Abstract
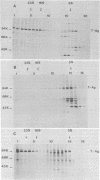
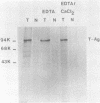
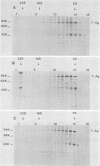
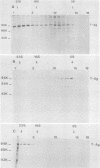
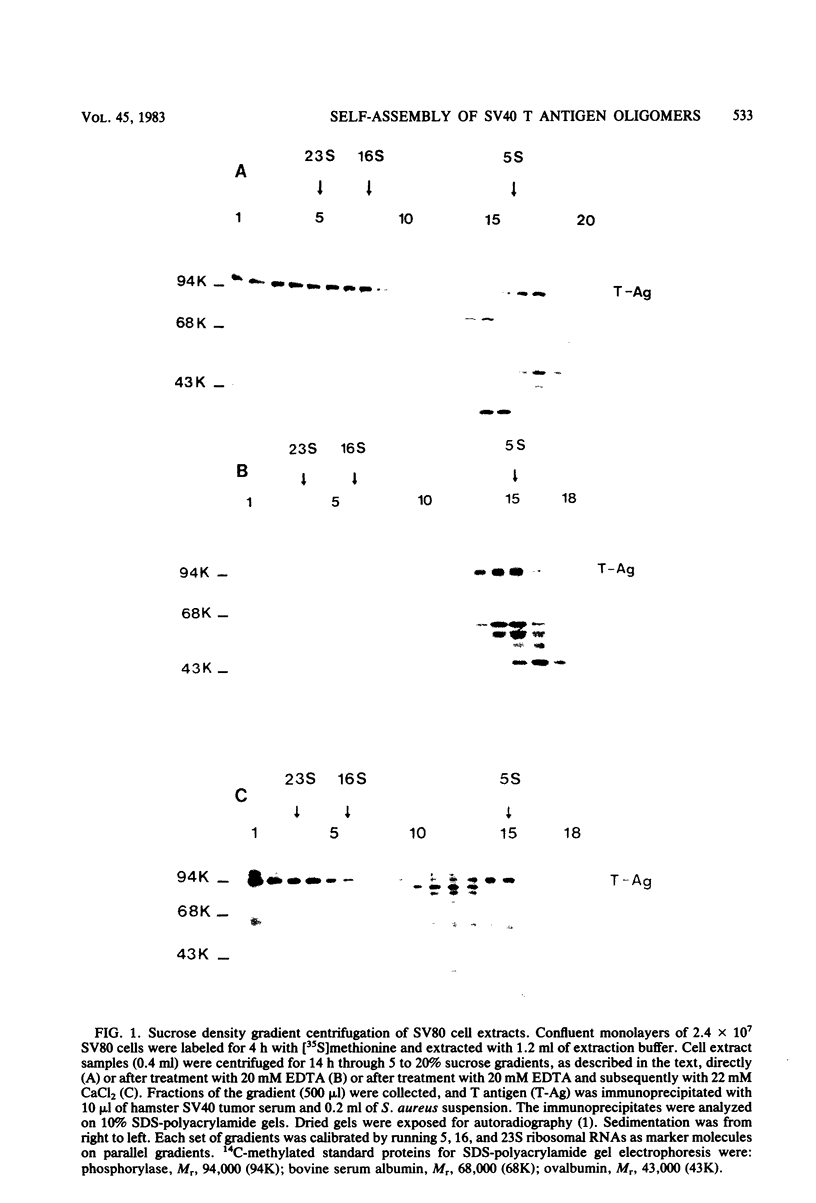
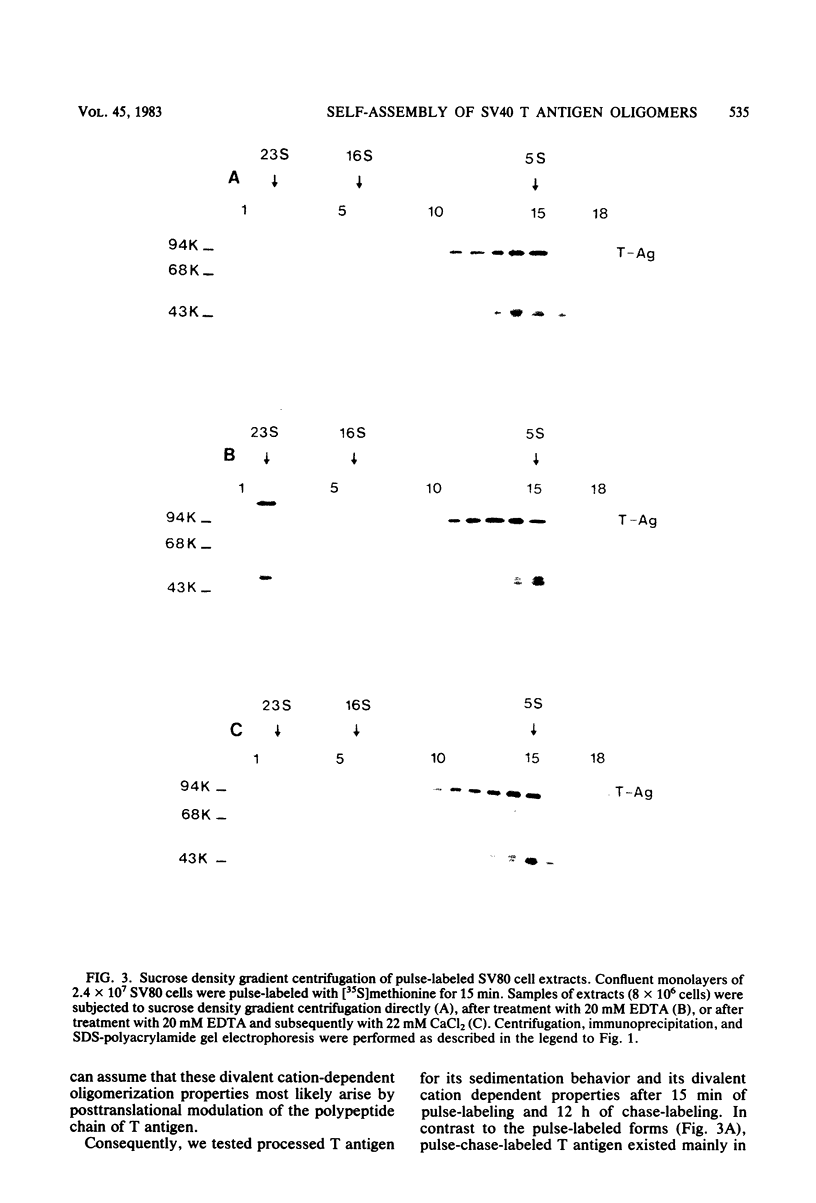
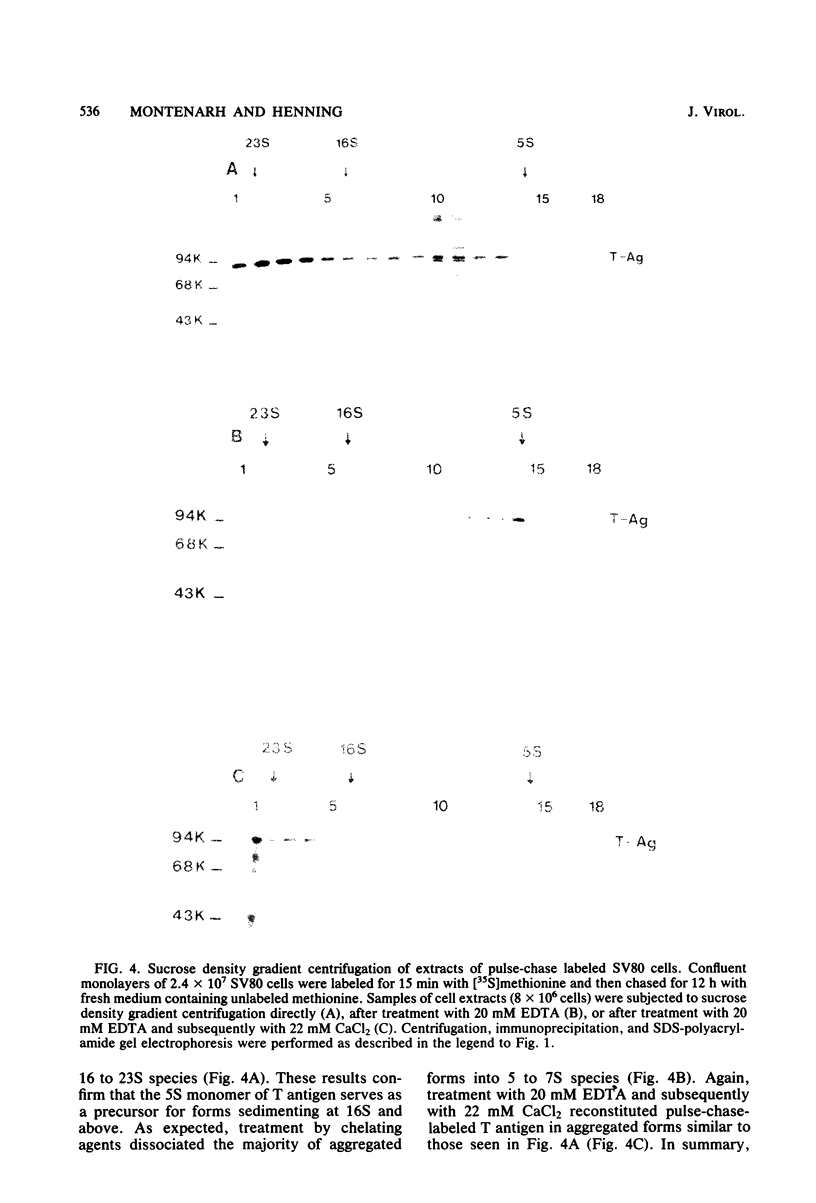
In simian virus 40-transformed cells, simian virus 40 large T antigen can be detected in different forms separable by sucrose density gradient centrifugation. In our experiments, light forms sedimented around 5 to 7S, oligomers such as tetramers were detected around 16S, and higher aggregates sedimented in a broad distribution reaching above 23S. The oligomers sedimenting at and above 16S could be disassembled into the slowly sedimenting 5 to 7S forms by chelating agents [EDTA or ethylene bis(oxonitrilo)tetraacetate]. After the addition of divalent cations (CaCl2 or MgCl2) in excess of chelating agents, oligomeric forms reassembled and appeared in a sedimentation pattern resembling that observed before treatment with chelating agents. Time course studies permitted the identification of the 5 to 7S forms as precursors upon pulse-labeling (15 min); the 16S and higher oligomers were identified as the successors after a 14-h chase. Treatment of extracts of pulse-chase-labeled cells with chelating agents again disassembled the oligomers, whereas pulse-labeled precursors did not change their 5 to 7S sedimentation pattern. Adding an excess of divalent cations reassembled the pulse-chase-labeled T antigen to oligomers but did not influence the sedimentation behavior of pulse-labeled 5 to 7S precursors. It is therefore reasonable to assume that a posttranslational modulation induces divalent cation binding, leading finally to the oligomerization of T antigen. Thus, some of the multifunctional activities of T antigen can be dictated by divalent cation binding properties.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Bradley M. K., Griffin J. D., Livingston D. M. Relationship of oligomerization to enzymatic and DNA-binding properties of the SV40 large T antigen. Cell. 1982 Jan;28(1):125–134. doi: 10.1016/0092-8674(82)90382-8. [DOI] [PubMed] [Google Scholar]
- Clark R., Lane D. P., Tjian R. Use of monoclonal antibodies as probes of simian virus 40 T antigen ATPase activity. J Biol Chem. 1981 Nov 25;256(22):11854–11858. [PubMed] [Google Scholar]
- Crawford L. V., Pim D. C., Gurney E. G., Goodfellow P., Taylor-Papadimitriou J. Detection of a common feature in several human tumor cell lines--a 53,000-dalton protein. Proc Natl Acad Sci U S A. 1981 Jan;78(1):41–45. doi: 10.1073/pnas.78.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanning E., Burger C., Gurney E. G. Comparison of T antigen-associated host phosphoproteins from SV40-infected and -transformed cells of different species. J Gen Virol. 1981 Aug;55(Pt 2):367–378. doi: 10.1099/0022-1317-55-2-367. [DOI] [PubMed] [Google Scholar]
- Fanning E., Nowak B., Burger C. Detection and characterization of multiple forms of simian virus 40 large T antigen. J Virol. 1981 Jan;37(1):92–102. doi: 10.1128/jvi.37.1.92-102.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giacherio D., Hager L. P. A poly(dT)-stimulated ATPase activity associated with simian virus 40 large T antigen. J Biol Chem. 1979 Sep 10;254(17):8113–8116. [PubMed] [Google Scholar]
- Gidoni D., Scheller A., Barnet B., Hantzopoulos P., Oren M., Prives C. Different forms of simian virus 40 large tumor antigen varying in their affinities for DNA. J Virol. 1982 May;42(2):456–466. doi: 10.1128/jvi.42.2.456-466.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman N., Brown M., Khoury G. Modification of SV40 T antigen by poly ADP-ribosylation. Cell. 1981 May;24(2):567–572. doi: 10.1016/0092-8674(81)90347-0. [DOI] [PubMed] [Google Scholar]
- Greenspan D. S., Carroll R. B. Complex of simian virus 40 large tumor antigen and 48,000-dalton host tumor antigen. Proc Natl Acad Sci U S A. 1981 Jan;78(1):105–109. doi: 10.1073/pnas.78.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin J. D., Light S., Livingston D. M. Measurements of the molecular size of the simian virus 40 large T antigen. J Virol. 1978 Jul;27(1):218–226. doi: 10.1128/jvi.27.1.218-226.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin J. D., Spangler G., Livingston D. M. Enzymatic activities associated with the SV40 large T antigen. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 1):113–122. doi: 10.1101/sqb.1980.044.01.013. [DOI] [PubMed] [Google Scholar]
- Griffin J. D., Spangler G., Livingston D. M. Protein kinase activity associated with simian virus 40 T antigen. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2610–2614. doi: 10.1073/pnas.76.6.2610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlow E., Pim D. C., Crawford L. V. Complex of simian virus 40 large-T antigen and host 53,000-molecular-weight protein in monkey cells. J Virol. 1981 Feb;37(2):564–573. doi: 10.1128/jvi.37.2.564-573.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson I. C., Livingston D. M. Partial purification and characterization of the SV40 T antigen. Cell. 1974 Sep;3(1):65–70. doi: 10.1016/0092-8674(74)90041-5. [DOI] [PubMed] [Google Scholar]
- Kessler S. W. Rapid isolation of antigens from cells with a staphylococcal protein A-antibody adsorbent: parameters of the interaction of antibody-antigen complexes with protein A. J Immunol. 1975 Dec;115(6):1617–1624. [PubMed] [Google Scholar]
- Khandjian E. W., Loche M., Darlix J. L., Cramer R., Türler H., Weil R. Simian virus 40 large tumor antigen: a "RNA binding protein"? Proc Natl Acad Sci U S A. 1982 Feb;79(4):1139–1143. doi: 10.1073/pnas.79.4.1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick F., Clark R., Harlow E., Tjian R. SV40 T antigen binds specifically to a cellular 53 K protein in vitro. Nature. 1981 Jul 2;292(5818):63–65. doi: 10.1038/292063a0. [DOI] [PubMed] [Google Scholar]
- McCormick F., Harlow E. Association of a murine 53,000-dalton phosphoprotein with simian virus 40 large-T antigen in transformed cells. J Virol. 1980 Apr;34(1):213–224. doi: 10.1128/jvi.34.1.213-224.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montenarh M., Deppert W., Henning R. Mapping of a DNA-binding domain of simian virus 40 T-antigen using non-defective adenovirus 2--simian virus 40 hybrid viruses. FEBS Lett. 1982 Jun 1;142(1):129–132. doi: 10.1016/0014-5793(82)80235-4. [DOI] [PubMed] [Google Scholar]
- Montenarh M., Henning R. The binding of simian virus 40 large T antigen to the polyphosphate backbone of nucleic acids. Biochim Biophys Acta. 1982 Jun 30;697(3):322–329. doi: 10.1016/0167-4781(82)90095-1. [DOI] [PubMed] [Google Scholar]
- Nockolds C. E., Kretsinger R. H., Coffee C. J., Bradshaw R. A. Structure of a calcium-binding carp myogen. Proc Natl Acad Sci U S A. 1972 Mar;69(3):581–584. doi: 10.1073/pnas.69.3.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POPE J. H., ROWE W. P. DETECTION OF SPECIFIC ANTIGEN IN SV40-TRANSFORMED CELLS BY IMMUNOFLUORESCENCE. J Exp Med. 1964 Aug 1;120:121–128. doi: 10.1084/jem.120.2.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw S. B., Tegtmeyer P. Binding of dephosphorylated A protein to SV40 DNA. Virology. 1981 Nov;115(1):88–96. doi: 10.1016/0042-6822(81)90091-x. [DOI] [PubMed] [Google Scholar]
- Smith A. E., Smith R., Paucha E. Extraction and fingerprint analysis of simian virus 40 large and small T-antigens. J Virol. 1978 Oct;28(1):140–153. doi: 10.1128/jvi.28.1.140-153.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tegtmeyer P., Rundell K., Collins J. K. Modification of simian virus 40 protein A. J Virol. 1977 Feb;21(2):647–657. doi: 10.1128/jvi.21.2.647-657.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todaro G. J., Green H., Swift M. R. Susceptibility of human diploid fibroblast strains to transformation by SV40 virus. Science. 1966 Sep 9;153(3741):1252–1254. doi: 10.1126/science.153.3741.1252. [DOI] [PubMed] [Google Scholar]