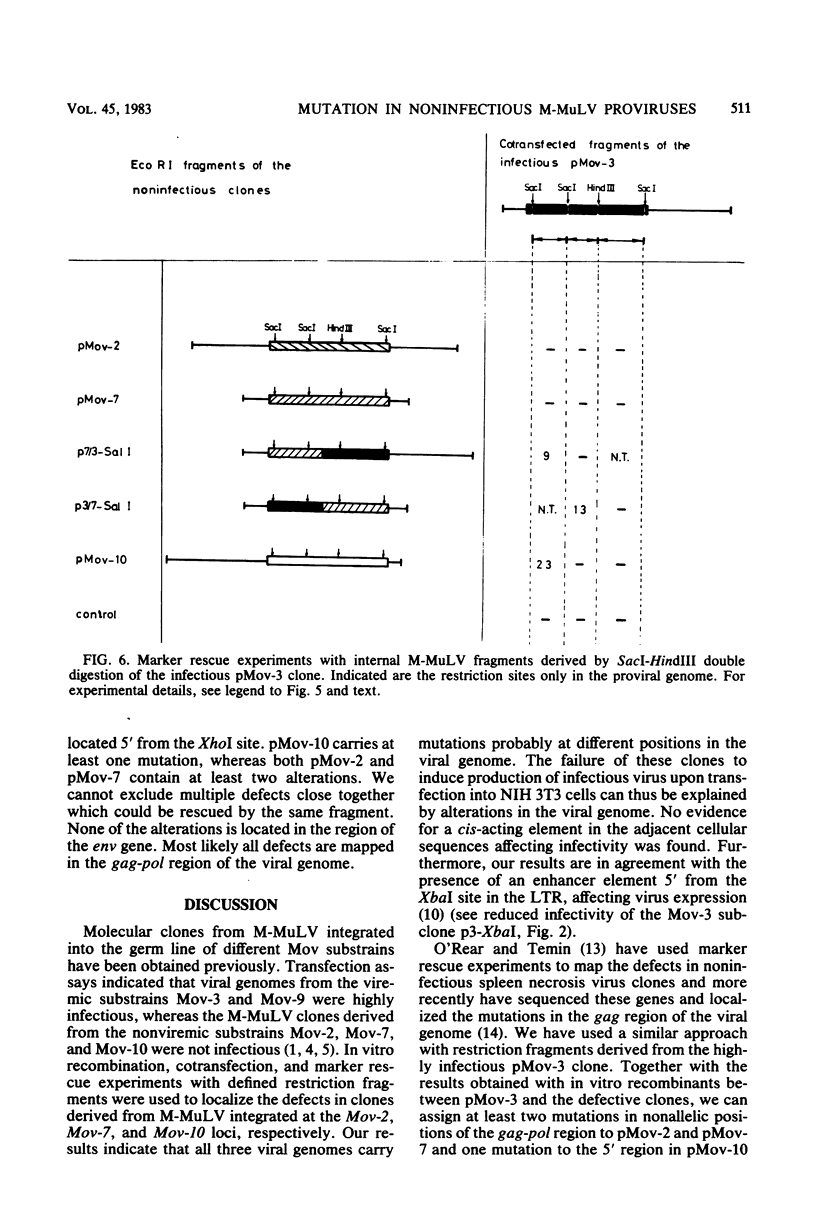
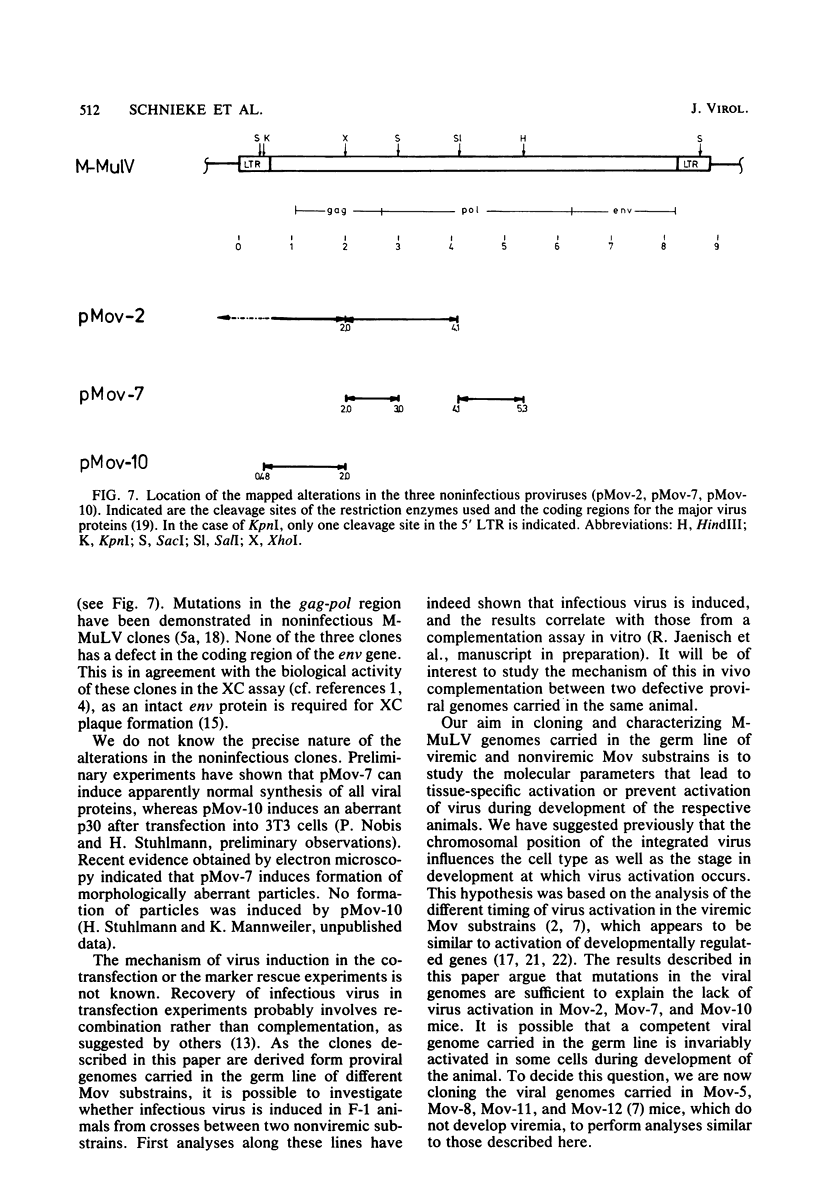
Substrains of mice carrying Moloney murine leukemia virus as a Mendelian gene (Mov locus) have been derived previously. Some of these strains, i.e., Mov-3 and Mov-9, develop viremia, whereas others, i.e., Mov-2, Mov-7, and Mov-10, do not regularly activate virus. We previously have molecularly cloned the respective Mov loci and shown that proviral clones derived from the different viral loci were either infectious (Mov-3, Mov-9) or failed to induce infectious virus (Mov-2, Mov-7, Mov-10) in a transfection assay. To analyze the sites affecting infectivity of the latter clones, complementation assays, in vitro recombinations, and marker rescue experiments were performed. Our results show that the three endogenous Moloney murine leukemia virus clones derived from Mov-2, Mov-7, and Mov-10 carry different mutations in the gag-pol region of the proviral genome. No inhibitory effect of flanking mouse sequences on provirus infectivity was observed.
Full text
PDF
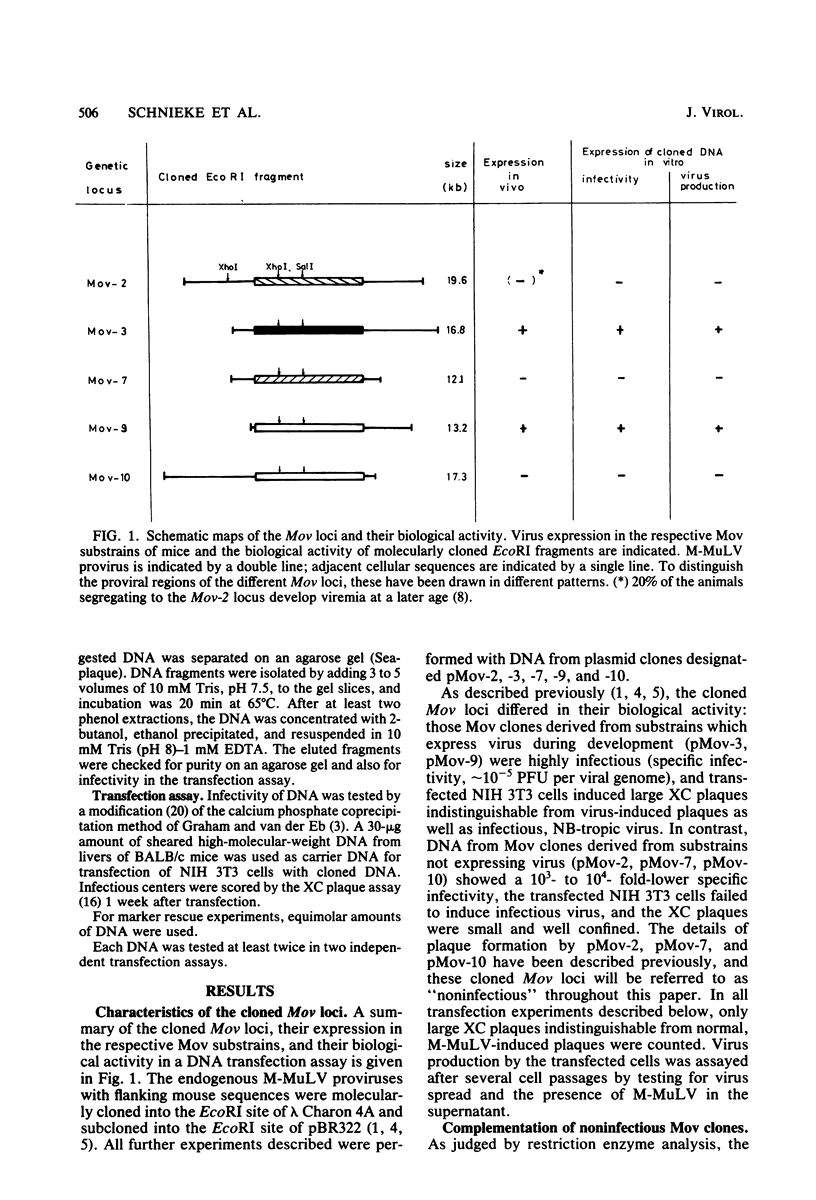

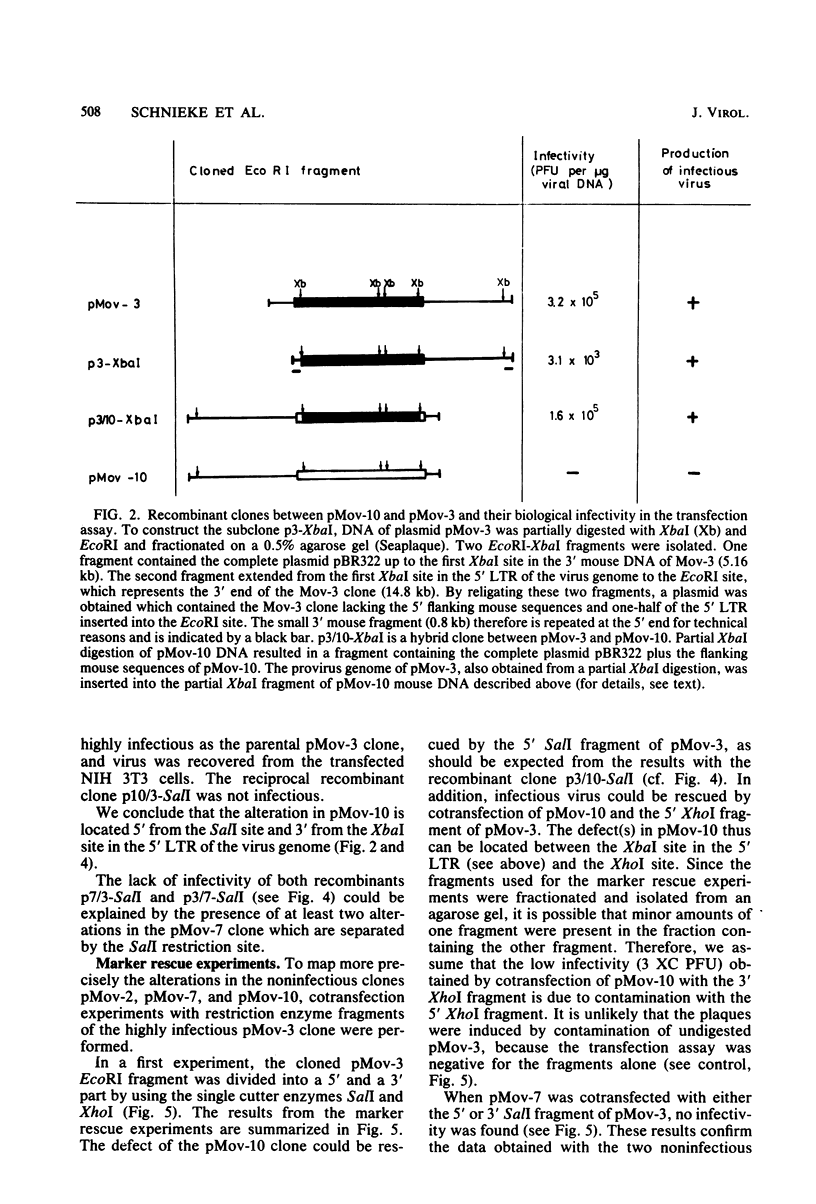

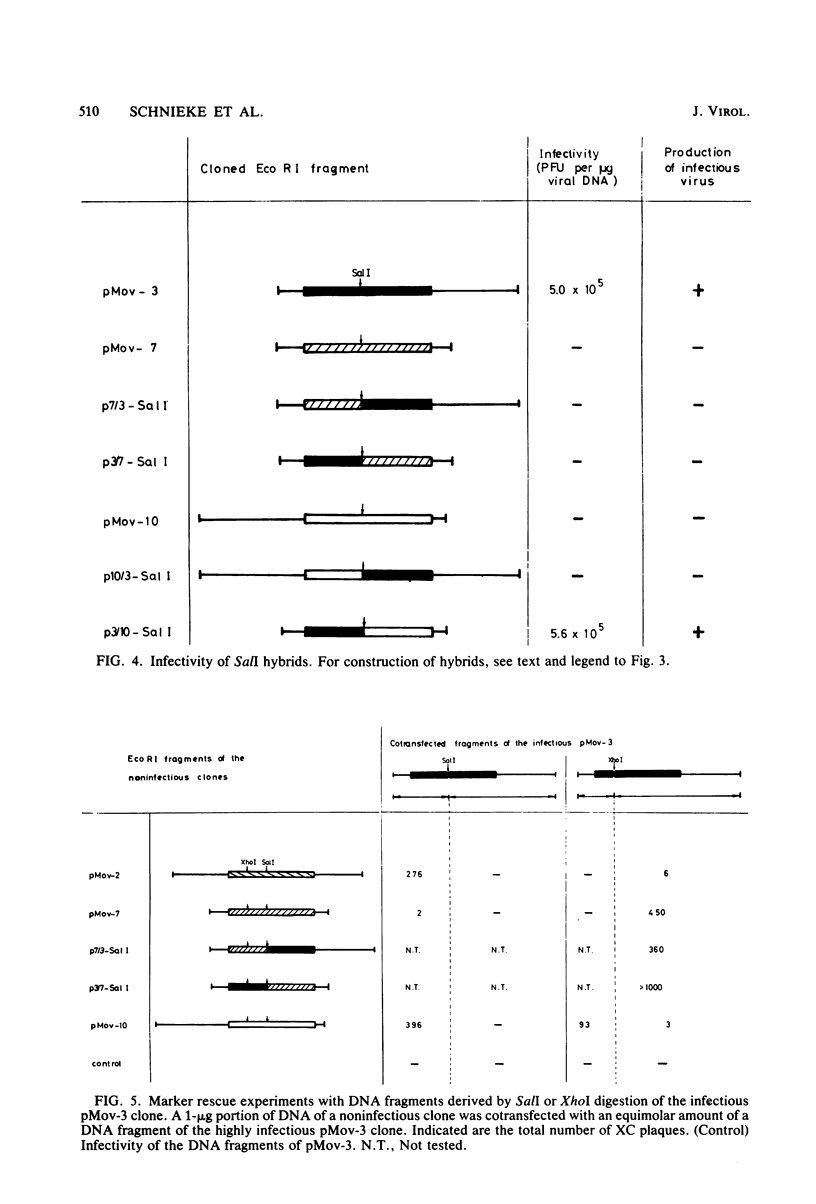

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chumakov I., Stuhlmann H., Harbers K., Jaenisch R. Cloning of two genetically transmitted Moloney leukemia proviral genomes: correlation between biological activity of the cloned DNA and viral genome activation in the animal. J Virol. 1982 Jun;42(3):1088–1098. doi: 10.1128/jvi.42.3.1088-1098.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiedler W., Nobis P., Jähner D., Jaenisch R. Differentiation and virus expression in BALB/Mo mice: endogenous Moloney leukemia virus is not activated in hematopoietic cells. Proc Natl Acad Sci U S A. 1982 Mar;79(6):1874–1878. doi: 10.1073/pnas.79.6.1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Harbers K., Schnieke A., Stuhlmann H., Jaenisch R. Infectivity and structure of molecular clones obtained from two genetically transmitted Moloney leukemia proviral genomes. Nucleic Acids Res. 1982 Apr 24;10(8):2521–2537. doi: 10.1093/nar/10.8.2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harbers K., Schnieke A., Stuhlmann H., Jähner D., Jaenisch R. DNA methylation and gene expression: endogenous retroviral genome becomes infectious after molecular cloning. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7609–7613. doi: 10.1073/pnas.78.12.7609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann J. W., Steffen D., Gusella J., Tabin C., Bird S., Cowing D., Weinberg R. A. DNA methylation affecting the expression of murine leukemia proviruses. J Virol. 1982 Oct;44(1):144–157. doi: 10.1128/jvi.44.1.144-157.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaenisch R. Germ line integration and Mendelian transmission of the exogenous Moloney leukemia virus. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1260–1264. doi: 10.1073/pnas.73.4.1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaenisch R., Jähner D., Nobis P., Simon I., Löhler J., Harbers K., Grotkopp D. Chromosomal position and activation of retroviral genomes inserted into the germ line of mice. Cell. 1981 May;24(2):519–529. doi: 10.1016/0092-8674(81)90343-3. [DOI] [PubMed] [Google Scholar]
- Jähner D., Jaenisch R. Integration of Moloney leukaemia virus into the germ line of mice: correlation between site of integration and virus activation. Nature. 1980 Oct 2;287(5781):456–458. doi: 10.1038/287456a0. [DOI] [PubMed] [Google Scholar]
- Katz L., Kingsbury D. T., Helinski D. R. Stimulation by cyclic adenosine monophosphate of plasmid deoxyribonucleic acid replication and catabolite repression of the plasmid deoxyribonucleic acid-protein relaxation complex. J Bacteriol. 1973 May;114(2):577–591. doi: 10.1128/jb.114.2.577-591.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levinson B., Khoury G., Vande Woude G., Gruss P. Activation of SV40 genome by 72-base pair tandem repeats of Moloney sarcoma virus. Nature. 1982 Feb 18;295(5850):568–572. doi: 10.1038/295568a0. [DOI] [PubMed] [Google Scholar]
- Mullins J. I., Casey J. W., Nicolson M. O., Burck K. B., Davidson N. Sequence arrangement and biological activity of cloned feline leukemia virus proviruses from a virus-productive human cell line. J Virol. 1981 May;38(2):688–703. doi: 10.1128/jvi.38.2.688-703.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Rear J. J., Mizutani S., Hoffman G., Fiandt M., Temin H. M. Infectious and noninfectious recombinant clones of the provirus of SNV differ in cellular DNA and are apparently the same in viral DNA. Cell. 1980 Jun;20(2):423–430. doi: 10.1016/0092-8674(80)90628-5. [DOI] [PubMed] [Google Scholar]
- O'Rear J. J., Temin H. M. Mapping of alterations in noninfectious proviruses of spleen necrosis virus. J Virol. 1981 Jul;39(1):138–149. doi: 10.1128/jvi.39.1.138-149.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Rear J. J., Temin H. M. Spontaneous changes in nucleotide sequence in proviruses of spleen necrosis virus, an avian retrovirus. Proc Natl Acad Sci U S A. 1982 Feb;79(4):1230–1234. doi: 10.1073/pnas.79.4.1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rein A., Lowy D. R., Gerwin B. I., Ruscetti S. K., Bassin R. H. Molecular properties of a gag- pol- env+ murine leukemia virus from cultured AKR lymphoma cells. J Virol. 1982 Feb;41(2):626–634. doi: 10.1128/jvi.41.2.626-634.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe W. P., Pugh W. E., Hartley J. W. Plaque assay techniques for murine leukemia viruses. Virology. 1970 Dec;42(4):1136–1139. doi: 10.1016/0042-6822(70)90362-4. [DOI] [PubMed] [Google Scholar]
- Shen C. K., Maniatis T. Tissue-specific DNA methylation in a cluster of rabbit beta-like globin genes. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6634–6638. doi: 10.1073/pnas.77.11.6634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields A., Witte W. N., Rothenberg E., Baltimore D. High frequency of aberrant expression of Moloney murine leukemia virus in clonal infections. Cell. 1978 Jul;14(3):601–609. doi: 10.1016/0092-8674(78)90245-3. [DOI] [PubMed] [Google Scholar]
- Stuhlmann H., Jähner D., Jaenisch R. Infectivity and methylation of retroviral genomes is correlated with expression in the animal. Cell. 1981 Oct;26(2 Pt 2):221–232. doi: 10.1016/0092-8674(81)90305-6. [DOI] [PubMed] [Google Scholar]
- Weintraub H., Larsen A., Groudine M. Alpha-Globin-gene switching during the development of chicken embryos: expression and chromosome structure. Cell. 1981 May;24(2):333–344. doi: 10.1016/0092-8674(81)90323-8. [DOI] [PubMed] [Google Scholar]
- van der Ploeg L. H., Flavell R. A. DNA methylation in the human gamma delta beta-globin locus in erythroid and nonerythroid tissues. Cell. 1980 Apr;19(4):947–958. doi: 10.1016/0092-8674(80)90086-0. [DOI] [PubMed] [Google Scholar]