Abstract
Schizosaccharomyces pombe cells respond to nutrient deprivation by altering G2/M cell size control. The G2/M transition is controlled by activation of the cyclin-dependent kinase Cdc2p. Cdc2p activation is regulated both positively and negatively. cdr2+ was identified in a screen for regulators of mitotic control during nutrient deprivation. We have cloned cdr2+ and have found that it encodes a putative serine-threonine protein kinase that is related to Saccharomyces cerevisiae Gin4p and S. pombe Cdr1p/Nim1p. cdr2+ is not essential for viability, but cells lacking cdr2+ are elongated relative to wild-type cells, spending a longer period of time in G2. Because of this property, upon nitrogen deprivation cdr2+ mutants do not arrest in G1, but rather undergo another round of S phase and arrest in G2 from which they are able to enter a state of quiescence. Genetic evidence suggests that cdr2+ acts as a mitotic inducer, functioning through wee1+, and is also important for the completion of cytokinesis at 36°C. Defects in cytokinesis are also generated by the overproduction of Cdr2p, but these defects are independent of wee1+, suggesting that cdr2+ encodes a second activity involved in cytokinesis.
INTRODUCTION
The eukaroytic cell cycle is regulated by a number of evolutionarily conserved gene products. The fission yeast, Schizosaccharomyces pombe, has been used extensively as a model organism to isolate and study these genes, especially those involved in the progression from G2 to M. The best described of these is cdc2+, which encodes a cyclin- dependent protein kinase, whose periodic activation controls progression into both M and S phases of the cell cycle (Nurse and Bissett, 1981; Piggot et al., 1982). Mutational analyses of S. pombe have identified temperature-sensitive alleles of cdc2 that arrest in G2, unable to progress into M phase, as well as alleles that arrest in both G2 and G1, unable to progress into M or S phase (Nurse and Bissett, 1981).
Other regulators of G2/M progression have also been identified, and several of these encode regulators of Cdc2p (reviewed by Berry and Gould, 1996). cdc13+ encodes a B-type cyclin that binds to Cdc2p as a positive regulatory subunit (Booher and Beach, 1988; Hagan et al., 1988; Booher et al., 1989). The gene products of wee1+ and cdc25+ regulate activation of Cdc2p by phosphorylation (inhibitory) and dephosphorylation (activating) of Cdc2p Y15, respectively, which sets the timing of mitosis (reviewed by Morgan, 1995; Berry and Gould, 1996). Y15 phosphorylation is not only a critical control point for G2/M progression, but it also determines cell size at mitosis (Gould and Nurse, 1989) and thus provides a link between mitotic control and growth.
S. pombe cells grow by tip extension, such that increasing cell mass is reflected by increasing cell length (Mitchison, 1957). At the end of the cycle, cells “fission” or divide medially, generating two identical daughter cells. S. pombe cells are also able to adjust cell length by changing the timing of mitosis (Fantes, 1977). For a population of cells to be able to maintain an average cell size, cells born with a smaller-than-average length must spend more time growing, before initiating mitosis and dividing, than do cells born with an average cell length. The reverse is true for cells born with a larger-than-average cell length.
The sizing mechanism linking growth to division, or the G2/M cell size checkpoint, has been studied extensively in S. pombe through the analysis of mutant alleles of wee1 (Nurse, 1975; Fantes and Nurse, 1978; Thuriaux et al., 1978; Nurse and Thuriaux, 1980; Fantes, 1981; Russell and Nurse, 1987a). The G2/M cell size checkpoint is missing in wee1 mutants, so that cells initiate mitosis at a length much shorter than wild type (Fantes and Nurse, 1978). Because wee1 cells spend a longer time than wild type in G1, it was also determined that a G1/S cell size checkpoint exists, forcing cells to achieve a critical cell length in G1 before progressing into S phase. Since the G2/M cell size checkpoint is missing in wee1 mutants, it is thought that this checkpoint operates through Wee1p-inhibitory phosphorylation of Cdc2p Y15.
For both immediate and long-term survival, S. pombe cells must be able to respond to changes in nutrition by changing the rate of growth and division (Fantes and Nurse, 1977). Nutritional changes trigger the G2/M cell size control mechanism, shifting the size required for mitotic progression so that cells growing in rich medium divide with a longer cell size, whereas cells growing in poor medium divide with a smaller cell size. Also, when faced with severe shortages of nitrogen, S. pombe cells are able to adapt by altering G2/M cell size control so that cells arrest in G1 with a much reduced cell size. If the cells are sexually competent and the proper mating partner is present, they differentiate sexually and mate (reviewed by Egel, 1989). However, if there is no sexual partner present, the arrested cells enter a long-term state of dormancy, also referred to as G0 or quiescence. Cells arrested in G0 are able to survive over extended periods of time and are resistant to environmental stresses such as heat shock.
In a search for potential regulators of the G2/M cell size control, Young and Fantes (1984, 1987) carried out a genetic screen in which they isolated mutants that were unable to alter G2/M size control in response to nitrogen deprivation. It was anticipated that such a screen would identify genes involved in G2/M progression control and also those involved in nutritional sensing and monitoring. To date, only one complementation group from this screen, cdr1+ (changed division response), has been further characterized (Russell and Nurse, 1987b; Feilotter et al., 1991; Coleman et al., 1993; Parker et al., 1993; Wu and Russell, 1993; Wu et al., 1996; Belenguer et al., 1997; Rupes et al., 1997; Wu and Russell, 1997). cdr1+ was also isolated as nim1+ (new inducer of mitosis), a multicopy suppressor of cdc25-22 (Russell and Nurse, 1987b). Like all cdr mutants, cdr1/nim1 mutant cells are unable to respond properly to nitrogen deprivation and arrest as large cells in G2 rather than as smaller cells in G1 (Young and Fantes, 1984, 1987; Belenguer et al., 1997; Wu and Russell, 1997). Moreover, cycling cdr1/nim1 cells are longer than wild type, suggesting that their mitotic control has been altered. Molecularly, it has been shown that cdr1/nim1 encodes a serine-threonine protein kinase and acts as a mitotic inducer by negatively regulating Wee1p activation; thus, it is a component of mitotic control (Russell and Nurse, 1987b; Feilotter et al., 1991; Coleman et al., 1993; Parker et al., 1993; Wu and Russell, 1993).
We present the characterization of a second complementation group isolated in the Young and Fantes screen. Similar to cdr1/nim1, cdr2 mutant cells initiate mitosis with a cell length longer than wild type and arrest in G0 from G2 with a large cell size in response to nitrogen deprivation (Young and Fantes, 1984, 1987; Rupes et al., 1997). We have found that cdr2+ is nonessential and is predicted to encode a serine-threonine protein kinase. We present genetic evidence to demonstrate that cdr2+ functions in G2 as a negative regulator of wee1+. We also show that cdr2+ has an additional role in cell division. Cells lacking Cdr2p or overexpressing cdr2+ fail to undergo cytokinesis and septation normally; these defects are independent of Wee1p function.
MATERIALS AND METHODS
Yeast Methods and Strains
S. pombe strains used in this study are listed in Table 1. Strains were grown in yeast extract medium, minimal medium with appropriate supplements, or minimal medium lacking ammonium chloride as the nitrogen source (Moreno et al., 1991). Crosses were performed on glutamate medium (minimal medium lacking ammonium chloride and containing 0.01 M glutamate, pH 5.6). Tetrad analysis was performed as described (Moreno et al., 1991). Yeast transformations were performed by electroporation (Prentice, 1991). Genomic DNA was isolated as described (Moreno et al., 1991; Hoffman, 1993).
Table 1.
S. pombe strain list
| Strain | Genotype | Source | |
|---|---|---|---|
| GL122 | h− | cdc25-22::pRIP2cdc25+leu1-32 ura4-D18his3-237 | Paul Russell |
| Q1045 | h+ | cdc25-22r1 | This study |
| Q1046 | h− | cdr2+::pcdr2.2leu1-32 | This study |
| KGY1 | h− | leu 1-32 | Paul Nurse |
| KGY4 | h− | wee1::ura4-+ura4-D18 leu1-32 | Paul Nurse |
| KGY19 | h− | cdc2-33 leu1-32 | Paul Nurse |
| KGY43 | h+ | cdc25-22 leu1-32 | Paul Nurse |
| KGY68 | h90 | Paul Nurse | |
| KGY69 | h+ | 975 | Paul Nurse |
| KGY137 | h+/h− | ura4-D18/ura4-D18 leu1-32/leu1-32 ade6-210/ade6-216 | Paul Nurse |
| KGY246 | h− | leu1-32, ura4-D18, ade6-210 | Paul Nurse |
| KGY347 | h− | cdc13-117 ura4-D18 leu1-32 ade6-216 | Paul Nurse |
| KGY460 | h+ | wee1-50 ura4-D18 leu1-32 | Paul Nurse |
| KGY519 | h− | cdr2-96 leu1-32 | Young and Fantes, 1987 |
| KGY531 | h− | cdc2-22 ura4-D18 leu1-32 ade6-210 | Paul Nurse |
| KGY1475 | h+ | cdr2::ura4+ura4D18 | This study |
| KGY1476 | h− | cdr2::ura4+ura4D18 | This study |
| KGY1480 | h90 | cdr2::ura4+ura4D18 | This study |
| KGY1519 | h− | cdr2::ura4+ura4-D18 leu1-32 | This study |
| KGY1628 | h− | HAcdr2+ | This study |
| KGY1630 | h+/h− | cdr2+/cdr2::ura4+ura4-D18/ura4-D18 leu1-32/leu1-32 ade6-210/ade6-216 | This study |
| KGY1631 | h− | cdc25-22 cdr2::ura4+ | This study |
| KGY1632 | h− | cdc2-33 cdr2::ura4+ | This study |
| KGY1633 | h− | cdc2-22 cdr2::ura4+ | This study |
| KGY1634 | h− | cdc13-117 cdr2::ura4+ | This study |
| KGY1635 | h− | wee1::ura4+cdr2:;ura4+ | This study |
| KGY1636 | h− | wee1-50 cdr2::ura4+ | This study |
| KGY1637 | h− | cdc25-22::pRIP2cdc25+cdr2::ura4+ | This study |
Molecular Biology Techniques
All plasmid manipulations and bacterial transformations were by standard techniques (Sambrook et al., 1989). Essential features of plasmid construction are described below. All sequencing was performed using Sequenase 2.0 (United States Biochemical, Cleveland, OH) according to manufacturer’s instructions. All PCR reactions except those for the construction of epitope-tagged strains were performed using Taq DNA polymerase and the GeneAmp PCR kit (Perkin Elmer-Cetus, Norwalk, CT) in a PTC-100 programmable thermal controller (PTC-100; MJ Research, Watertown, MA) programmed as follows: 94°C, 1 min; 50°C, 2 min; 72°C, 2 min (40 cycles); 72°C, 10 min. TaqPlus Precision (Stratagene, La Jolla, CA) was used to amplify the cdr2HA sequence in a PTC-100 programmable thermal controller (PTC-100; MJ Research) programmed as follows: 95°C, 1 min; 65°C–1°C per cycle, 1 min; 72°C, 5 min (15 cycles); 95°C, 1 min; 50°C, 1 min; 72°C, 5 min (30 cycles); 72°C, 10 min.
Physiological Experiments
For analysis of synchronous cell populations, 4 l of cells were grown to midlog phase (8 × 106 cells/ml) at 30°C in minimal medium. Cells were separated on the basis of size by centrifugal elutriation in an elutriator rotor (JE 5.0; Beckman, Fullerton, CA). Cells synchronized in early G2 were collected in minimal medium and split into two cultures. Cells from each culture were collected on filters and released into either minimal medium or minimal medium lacking nitrogen at 30°C. Synchrony was monitored at 20- and 30-min intervals by scoring 100 cells for the presence of a septum. Samples were collected periodically for determination of cell length, DNA content, cell number increase, binculeate cells, and protein analysis.
For asynchronous nitrogen deprivation experiments, cells were grown to midlog phase in minimal medium at 30°C. Cells were collected on filters and then inoculated into minimal medium lacking nitrogen. Samples were collected periodically and were processed to determine DNA content.
To determine long-term viability and resistance to heat shock in response to nitrogen deprivation, cells were grown and deprived of nitrogen as described (Su et al., 1996). For viability, samples were collected periodically over a 20-d time course, diluted appropriately, and plated in triplicate on YE plates at 25°C, and colonies were counted 5 d later. For heat shock, cells were incubated at 42°C for 5 min, diluted appropriately, and plated in triplicate on YE plates at 25°C, and colonies were counted 5 d later.
To determine total cell number, cells were collected and fixed in 0.12 M NaCl, 3% formaldehyde, diluted appropriately, sonicated briefly, and then counted in triplicate using the Coulter Multisizer II (Coulter Electronics, Hialeah, FL). Total cell number was taken as the average of each triplicate.
To determine DNA content, cells were fixed in ice-cold 70% ethanol, washed in 50 mM sodium citrate, incubated with 0.1 μg/ml RNase A in 50 mM sodium citrate for 2 h at 37°C, and then stained with 1 μM Sytox green (Molecular Probes, Sunnyvale, CA) for 2 h. Cells were sonicated and analyzed by flow cytometry as described (Sazer and Sherwood, 1990).
To determine cell length, fixed cells were measured microscopically using phase optics and a 100× objective with an eyepiece drum micrometer. To determine average cell length, 100 cells were measured. To determine septation length, 50 cells containing a septum were measured.
Microscopy
All light and fluorescence microscopy was performed on a Zeiss microscope (Axioskop; Carl Zeiss, Thornwood, NY) using appropriate filters. Cells were either fixed in 100% methanol at −20°C for 8 min or in 30% formaldehyde for 10 min at room temperature and then washed with PBS and processed as described by Balasubramanian et al. (1997). To visualize DNA and/or cell and septal material, cells were fixed in methanol or formaldehyde and stained with DAPI and/or Calcofluor.
Cloning and DNA Sequence of cdr2+
The cdr2-96 cdc25-22r1 leu1-32 (Q1045) strain was transformed with a S. pombe genomic library that was prepared from HindIII and Sau3A partially digested genomic DNA inserted into pWH5 (Wright et al., 1986; Hudson et al., 1990; Young and Beach, unpublished results). Transformants were selected at 25°C for 2 d on minimal medium lacking leucine and then shifted to 35°C. Plasmids were recovered from viable colonies and were transformed into the cdr2-96 leu1-32 strain (KGY519). Only one plasmid (pcdr2.1) was able to restore the nitrogen deprivation response of cdr2-96, and this plasmid was subjected to further subcloning to identify a minimal complementing fragment of 3.0 kilobases (kb).
To characterize the complementing DNA, the 3.0-kb genomic fragment was further subcloned, and appropriate plasmids were sequenced in both directions to generate 2656 base pairs (bp) of sequence. This sequence contained a single open reading frame (ORF) of 2247 bp but terminated without a stop codon. Comparison of this sequence to the S. pombe sequence database revealed that this sequence was identical to a sequence contained on cosmid c57A10 from chromosome I. Cosmid c57A10 contained the complete ORF of 2325 bp.
For integration mapping, a leu1-32 strain (KGY1) was transformed with linearized pcdr2.2. Transformants were selected on minimal medium lacking leucine. The resulting Leu+ strain (Q1046) was crossed to cdr2-96 leu1-32 (KGY519), to verify cosegregation of the Cdr2+ phenotype with the Leu+ phenotype. Only Cdr2+ Leu+ and Cdr2− Leu− progeny were produced, indicating that pcdr2.2 had integrated within or very close to the cdr2+ locus.
Deletion of cdr2+
To generate a full-length cdr2+ construct containing 5′- and 3′-flanking genomic sequence, the 3.0-kb fragment containing 2247 bp of cdr2+ sequence and 400 bp of 5′-flanking sequence was subcloned into pSK (Stratagene) generating pMB17. Next, a NdeI site was introduced at the initiating codon of the cdr2+ sequence, and two endogenous NdeI sites were removed by site-directed mutagenesis (Chameleon Double Stranded Mutagenesis Kit; Stratagene) in pMB17 generating pKG939. To generate the 3′-coding region missing from pMB17, the oligonucleotides CSB51 (5′-CGTATGAATGAGAATGA-3′) and CSB52 (5′-CCTTGCTCTAGACATGCAAG-3′) were used to PCR amplify from genomic DNA a 1300-bp fragment consisting of 876 bp of the 3′-cdr2+ coding region plus 424 bp of 3′-flanking genomic sequence. After digestion with XbaI, the 3′-fragment was subcloned into pMB939, exchanging the incomplete 3′-end of the cdr2+ coding region with the complete 3′-coding sequence plus flanking sequence, generating pKG943. pKGY943 was sequenced to verify accuracy of the cdr2+ coding region.
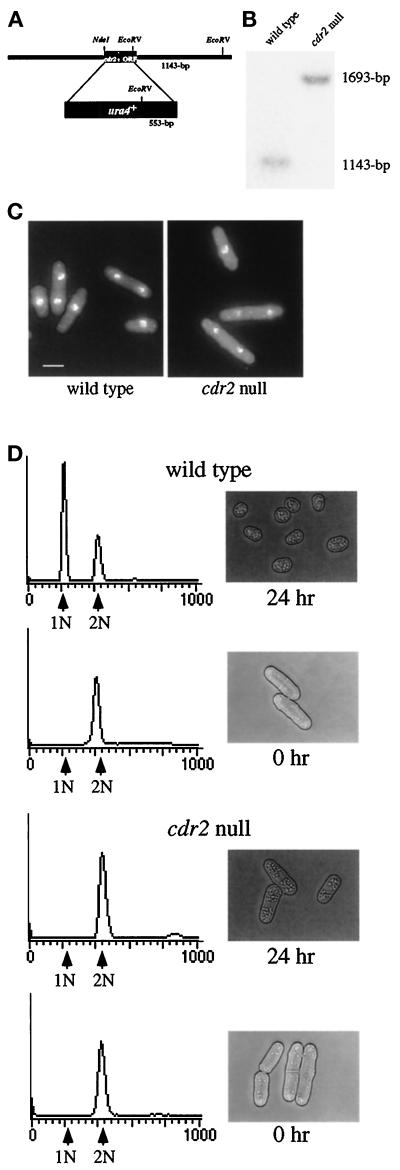
To generate a deletion construct containing cdr2+ flanking sequence but lacking the cdr2+ coding region, the cdr2+ coding sequence plus flanking sequences were first shuttled into pTZ from pKG943 as a SacI–SmaI fragment to generate a construct containing a unique EcoRV site (pKG935). pKG935 was digested with NdeI and EcoRV, removing all but the last 289 bases of the cdr2+ coding sequence, and replaced with a 1.8-kb fragment containing the ura4+ gene, generating pKG1275 (see Figure 2A). Digestion of pKG1275 with SmaI and SacI liberated the deletion fragment, which was transformed into a ura4-D18/ura4-D18 diploid strain (KGY137). Ura+ diploid transformants were selected on minimal medium lacking uracil and were subjected to tetrad analysis. All spores were viable, and the Ura+ prototrophs were subjected to Southern hybridization analysis to verify deletion of the cdr2+ sequence.
Figure 2.
Figure 2. Deletion of cdr2+. (A) Diagram of the cdr2 deletion construct. (B) Southern blot of a wild-type and a cdr2::ura4+ strain (KGY246 and KGY1519). The 1143-bp band represents the wild- type cdr2+ locus while the 1693-bp band represents the disrupted cdr2::ura4+ locus. (C) Phenotype of the cdr2 null cells. Wild-type and cdr2::ura4+ strains (KGY69 and KGY1475) were grown at 30°C to midlog in YE, fixed with formaldehyde, and stained with DAPI to visualize DNA. Scale bar, 5 μm. (D) Response of cdr2 null and wild-type cells to nitrogen deprivation. Wild-type (upper panels) and cdr2 null (lower panels) strains (KGY69 and KGY1475) were grown at 30°C to midlog, harvested by filtration, washed, and released into minimal medium lacking nitrogen. Samples were collected at 0 and 24 h for DNA content (left-hand panels) and to visualize the phenotype of the cells by light microscopy (right-hand panels).
Conjugation Efficiency Assay
To determine conjugation efficiency, homothallic cdr2::ura4+ (KGY1480) and wild-type homothallic (KGY68) strains were plated onto glutamate agar for 48 h. Samples were analyzed under light microscopy, and conjugation efficiency was determined as described (Wu and Russell, 1997).
Overexpression Analysis
To determine whether the nitrogen deprivation defect of the cdr2 null could be rescued by overexpression of genes encoding mitotic components, a cdr2::ura4+ ura4-D18 leu1-32 strain (KGY1519) was transformed with plasmids containing cDNAs encoding cdc2+, cdc13+, and cdr2+ under control of the S. pombe nmt1 (no message in thiamine) thiamine repressible promoter (Maundrell, 1993). To generate pREP1cdr2+ (KG947), pKG939 was digested with XbaI, treated with Klenow, and then digested with NdeI. The resulting fragment was subcloned into NdeI/SmaI-digested pREP1. The transformed strains were grown to midlog at 30°C in minimal medium containing thiamine, recovered on filters, released into minimal medium containing thiamine but lacking nitrogen, and incubated at 30°C for 48 h. Rescue was determined by phenotype and by DNA content. To determine whether overexpression of cdc25+ could rescue the nitrogen deprivation defect of the cdr2 null, a cdc25-22 strain containing cdc25+ under control of the nmt1 promoter integrated at the cdc25 locus (GL122) was crossed to the cdr2 null. The resulting double mutant (KGY1637) was grown in minimal medium containing thiamine to midlog at 25°C, washed to remove the thiamine, and then incubated in minimal medium for 18 h to induce expression of cdc25+. Cells were then collected on filters, released into minimal medium lacking nitrogen, and incubated for 48 h at 25°C.
To analyze the phenotype of cdr2+ overexpression in wild-type cells and in the absence of wee1+ activity, a wild-type strain (KGY246) and the wee1::ura4 ura4-D18 leu1-32 strain (KGY4) were each transformed with pREP1 vector alone and pREP1cdr2+ (KG947). The transformed strains were grown at 25°C in minimal medium containing thiamine to midlog, collected, washed to remove the thiamine, and then released into minimal medium lacking thiamine for 18 h.
Construction of Epitope-tagged Strains
To construct a genomic 3′-3XHA epitope-tagged cdr2+ strain (KGY1628), a PCR amplification-based strategy was utilized as described previously (Bähler et al., 1998). Oligonucleotides 5′-cdr2long (5′-CGGCATCCAGACCTGTTTCTCGAATGAGTGTAAGTAGTAGTCCTTTTGCTGTATTTCGTCAACGACAATCCGTCCAAAGTCGGATCCCCGGGTTAATTAA-3′) and 3′-cdr2long (5′-CCAAAGCATCACGAGAAAAATGAAGTTTGCAAAGGTTTTGGAGAATCAAAAAAAAATGATAATAATAATAATAAAAGAATGAATTCGAGCTCGTTTAAAC-3′) were used to PCR amplify a fragment containing a 3XHA epitope tag kanamycin resistance (kanMX6) cassette flanked on either side with cdr2+ 3′-genomic sequence. A wild-type strain (KGY246) was transformed with the amplified fragment, plated onto YE medium overnight, and then replica plated onto YE medium containing the drug G418 to select for recombinants. Recombinants were outcrossed to wild type to confirm segregation of the kanMX6 marker and were screened by immunoblotting with the 12CA5 monoclonal antibody specific to the hemagglutinin (HA) epitope. Proper integration of the HA epitope in cdr2+ was confirmed by Southern hybridization analysis and functionality was assessed by phenotype.
Southern Hybridization Analysis
Approximately 0.5 μg of genomic DNA was digested overnight at 37°C, size fractionated on an 0.8% agarose gel, and transferred to a GeneScreen Plus membrane (New England Nuclear Life Science Products, Boston, MA) overnight. The membrane was treated for 1 h in hybridization buffer (5× Denhardt’s solution, 0.5% SDS, 5× SSPE, and 100 μg of hydrolyzed yeast RNA per ml), and then overnight at 65°C in hybridization buffer containing a random-primed α-[32P]dCTP-labeled probe (rediPrime; Amersham, Arlington Heights, IL). The membrane was then washed two times for 30 min at 65°C in 2× SSC–0.2% SDS. Hybridizing bands were detected by autoradiography.
Immunoblotting
S. pombe denatured lysates were prepared as described (Gould et al., 1991). Approximately 2.4 × 108 cells were lysed, and total protein in each lysate was determined by BCA (BCA Protein Assay Reagent Kit; Pierce Chemical, Rockford, IL). For Western blot analysis, lysates were resolved by SDS-PAGE and electrophoretically transferred to a polyvinylidene difluoride membrane (Immobilon-P, Millipore, Bedford, MA). Blots were probed with either monoclonal antibodies specific to the HA epitope (12CA5) at 2 μg/ml, PSTAIRE domain of cyclin-dependent kinases (Yamashita et al., 1991; Sigma Chemical, St. Louis, MO) at 2 μg/ml, or a polyclonal antibody specific to S. pombe Cdc13p (GJG56, Den Haese and Gould, unpublished results). Antibody GJG56 was affinity purified as described previously (Olmsted, 1981) and used at 1:100 dilution. In S. pombe, the PSTAIRE antibody detects two PSTAIRE motif-containing proteins, p34 (Cdc2p) and p31, which encodes a PSTAIRE-related protein (Tournier et al., 1997). Primary antibodies were followed with the appropriate peroxidase-conjugated secondary antibody (Sigma). Reactive proteins were visualized by enhanced chemiluminescence (Amersham Life Sciences). For quantitation of immunoblotting data, ECL Plus reagents (Amersham Life Sciences) were used. Data were collected on a Molecular Dynamics Storm instrument and quantified by ImageQuant version 1.1.
RESULTS
cdr2+ Encodes a Putative Serine-Threonine Protein Kinase
Since none of the previously characterized cdr2 mutant alleles imparted a conditional phenotype, we took advantage of the strong negative interaction between cdr2 and cdc25 mutant alleles to clone the cdr2+ gene (Young and Fantes, 1987). A double mutant between cdr2-96 and a non-temperature–sensitive allele of cdc25, cdc25-22r1 (Hudson et al., 1990), was constructed. While viable at 25°C, cdr2-96 cdc25-22r1 is temperature sensitive for growth at 35°C. This strain was transformed with a S. pombe genomic library (Wright et al., 1986; Young and Beach, unpublished results), and only one transformant that grew at 35°C contained a plasmid that was also able to restore the ability of cdr2-96 to arrest with a small cell size in response to nitrogen deprivation. The complementing plasmid was further characterized to identify a minimal rescuing fragment of 3.0 kb. Integration mapping confirmed that the 3.0-kb fragment contained the cdr2+ gene and not a high-copy suppressor.
Sequencing of the 3.0-kb genomic rescuing fragment uncovered a continuous ORF of 2247 bp that did not contain a stop codon. When this ORF was compared with the S. pombe sequence database, an identical sequence was found on cosmid c57A10 from S. pombe chromosome I. The complete cdr2+ ORF encoded a protein of 775 amino acids with a predicted molecular weight of 85,917 (Figure 1A). Further comparison of the Cdr2p amino acid sequence to sequences in the databases revealed the presence of amino- terminal motifs that are signatures of protein kinase catalytic domains (Hanks et al., 1988). The putative catalytic domain of Cdr2p showed highest sequence similarity to the Snf1p subfamily of serine-threonine protein kinases of which the Saccharomyces cerevisiae carbon catabolite derepressing kinase Snf1p is the prototypical member. Members of the Snf1p subfamily to which Cdr2p has high similarity in the catalytic domain include the S. cerevisiae protein Gin4p, Cdr1p/Nim1p in S. pombe, and the Cdr1p/Nim1p S. cerevisiae homologue, Hsl1p (Figure 1B) (Russell and Nurse, 1987b; Feilotter et al., 1991; Ma et al., 1996; Atman and Kellogg, 1997). Although limited, Cdr2p also has sequence similarity to Gin4p outside the catalytic domain (Figure 1C).
Figure 1.
Sequence analysis of cdr2+. (A) Nucleotide sequence and predicted amino acid sequence of cdr2+. Sequencing of the cdr2+ complementing DNA generated bases −409 to +2247 that did not include a stop codon. Comparison of this sequence to the S. pombe sequence database revealed that it was identical to a ORF on cosmid c57A10 from chromosome I. Bases +2248 to +2872 were obtained solely from the sequence of cosmid c57A10. The GenBank accession number for the cdr2+ sequence is AF092508. (B) Alignment of the Cdr2p putative catalytic domain with the Snf1p subfamily members S. cerevisiae proteins Hsl1p and Gin4p and S. pombe Cdr1p/Nim1p. Subdomains conserved among protein kinases I–XI are indicated by black lines (Hanks et al., 1988). Amino acid residues conserved in three of four sequences are highlighted in black. (C) Amino acid sequence alignment of Cdr2p and Gin4p. Identical residues are indicated by colons (:). Similar residues are indicated by a period (.).
Analysis of a cdr2 Null Mutant
To determine the phenotype of a strain in which cdr2+ has been deleted, the one-step gene disruption method was used to replace one copy of the cdr2+ coding sequence with the S. pombe ura4+ selectable marker as described in MATERIALS AND METHODS. We found that cdr2+ is a nonessential gene; Southern hybridization analysis of genomic DNA isolated from a Ura+ haploid colony confirmed that the cdr2+ coding sequence had been replaced by ura4+ (Figure 2B). However, cells lacking cdr2+ were longer than wild type, septating at an average length of 19.5 μm in minimal medium, the same length at which cdr2-96 septates, while wild-type cells septated at an average of 13.0 μm (Figure 2C).
The original cdr2 mutant alleles were isolated by virtue of their inability to respond normally to nitrogen deprivation (Young and Fantes, 1984, 1987). Subsequent analyses demonstrated that the cdr2 mutants were longer than wild-type cells under all growth conditions. Moreover, it was determined that during nitrogen deprivation, cdr2 mutants failed to arrest in G1 and instead arrested in G2 (Young and Fantes, 1984, 1987; Rupes et al., 1997). To determine whether the cdr2 null strain behaved similarly, we performed flow cytometry on cdr2 null and wild-type strains undergoing nitrogen deprivation. Over the course of the experiment, wild-type cells became short and rounded (Figure 2D). The majority of the cells contained a 1 N content of DNA, demonstrating that these cells had arrested in G1. In contrast, the majority of cdr2 null cells remained longer than wild-type cells and contained a 2N content of DNA. Hence, the cdr2 null cells behaved identically to cdr2-96 cells in these assays.
cdr2 Mutants Arrest in G0 in Response to Nitrogen Deprivation
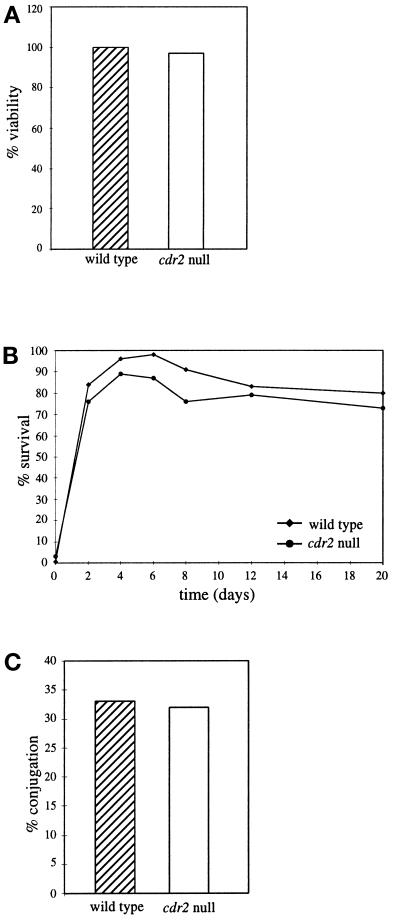
S. pombe cells responding to nitrogen deprivation have two choices: they either enter G0, or they initiate sexual differentiation if the proper mating partner is present. Cells arrested in G0 are able to maintain viability over long periods of time and are able to withstand extracellular stresses such as heat shock (reviewed by Egel, 1989). To examine the ability of cdr2 cells to enter G0 from G2, long-term viability and heat-shock tolerance were measured in cdr2 null and wild-type strains undergoing nitrogen deprivation. There was no significant difference in the long-term viability of the cdr2 null strain compared with wild type (Figure 3A), nor was there a difference in their ability to withstand heat shock over time (Figure 3B). This demonstrates that the cdr2 null is able to enter G0 from G2, which is similar to the phenotype exhibited by cdr1/nim1 and also a subset of sterile mutants such as nuc2 (Kumada et al., 1995; Belenguer et al., 1997; Wu and Russell, 1997).
Figure 3.
Cells lacking cdr2+ are able to enter quiescence upon nitrogen deprivation. Wild-type and cdr2 null strains (KGY69 and KGY1475) were grown at 30°C to midlog, filtered, washed, and released into minimal medium lacking nitrogen. Cells were incubated for a total of 20 d with samples collected periodically to determine total viability (A) and heat shock resistance (B). (C) Conjugation assay. Homothallic wild- type and cdr2 null strains were plated onto glutamate agar and incubated at 25°C for 48 h.
cdr2+ Is Not Required for Sexual Differentiation
In wild-type S. pombe, nutrient deprivation is a necessary prerequisite for sexual differentiation. Lack of nitrogen lowers intracellular cAMP levels by 50% (Maeda et al., 1990; Kawamukai et al., 1991; Mochizuki and Yamamoto, 1992). This, in turn, triggers the induction of ste11+, whose protein product induces transcription of genes required for sexual differentiation (Sugimoto et al., 1991). To determine whether cdr2+ played a role in this response, we examined the induction of ste11+ mRNA. We found that the transcription of ste11+ mRNA was up-regulated in both wild-type and cdr2 null strains upon nitrogen deprivation (our unpublished results).
In order for S. pombe cells to mate, they must first arrest in G1 in response to nitrogen deprivation. This is a critical step because each cell must have a 1C DNA content before undergoing conjugation and nuclear fusion. Although the induction of ste11+ appeared normal, we still might expect cdr2 null cells to have a mating defect because of their inability to arrest in G1. To examine this question, we generated a homothallic, or self-mating, cdr2 null strain and compared its mating efficiency to a wild-type homothallic strain. As illustrated in Figure 3C, there was no significant difference between the mating efficiencies of wild type and cdr2 null strains. The ability of the cdr2 null cells to mate at levels similar to wild type suggests that the cdr2 null cells must be able to arrest in G1 under conditions suitable for mating, i.e., low nitrogen levels and the presence of pheromones expressed from cells of the opposite mating type (reviewed by Neilsen and Davey, 1995).
The G2 Delay Exhibited by the cdr2 Null Is Exacerbated during Nitrogen Deprivation
Since the cdr2 null was not defective in undergoing sexual differentiation in limiting nitrogen, or arresting in G0 in response to nitrogen deprivation, it seemed unlikely that cdr2+ functioned as a nutritional sensor or monitor. Another possibility for the inability of the cdr2 null cells to arrest in G1 is a defect in G2/M progression. The longer cell length of the cdr2 null suggests that it takes a longer period of time for cells lacking cdr2+ to initiate mitosis. Such a delay might inhibit the ability of the cdr2 null cells to alter mitotic control in response to nitrogen deprivation.
To carefully measure the ability of cells to undergo mitosis in response to nitrogen deprivation, we utilized cultures synchronized in the cell cycle by centrifugal elutriation. Either wild-type or cdr2 null strains were grown to midlog phase in minimal medium at 30°C. Newborn cells, which we found using the new DNA dye, Sytox green, were actually in S phase (see Figure 4F), were selected by centrifugal elutriation and collected on filters, and then released immediately into either minimal medium or minimal medium lacking a nitrogen source. Samples were collected periodically during a 48-h time course to follow cell length, DNA content, cell number increase, and time of septation, and for protein analysis.
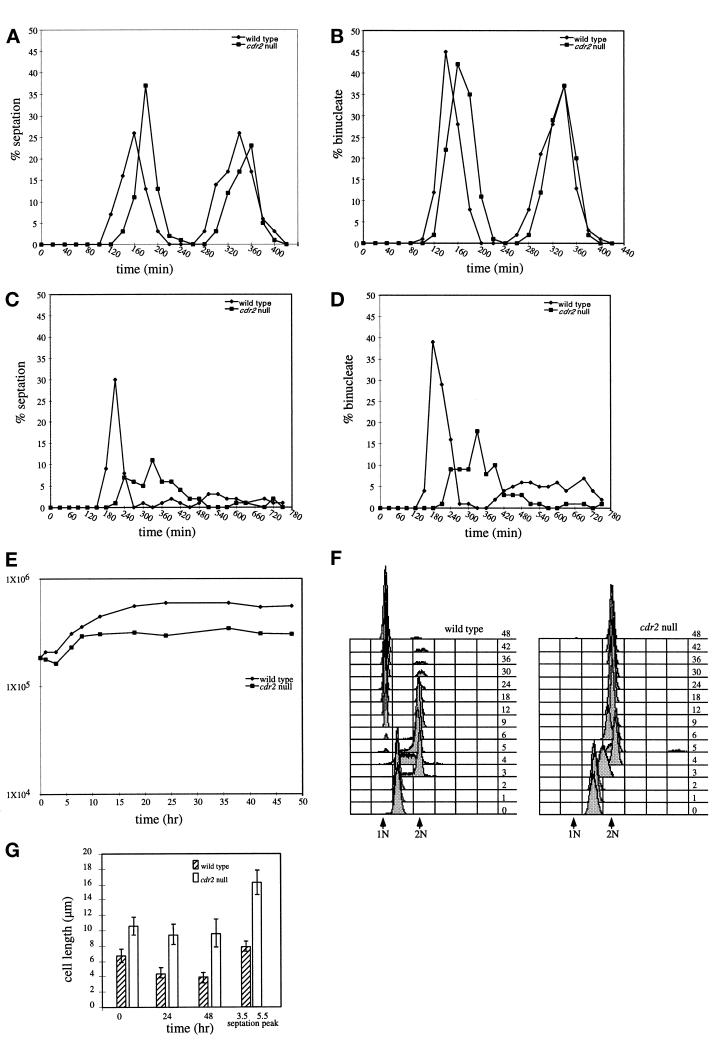
Figure 4.
Mitotic response to nitrogen deprivation. Wild-type and cdr2 null strains (KGY69 and KGY1475) were grown to midlog phase in minimal medium, and then separated on the basis of size by centrifugal elutriation. The smallest newborn cells were collected and split between two cultures, and each half was filtered, washed, and released into minimal medium (A and B) or minimal medium lacking nitrogen (C, D, E, F, and G). Samples were collected initially every 20 min for 6 h (A and B) or every 30 min for 12 h (C and D), and the percentage of binucleate (B and D) and cells containing a septum (A and C) were determined microscopically. From 12–48 h, samples were collected less frequently, and total cell number (E) and DNA content (F) were determined by Coulter counting and flow cytometry, respectively. Samples of cells grown in medium lacking nitrogen were also collected to measure average cell length at 0, 24, and 48 h and cell length at the first peak of septation (3.3 h for wild type and 5.5 h for cdr2 null) (G).
Wild-type cells inoculated into minimal medium began to septate at 100 min, with a septation peak at 160 min for the first cell cycle and at 340 min for the second cell cycle (Figure 4A). The peaks of binucleate cells were at 140 and 340 min (Figure 4B). Similarly, the cdr2 null cells inoculated into minimal medium exhibited a peak of septation at 180 min in the first cell cycle and at 360 min in the second (Figure 4A). The binucleate peaks were at 160 and 340 min (Figure 4B).
Cells deprived of nitrogen behaved differently. Nitrogen-deprived wild-type cells exhibited a significant delay in septation: they did not begin to septate until 180 min, exhibiting a peak of septation at 210 min, after which the synchrony of the culture was lost (Figure 4C). The peak of binucleates was similarly delayed, exhibiting a first peak at 180 min (Figure 4D). The cdr2 null cells inoculated into minimal medium without nitrogen did not begin to septate until 210 min, and then only a few cells were able to septate at any given time, suggesting that the synchrony of the culture was lost by the time the first cells initiated septation (Figure 4C). The binucleate peak also appeared later (Figure 4D). That the cell cycle delays occurred in G2 rather than in mitosis was confirmed by determining that the appearance of mitotic spindles was also delayed (our unpublished results).
By determining cell number increases and DNA content of the cells throughout this experiment, we found that wild-type cells deprived of nitrogen underwent two rounds of cell division; cell number approximately quadrupled (Figure 4E). Interestingly, using the improved DNA stain, Sytox green, rather than propidium iodide, we were able to discern in this and other experiments (our unpublished results) that the smallest S. pombe cells isolated by centrifugal elutriation were in S phase rather than G2. After completion of DNA replication, they divided synchronously and then underwent a final cell cycle and arrested in G1 (Figure 4F). In contrast to wild-type cells, the cdr2 null cells underwent only one round of cell division, doubling their cell number over the course of the experiment (Figure 4E). Because they did not go through another round of mitosis, they were arrested in G2 (Figure 4F).
As mentioned above, wild-type cells deprived of nitrogen alter cell size control and divide at a reduced cell size (Fantes and Nurse, 1977). To determine whether cdr2 null cells were similarly able to alter cell size control upon nitrogen deprivation, mean cell lengths were determined throughout this synchronous cell experiment (Figure 4G). The newborn wild-type cells isolated by centrifugal elutriation were, on average, 6.7 μm in length. At the first round of division, the average length of cells containing a septum was 7.9 μm. This represents a reduction from ∼13.0 μm at septation of wild-type cells grown in minimal medium. After 24 h and 48 h of nitrogen starvation, the mean cell lengths of wild-type cells had diminished to 4.4 μm and 3.9 μm, respectively. Newborn cdr2 null cells isolated by centrifugal elutriation were 11.0 μm in length. At the first round of division, the average length of cells containing a septum was 16.0 μm. This is somewhat reduced from the average septation length of 19.5 μm in nitrogen-containing minimal medium. After 24 and 48 h of nitrogen starvation, the mean cell lengths of the cdr2 null cells were 9.4 and 9.6, respectively. Thus, cdr2 null cells do adjust cell size at division in response to nitrogen deprivation but not to the same proportion as wild-type cells.
Genetic Interactions with Mitotic Control Genes
To better understand the role of cdr2+ in mitotic control, we examined genetic interactions between the cdr2 null and a variety of mitotic control mutants. cdr2 mutants display strong negative interactions with mutations in cdc25 and lower the restrictive temperature of alleles of cdc2 and cdc13 (Young and Fantes, 1987). As mentioned previously, the cdr2 null appeared phenotypically similar to the initially characterized cdr2 mutant alleles. We found that the cdr2 null displayed genetic interactions with alleles of cdc25, cdc2, and cdc13 similar to those found with the cdr2 mutant alleles (Table 2). Interestingly, mutations in wee1 or dominant alleles of cdc2 are epistatic to mutations in cdr2, including the cdr2 null; cells lacking both wee1 and cdr2 were small in length (Young and Fantes, 1987; Figure 6B). This result would be consistent with the possibility that Cdr2p acts as a negative regulator of Wee1p.
Table 2.
Genetic interactions between cdr2 null and mitotic control genes
| Strain | 25°C | 29°C | 32°C | 36°C |
|---|---|---|---|---|
| Wild type | + | + | + | + |
| cdr2 null | + | + | + | + |
| cdc13-117 | + | + | + | − |
| cdc2-22 | + | + | + | − |
| cdr2 null cdc13-117 | + | + | − | − |
| cdr2 null cdc2-22 | + | + | − | − |
Strains were streaked out onto YE media and incubated for 2 d at 25°C, replica plated to YE, and shifted to the indicated temperature for 24 h. + Indicates that the colonies were viable at the indicated temperature; − indicates that the colonies were inviable at the indicated temperature.
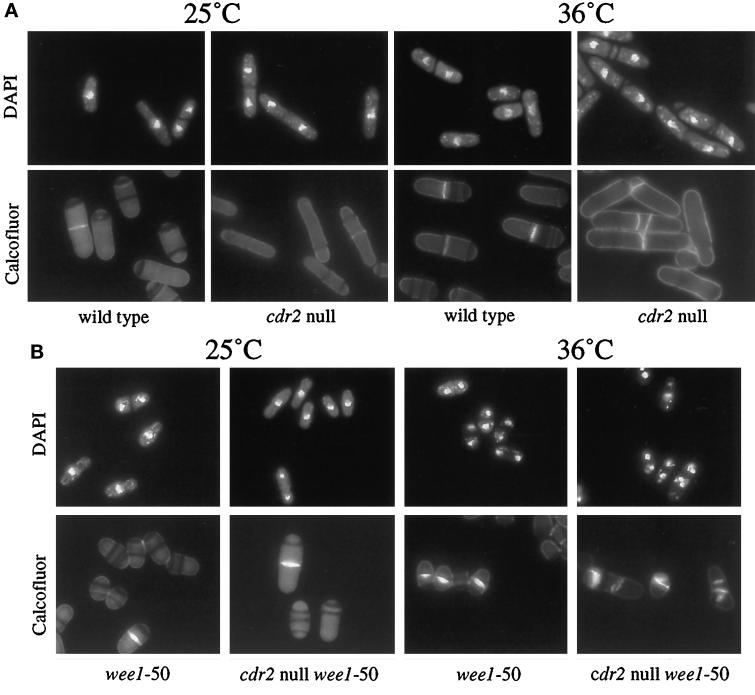
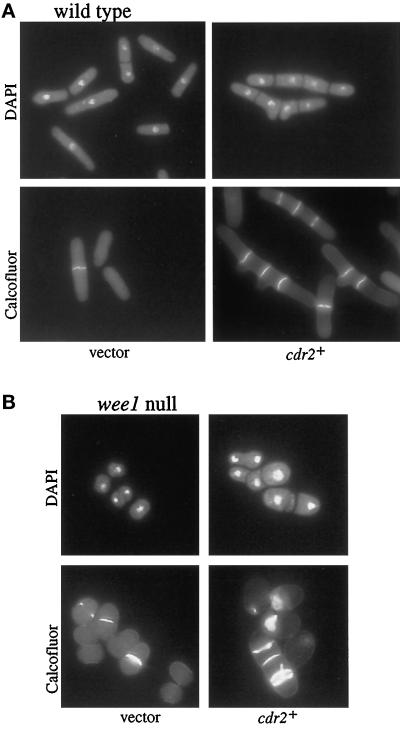
Figure 6.
cdr2 null and cdr2 null wee1-50 strains at 36°C. (A) cdr2 null at 36°C. Wild-type and cdr2 null strains (KGY69 and KGY1475) were grown to midlog at 25°C (left panels) and to midlog at 36°C (right panels). Cells were fixed in methanol and then stained with DAPI to visualize the DNA (top panels) or with Calcofluor to visualize cell walls and septa (bottom panels). (B) wee1-50 and cdr2 null wee1-50 strains at 36°C. wee1-50 and cdr2 null wee1-50 strains (KGY460 and KGY1636) were grown to midlog at 25°C (left panels) and then shifted to 36°C for 6 h (right panels). Cells were fixed in methanol and then stained with DAPI to visualize the DNA (top panels) or with Calcofluor to visualize cell walls and septa (bottom panels).
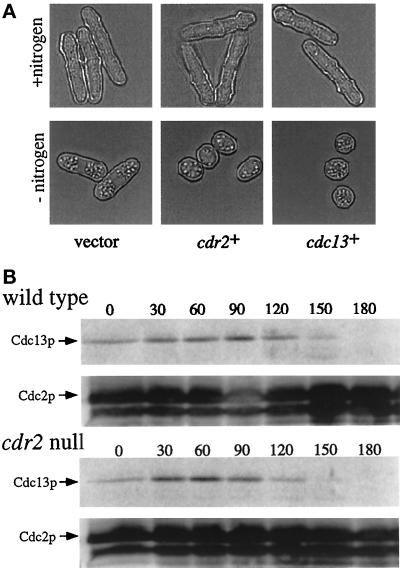
To determine whether overexpression of several known mitotic control genes could compensate for the lack of cdr2+, the cDNAs encoding cdr2+, cdc2+, cdc13+, and cdc25+, under control of the S. pombe nmt1 (no message in thiamine) thiamine-repressible promoter (Maundrell, 1993), were overexpressed in the cdr2 null strain. Only overexpression of cdr2+ or cdc13+ could restore the wild-type response to nitrogen deprivation; these cells arrested in G0 with a small cell size that was indistinguishable from wild type (Figure 5A).
Figure 5.
Cdc13p levels in the cdr2 null. (A) Overexpression of cdc13+ in the cdr2 null. A cdr2 null leu1-32 strain was transformed with either empty pREP1 vector (left panel), pREP1cdr2+ (middle panel), or pREP1cdc13+ (right panel). Transformants were selected and grown to midlog in selective medium. Transformants were then collected, filtered, washed, and released into selective medium lacking nitrogen for 48 h. Micrographs were taken just before nitrogen deprivation (top panels; +nitrogen) and after 48 h (bottom panels; −nitrogen). (B) Detection of endogenous Cdc13p during nitrogen deprivation. Cell samples acquired from the experiment described in Figure 4 were analyzed for Cdc13p levels at 30-min intervals for 3 h. Cdc2p served as a loading control.
The ability of additional Cdc13p to restore the wild-type response to nitrogen deprivation suggested that Cdc13p levels may be altered in the cdr2 null. The level of Cdc13p is highly regulated. During mitosis, Cdc13p levels decline (Booher et al., 1989; Hayles and Nurse, 1995; Creanor and Mitchison, 1996). Also, Cdc13p is degraded in response to nitrogen deprivation (Broek et al., 1991). To determine whether Cdc13p levels are perturbed in the cdr2 null strain, Cdc13p levels were assayed from samples collected from the same experiment described in Figure 4. We found that Cdc13p was degraded with similar kinetics in both the wild-type and the cdr2 null strains (Figure 5B). Quantification of the Cdc13p signal standardized with the Cdc2p loading control indicated that in both wild-type and cdr2 strains, the percent of Cdc13p signal dropped to 8 and 10% of starting levels, respectively, by 180 min. Since cdr2 null cells must grow substantially before initiating septation even during nitrogen deprivation, as shown in Figure 4, this timely degradation of Cdc13p partially explains why cdr2 null cells struggle to initiate mitosis after nitrogen deprivation and also might explain why increased levels of Cdc13p are sufficient to rescue this defect of the cdr2 null.
cdr2 Mutants Have an Additional Defect in Cell Division
Although no temperature-sensitive phenotype was initially ascribed to the cdr2 mutants (Young and Fantes, 1987), we observed that the cdr2 null strain exhibited temperature-associated growth abnormalities. When incubated at 36°C, cdr2 null cells were more elongated and showed defects in cytokinesis; cells contained multiple septa and misplaced septa and septum material (Figure 6A). However, these defects did not result in lethality and were only observed when cells were incubated in YE. Moreover, the defects at 36°C were suppressed by the addition of sorbitol to the medium (our unpublished results).
Since cdr2+ activity is not required in the absence of wee1+ with respect to cell length, we wanted to know whether loss of wee1+ activity also suppressed the cytokinesis defect of the cdr2 null. To determine this, the cdr2 null wee1–50 double mutant was incubated at 36°C in YE. As can be seen in Figure 6B, loss of wee1+ did not suppress the cytokinesis defect associated with loss of cdr2+. The double mutant not only had defects in cytokinesis but also accumulated multiple nuclei. The same was found to be true in a cdr2 null wee1 null double mutant (our unpublished results). The inability of the loss of wee1 function to suppress the cytokinesis defect of the cdr2 null suggested the possibility that cdr2+ has two separate roles: one in the decision to enter mitosis, dependent on wee1+, the other in cytokinesis, independent of wee1+.
Overexpression of cdr2+ Has a Dominant Negative Effect
To further characterize the function of cdr2+, we overexpressed the cdr2+ gene in a wild-type strain. If cdr2+ functions as a negative regulator of wee1+, similar to cdr1+/nim1+, then we might expect overexpression of cdr2+ to cause cells to become wee in size, as in the case of cdr1+ overexpression (Russell and Nurse, 1987b). Contrary to this expectation, overexpression of cdr2+ in the wild-type strain was lethal and generated elongated, highly branched cells that contained two or more septa (Figure 7A). Thus, the overexpression of cdr2+ has an apparent dominant negative effect.
Figure 7.
Overexpression of cdr2+ in wild-type and wee1 null cells. A leu1-32 strain (KGY246; panel A) and a wee1 null leu 1-32 strain (KGY4; panel B) were transformed with either empty pREP1 vector (left panels) or pREP1cdr2+ (right panels). Transformants were selected and grown to midlog in selective medium containing thiamine. Cells were collected, washed, and released into selective medium lacking thiamine for 18 h. Samples were fixed with methanol and stained with DAPI (top panels) to visualize the DNA or Calcofluor (bottom panels) to visualize cell walls and septa.
To determine whether the cdr2+ overexpression phenotype was dependent on wee1+, we overexpressed cdr2+ in the wee1 null strain. Overexpression of cdr2+ in the wee1 null was lethal, and these cells displayed septation defects that were similar to the defects that resulted from overexpression of cdr2+ in wild-type cells (Figure 7B). However, the wee1 null cells overexpressing cdr2+ did not become elongated, supporting the idea that cdr2+ function is dependent on wee1+ with respect to cell length, but is independent of wee1+ with respect to its role in cytokinesis.
Cdr2p Abundance Is Regulated during Nitrogen Deprivation
The results from the experiments above suggested that Cdr2p is required for cells to initiate mitosis at the appropriate time. Thus, we might expect Cdr2p abundance to be regulated during the cell cycle and/or during nitrogen deprivation. We first determined that the level of cdr2+ mRNA did not vary during the cell cycle or during nitrogen deprivation (our unpublished results).
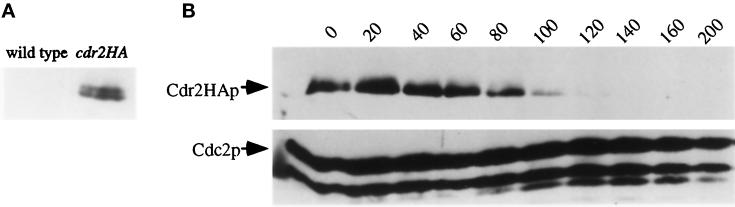
To measure Cdr2p protein abundance, we generated a C-terminal HA epitope-tagged cdr2+. The resulting Cdr2p-HA protein was determined to be functional as the tagged strain was indistinguishable from wild type in terms of cell length and response to nitrogen deprivation (our unpublished results). The Cdr2p-HA protein was detected as a doublet migrating at a molecular mass of ∼90 kDa using monoclonal antibodies specific to the HA epitope (12CA5). This doublet was detected only in lysates from the tagged strain (Figure 8A).
Figure 8.
Regulation of Cdr2p levels. (A) Detection of Cdr2p-HA. Protein generated from a HA epitope-tagged cdr2+ strain (cdr2HA+; KGY1628) and a wild-type strain (KGY68) was probed with the HA antibody (12CA5) to detect Cdr2p-HA. (B) Detection of Cdr2p-HA levels during nitrogen deprivation. The cdr2HA+ strain (KGY1628) was grown to midlog in minimal medium, and then separated on the basis of size by centrifugal elutriation. Early G2 cells were collected, filtered, washed, and released into minimal medium lacking nitrogen. Time points were taken every 20 min for 3 h to analyze Cdr2p-HA levels. Cdc2p served as a loading control.
A synchronous cell population was used to determine the abundance of Cdr2p-HA throughout the cell cycle and during nitrogen deprivation. While Cdr2p-HA levels remained constant throughout the cell cycle (our unpublished results), the level of Cdr2p-HA decreased during nitrogen deprivation but was detectable up to 100 min (Figure 8B). This is contrast to Cdr1p/Nim1p, which is degraded immediately upon release into medium lacking nitrogen (Wu and Russell, 1997). These data also support the hypothesis that Cdr2p acts as a mitotic inducer; Cdr2p is present at the time in which cells are initiating mitosis.
DISCUSSION
cdr2+ was isolated in a screen for genes required for the proper mitotic response to nitrogen deprivation in S. pombe. Because the cdr mutants were not able to respond properly to nitrogen deprivation, it was predicted that the cdr+ genes would be involved in either nutritional sensing and/or mitotic control (Young and Fantes, 1984, 1987). Early evidence supported both predictions; the two complementation groups characterized, cdr1 and cdr2, initiated mitosis with a longer cell length than wild type, indicating a delay in G2, and they were unable to respond properly to nitrogen deprivation. cdr1+, also known as nim1+, has been shown to encode a conserved serine-threonine protein kinase that promotes G2/M progression through the inhibitory phosphorylation of the wee1+ gene product, thus fulfilling one of the predictions of the cdr screen (Russell and Nurse, 1987b; Feilotter et al., 1991; Coleman et al., 1993; Parker et al., 1993; Wu and Russell, 1993). However, cdr1+/nim1+ does not appear to have a role in nutritional sensing, and the defect in responding to nitrogen deprivation by cdr1+/nim1+ mutants is a result of a delay in G2 (Belenguer et al., 1997; Wu and Russell, 1997). We have continued the characterization of cdr2+ and have found that similar to cdr1+/nim1+, cdr2+ acts as an inducer of mitosis. However, unlike cdr1+/nim1+, cdr2+ has an additional role in cytokinesis.
We have found that cdr2+ is not an essential gene. The cdr2 null is phenotypically similar to cdr2 mutants; cdr2 null cells septate at the same length as the cdr2-96 mutant. Utilizing the cdr2 null strain, we dissected the involvement of cdr2+ in the response of S. pombe cells to nitrogen deprivation. We found that, like cdr1/nim1 mutants, the cdr2 null mutant arrested in G2 instead of G1 when starved for nitrogen and entered a state of quiescence or G0. Since S. pombe cells can enter G0 in response to nitrogen deprivation from either G1 or G2 (Castello et al., 1986), the latter usually occurring from carbon deprivation, it is not surprising that cdr2 mutants are able to enter G0 from G2. However, we also found that the cdr2 null was able to undergo sexual differentiation and produce viable haploid progeny, which is surprising if the cdr2 null cells had truly arrested in G2 under the conditions employed in mating assays. However, mating assays differ from nitrogen deprivation experiments in that low amounts of nitrogen are present. Also, pheromones, produced under limiting nutrient conditions (reviewed by Neilsen and Davey, 1995), may help to promote a G1 arrest in the mating assays. Thus, under these conditions, it is likely that the cdr2 null cells are able to transiently arrest in G1 and undergo sexual differentiation. These data indicate that cdr2+ is not required for nutritional sensing, and therefore it is not likely that cdr2+ encodes a nutritional sensor or monitor.
To show that cdr2+ functions in G2/M progression, we analyzed the mitotic response of wild-type and cdr2 null cells to nitrogen deprivation. It has been demonstrated previously that asynchronous populations of wild-type cells respond to nitrogen deprivation by immediately altering G2/M size control and entering mitosis with a reduced cell size (Fantes and Nurse, 1977). In our nitrogen deprivation experiments, wild-type cells entered mitosis with a reduced cell size as seen previously. However, there was an unexpected delay in G2 before these cells entered mitosis. Since the cells utilized in our experiments were synchronized early in the cell cycle, they had not yet reached the critical size for mitosis. Hence, these uniformly small cells were forced to grow until the proper size was reached, even though the G2/M size had indeed been reset in response to nitrogen deprivation. When we analyzed asynchronous populations of wild-type cells, we observed the acceleration into mitosis previously described (our unpublished results) (Fantes and Nurse, 1977).
The cdr2 null strain also exhibited a delay in G2 in response to nitrogen deprivation, but the delay in the cdr2 null cells was substantially longer than in wild-type cells. Furthermore, although cell size at mitosis was altered by nitrogen deprivation in the absence of Cdr2p function, the response was not proportionately as large as in wild-type cells. The cdr2 null cells did eventually undergo mitosis and divide, as reflected by the doubling of cell number, and arrested in G2 of the following cell cycle. Under the same regimen, wild-type cells nearly quadrupled in number, undergoing an additional round of mitosis and arresting in G1. These data demonstrate that Cdr2p acts as a mitotic inducer, which is especially important in conditions of limiting nitrogen.
To begin dissecting the molecular nature of Cdr2p’s role in promoting mitosis, we examined the genetic relationship between cdr2 and known mitotic control genes. From the initial characterization of cdr2+, it is known that the restrictive temperatures of alleles of cdc2, cdc25, and cdc13 mutants are lowered in combination with cdr2 mutants, demonstrating that they interact genetically (Young and Fantes, 1987). Moreover, we found that ectopic expression of cdc13+ was sufficient to rescue the nitrogen deprivation response of the cdr2 null. This suggested that Cdc13p might be limiting in the cdr2 null since Cdc13p association with Cdc2p is required for activation of Cdc2p and entry into mitosis (Booher et al., 1989). When the level of Cdc13p was examined in the cdr2 null, we found that it was the same as in wild type (our unpublished results). Because Cdc13p protein is turned over in response to nitrogen deprivation (Broek et al., 1991), we then tested the possibility that the kinetics of Cdc13p degradation were altered in the cdr2 null. The kinetics of Cdc13p degradation in response to nitrogen deprivation were similar in the cdr2 null and wild-type cells. Since the cdr2 null cells must grow proportionately longer than wild-type cells before initiating mitosis, the decrease in Cdc13p levels might contribute to their difficulty initiating mitosis. Because of the limitations of detection on immunoblots, we would not argue that these cells are initiating mitosis in the absence of Cdc13p but rather that Cdc13p becomes limiting under these conditions. Hence, addition of excess Cdc13p is sufficient to restore the wild-type response to nitrogen deprivation in cdr2 null cells.
Genetic interactions between cdr2 and wee1 mutants also provide some insight into the molecular role of cdr2+ in G2/M progression. It was demonstrated by Young and Fantes (1987) that wee1+ was epistatic to both cdr1+/nim1+ and cdr2+, and they suggested that the cdr genes may regulate wee1+ activity. Indeed, Cdr1p/Nim1p has been shown to inhibit Wee1p directly by phosphorylation (Coleman et al., 1993; Parker et al., 1993; Wu and Russell, 1993). cdr2+ also encodes a putative protein kinase with homology to the N-terminal catalytic domain of the Snf1p subfamily of serine-threonine protein kinases. Does cdr2+ function as a negative regulator of wee1+ as originally suggested? cdr2+ overexpression data suggests that it does. Overexpression of cdr2+ in wild-type cells led to a delay in G2, followed by defects in cytokinesis, but no G2 delay was detected when cdr2+ is overexpressed in cells lacking wee1+ activity. These data, combined with the epistatic relationship between wee1 and cdr2 mutants, demonstrate that cdr2+ activity is not required in the absence of wee1+ to promote mitosis, and possibly Cdr2p acts as an inhibitor of Wee1p. If cdr2+ is acting as an inducer of mitosis acting through wee1+, does it do so in a manner identical to cdr1+/nim1+? Our data suggest not. As mentioned above, overexpression of cdr2+ led to a delay in G2 followed by defects in cytokinesis. This is in sharp contrast to cdr1+/nim1+ overexpression, which promotes entry into mitosis at a reduced cell size (Russell and Nurse, 1987).
Genetic analysis of cdr1 and cdr2 mutants further suggests that cdr2+ functions in a pathway separate from cdr1+/nim1+. Even though a cdr2 null cdr1/nim1 null double mutant is phenotypically indistinguishable from the cdr2 null, both cdr2 and cdr1/nim1 mutants were sensitive to overexpression of the other (our unpublished results), which demonstrates a lack of dependence between cdr2+ and cdr1+/nim1+. These data suggest that cdr2+ and cdr1+/nim1+ do not function in a linear pathway and act independently to promote mitosis. Since Wee1p is the key inhibitor of Cdc2p activity before mitosis (Russell and Nurse, 1987a), there are probably numerous factors and signal transduction pathways, some yet uncharacterized, that regulate wee1+ activity.
The additional role of cdr2+ in cytokinesis also supports the idea that cdr2+ functions in a pathway separate from cdr1+/nim1+. Cells lacking cdr2+ and cells overproducing cdr2+ exhibited defects in forming septa and undergoing cell separation. It appears then that cdr2+ is required for proper septum formation and cell separation. At this time, we do not understand the molecular role of cdr2+ in cytokinesis, except that it is independent of wee1+ activity. Thus, if cdr2+ functions to negatively regulate wee1+, it is doing so in a manner different from that of cdr1+/nim1+. It is possible that cdr2+ acts indirectly to inhibit wee1+. Excess Cdr2p might sequester a Wee1p inhibitory factor that is normally activated by Cdr2p; thus, a G2 delay is produced when cdr2+ is overexpressed. Another possibility is that Cdr2p influences the level of Wee1p. In S. pombe, the level of Wee1p protein level is cell cycle regulated; Wee1p levels decrease at mitosis, and this reduced level is maintained into G1 (Aligue et al., 1997). In this scenario, Cdr2p may facilitate the degradation of Wee1p to promote Cdc2p activation and entry into mitosis.
Interestingly, Cdr2p is most similar to the S. cerevisiae protein kinase Gin4p. GIN4 encodes a nonessential protein kinase that is required for the ability of NAP1 and CLB2 to promote normal mitotic progression (Altman and Kellogg, 1997). S. cerevisiae CLB2 encodes a B-type cyclin analogous to S. pombe Cdc13p (Fitch et al., 1992; Richardson et al., 1992), whereas the NAP1 gene product was identified as a Clb2p-binding protein (Kellogg and Murray, 1995; Kellogg et al., 1995). Gin4p binds Nap1p, and Gin4p phosphorylation and activation in mitosis are dependent on both Nap1p and Clb2p (Altman and Kellogg, 1997). Since we have shown that cdr2+ also promotes mitosis, it will be interesting to see whether Cdr2p interacts with a protein similar to Nap1p in S. pombe and whether such an interaction influences its function. Our genetic data, however, suggest that at least one function of cdr2+ lies upstream of cdc2+/cdc13+ activation, rather than being dependent upon it.
In conclusion, we have begun characterizing the role of cdr2+ in S. pombe and have found that, like cdr1+/nim1+, cdr2+ functions as a mitotic inducer that is especially important under nutrient limiting conditions and not as a sensor of nutrient limitation. Unlike cdr1+/nim1+, which acts directly on wee1+ to regulate mitosis, the role of cdr2+ in cell cycle regulation is more complex; cdr2+ has an additional role in cytokinesis and is required for proper septum formation and cell separation.
ACKNOWLEDGMENTS
We thank Drs. J. Bähler and J.R. Pringle for providing us with the HA-kan tagging cassette, and Dr. Paul Russell for discussion of unpublished results and S. pombe strain GL122. All current and past members of the Gould laboratory, including Drs. Dan McCollum and L.D. Berry, are appreciated for their valuable discussions and technical advice. This work was supported by NIH grant GM-47728 to K.L.G.; C.S.B. was supported by National Cancer Institute grant T32 CA-09592. P.G.Y. was supported by the Natural Sciences and Engineering Research Council of Canada. K.L.G. is an associate investigator of the Howard Hughes Medical Institute.
REFERENCES
- Aligue R, Wu L, Russell P. Regulation of Schizosaccharomyces pombe Wee1 tyrosine kinase. J Biol Chem. 1997;272:13320–13325. doi: 10.1074/jbc.272.20.13320. [DOI] [PubMed] [Google Scholar]
- Atman R, Kellogg D. Control of mitotic events by Nap1 and the Gin4 kinase. J Cell Biol. 1997;138:119–130. doi: 10.1083/jcb.138.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bähler J, Wu J, Longtine MS, Shah NS, McKenzie A, III, Steever AB, Wach A, Philippsen P, Pringle JR. Heterologous molecules for efficient and versatile PCR-based gene targeting in Schizosaccharomyces pombe. Yeast. 1998;14:943–951. doi: 10.1002/(SICI)1097-0061(199807)14:10<943::AID-YEA292>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Balasubramanian MK, McCollum D, Gould KL. Cytokinesis in the fission yeast Schizosaccharomyes pombe. Methods Enzymol. 1997;283:494–506. doi: 10.1016/s0076-6879(97)83039-x. [DOI] [PubMed] [Google Scholar]
- Belenguer P, Pelloquin L, Oustrin ML, Ducommun B. Role of the fission yeast nim1 protein kinase in the cell cycle response to nutritional signals. Biochem Biophys Res Commun. 1997;232:204–208. doi: 10.1006/bbrc.1997.6253. [DOI] [PubMed] [Google Scholar]
- Berry LD, Gould KG. Regulation of Cdc2 activity by phosphorylation at T14/Y15. In: Meijer L, Guidet S, Vogel L, editors. Progress in Cell Cycle Research. Vol. 2. New York, NY: Plenum Press; 1996. pp. 99–105. [DOI] [PubMed] [Google Scholar]
- Booher R, Beach D. Involvement of cdc13+ in mitotic control in Schizosaccharomyces pombe: possible interaction of the gene product with microtubules. EMBO J. 1988;7:2321–2327. doi: 10.1002/j.1460-2075.1988.tb03075.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booher RN, Alfa CE, Hyams JS, Beach D. The fission yeast cdc2/cdc13/suc2 protein kinase regulation of catalytic activity and nuclear localization. Cell. 1989;58:485–497. doi: 10.1016/0092-8674(89)90429-7. [DOI] [PubMed] [Google Scholar]
- Broek D, Bartlett R, Crawford K, Nurse P. Involvement of p34cdc2 in establishing the dependency of S phase on mitosis. Nature. 1991;349:388–393. doi: 10.1038/349388a0. [DOI] [PubMed] [Google Scholar]
- Castello G, Rogers L, Beach D. Fission yeast enters the stationary phase G0 from either mitotic G1 or G2. Curr Genet. 1986;11:119–125. [Google Scholar]
- Coleman TR, Zang Z, Dunphy WG. Negative regulation of the Wee1 protein kinase by direct action of the Cdr1p/Nim1p mitotic inducer. Cell. 1993;72:919–929. doi: 10.1016/0092-8674(93)90580-j. [DOI] [PubMed] [Google Scholar]
- Creaner J, Mitchison JM. The kinetics of the B cyclin p56cdc13 and the phosphatase p80cdc25 during the cell cycle of the fission yeast Schizosaccharomyces pombe. J Cell Sci. 1996;109:1647–1653. doi: 10.1242/jcs.109.6.1647. [DOI] [PubMed] [Google Scholar]
- Egel R. General cytology of fission yeasts. In: Nasim A, Young P, Johnson BF, editors. Molecular Biology of the Fission Yeast. San Diego, CA: Academic Press; 1989. pp. 31–74. [Google Scholar]
- Fantes PA. Control of cell size and cycle time in Schizosaccharomyces pombe. J Cell Sci. 1977;24:51–67. doi: 10.1242/jcs.24.1.51. [DOI] [PubMed] [Google Scholar]
- Fantes PA. Isolation of cell size mutants by a new selective method: characterization of mutants and implications for division control markers. J Bacteriol. 1981;137:746–754. doi: 10.1128/jb.146.2.746-754.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantes PA, Nurse P. Control of cell size division in fission yeast by a growth-modulated size control over nuclear division. Exp Cell Res. 1977;107:377–386. doi: 10.1016/0014-4827(77)90359-7. [DOI] [PubMed] [Google Scholar]
- Fantes PA, Nurse P. Control of the timing of cell division in fission yeast by a growth-modulated size control over nuclear division. Exp Cell Res. 1978;115:317–329. doi: 10.1016/0014-4827(78)90286-0. [DOI] [PubMed] [Google Scholar]
- Feilotter H, Nurse P, Young P. Genetic and molecular analysis of the cdr1/nim1 in Schizosaccharomyces pombe. Genetics. 1991;127:309–318. doi: 10.1093/genetics/127.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitch I, Dahmann C, Surana U, Amon A, Nasmyth K, Goetsch B, Byers B, Futcher B. Characterization of four B-type cyclin genes of the budding yeast Saccharomyces cerevisiae. Mol Biol Cell. 1992;3:805–818. doi: 10.1091/mbc.3.7.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould KL, Moreno S, Owen DJ, Sazer S, Nurse P. Phosphorylation of Thr167 is required for Schizosaccharomyces pombe p34cdc2 function. EMBO J. 1991;10:3297–3309. doi: 10.1002/j.1460-2075.1991.tb04894.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould KL, Nurse P. Tyrosine phosphorylation of the fission yeast cdc2+ protein kinase regulates entry into mitosis. Nature. 1989;342:39–45. doi: 10.1038/342039a0. [DOI] [PubMed] [Google Scholar]
- Hagan I, Hayles J, Nurse P. Cloning and sequencing of the cyclin-related cdc13+ gene and a cytological study of its role in fission yeast mitosis. J Cell Sci. 1988;91:587–595. doi: 10.1242/jcs.91.4.587. [DOI] [PubMed] [Google Scholar]
- Hanks SK, Quinn AM, Hunter T. The protein kinase family: conserved features and deduced phylogeny of the catalytic domains. Science. 1988;241:42–52. doi: 10.1126/science.3291115. [DOI] [PubMed] [Google Scholar]
- Hayles J, Nurse P. A pre-start checkpoint preventing mitosis in fission yeast acts independently of p34cdc2 tyrosine phosphorylation. EMBO J. 1995;14:2760–2771. doi: 10.1002/j.1460-2075.1995.tb07276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman CS. Preparation of yeast DNA, In: Ausubel FA, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K, editors. Current Protocols in Molecular Biology. New York, NY: John Wiley and Sons; 1993. pp. 13.11.1–13.11.4. [Google Scholar]
- Hudson JD, Feilotter H, Young PG. stf1: non-wee mutations epistatic to cdc25 in the fission yeast Schizosaccharomyces pombe. Genetics. 1990;126:309–315. doi: 10.1093/genetics/126.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamukai M, Ferguson K, Wigler M, Young D. Genetic and biochemical analysis of the adenylyl cyclase of Schizosaccharomyces pombe. Cell Regul. 1991;2:155–164. doi: 10.1091/mbc.2.2.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellogg DR, Kikuchi T, Fujii-Nakata, Turck CW, Murray AW. Members of the NAP/SET family of proteins interact specifically with B-type cyclins. J Cell Biol. 1995;130:661–673. doi: 10.1083/jcb.130.3.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellogg DR, Murray AW. NAP1 acts with Clb2 to perform mitotic functions and suppress polar bud growth in budding yeast. J Cell Biol. 1995;130:675–685. doi: 10.1083/jcb.130.3.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumada K, Su S, Yanagida M, Toda T. Fission yeast TPR-family protein nuc2 is required for G1-arrest upon nitrogen starvation and is an inhibitor of septum formation. J Cell Sci. 1995;108:895–905. doi: 10.1242/jcs.108.3.895. [DOI] [PubMed] [Google Scholar]
- Ma XJ, Lu Q, Grunstein M. A search for proteins that interact genetically with histone H3 and H4 amino termini uncovers novel regulators of the Swe1 kinase in Saccharomyces cerevisiae. Genes Dev. 1996;10:1327–1340. doi: 10.1101/gad.10.11.1327. [DOI] [PubMed] [Google Scholar]
- Maeda T, Mochizuki N, Yamamoto M. Adenylyl cyclase is dispensable for vegetative cell growth in the fission yeast Schizosaccharomyces pombe. Proc Natl Acad Sci USA. 1990;87:7814–7818. doi: 10.1073/pnas.87.20.7814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maundrell K. Thiamine-repressible promoters pREP and pRIP for fission yeast. Gene. 1993;123:127–130. doi: 10.1016/0378-1119(93)90551-d. [DOI] [PubMed] [Google Scholar]
- Mitchison JM. The growth of single cells. I. Schizosaccharomyces pombe. Exp Cell Res. 1957;13:244–262. doi: 10.1016/0014-4827(57)90005-8. [DOI] [PubMed] [Google Scholar]
- Mochizuki N, Yamamoto M. Reduction in the intracellular cAMP level triggers initiation of sexual development in fission yeast. Mol Gen Genet. 1992;233:17–24. doi: 10.1007/BF00587556. [DOI] [PubMed] [Google Scholar]
- Moreno S, Klar A, Nurse P. Molecular genetic analysis of fission yeast Schizosaccharomyces pombe. Methods Enzymol. 1991;194:795–823. doi: 10.1016/0076-6879(91)94059-l. [DOI] [PubMed] [Google Scholar]
- Morgan DO. Principles of CDK regulation. Nature. 1995;374:131–134. doi: 10.1038/374131a0. [DOI] [PubMed] [Google Scholar]
- Neilsen O, Davey J. Pheromone communication in the fission yeast Schizosaccharomyces pombe. Semin Cell Biol. 1995;6:95–105. doi: 10.1016/1043-4682(95)90006-3. [DOI] [PubMed] [Google Scholar]
- Nurse P. Genetic control of cell size at cell division in fission yeast. Nature. 1975;256:547–551. doi: 10.1038/256547a0. [DOI] [PubMed] [Google Scholar]
- Nurse P, Bissett Y. Gene required in G1 for commitment to the cell cycle and G2 for control of mitosis in fission yeast. Nature. 1981;292:558–560. doi: 10.1038/292558a0. [DOI] [PubMed] [Google Scholar]
- Nurse P, Thuriaux P. Regulatory genes controlling mitosis in the fission yeast Schizosaccharomyces pombe. Genetics. 1980;96:627–637. doi: 10.1093/genetics/96.3.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olmsted JB. Affinity purification of antibodies from diazotized paper blots of heterogeneous protein samples. J Biol Chem. 1981;256:11955–11957. [PubMed] [Google Scholar]
- Parker LL, Walter SA, Young PG, Piwnica-Worms H. Phosphorylation and inactivation of the mitotic inhibitor Wee1 by the nim1/cdr1 kinase. Nature. 1993;363:736–738. doi: 10.1038/363736a0. [DOI] [PubMed] [Google Scholar]
- Piggot JR, Rai R, Carter BL. A bifunctional gene product involved in two phases of the yeast cell cycle. Nature. 1982;298:391–393. doi: 10.1038/298391a0. [DOI] [PubMed] [Google Scholar]
- Prentice HL. High efficiency transformation of the Schizosaccharomyces pombe by electroporation. Nucleic Acids Res. 1991;20:261. doi: 10.1093/nar/20.3.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson H, Lew DH, Henze M, Sugimoto K, Reed S. Cyclin B homologues in Saccharomyces cerevisiae function in S phase and in G2. Genes Dev. 1992;6:2021–2034. doi: 10.1101/gad.6.11.2021. [DOI] [PubMed] [Google Scholar]
- Rupes I, Jochova J, Young PG. Markers of cell polarity during and after nitrogen starvation in Schizosaccharomyces pombe. Biochem Cell Biol. 1997;75:697–708. doi: 10.1139/o97-084. [DOI] [PubMed] [Google Scholar]
- Russell P, Nurse P. Negative regulation of mitosis by wee1+, a gene encoding a protein kinase homologue. Cell. 1987a;49:559–567. doi: 10.1016/0092-8674(87)90458-2. [DOI] [PubMed] [Google Scholar]
- Russell P, Nurse P. The mitotic inducer nim1+ functions in a regulatory network of protein kinase homologues controlling the initiation of mitosis. Cell. 1987b;49:569–576. doi: 10.1016/0092-8674(87)90459-4. [DOI] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: A Laboratory Manual. 2nd ed. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- Sazer S, Sherwood SW. Mitochondrial growth and DNA synthesis occur in the absence of nuclear DNA replication in fission yeast. J Cell Sci. 1990;97:509–516. doi: 10.1242/jcs.97.3.509. [DOI] [PubMed] [Google Scholar]
- Su SSY, Tanaka Y, Samejima E, Tanaka K, Yanagida M. A nitrogen starvation-induced dormant G0 state in fission yeast: the establishment from uncommitted G1 state and its delay for return to proliferation. J Cell Sci. 1996;109:1347–1357. doi: 10.1242/jcs.109.6.1347. [DOI] [PubMed] [Google Scholar]
- Sugimoto A, Iino Y, Watanabe Y, Yamamoto M. Schizosaccharomyces pombe ste11+ encodes a transcription factor with an HMG motif that is a critical regulator of sexual development. Genes & Dev. 1990;5:1990–1999. doi: 10.1101/gad.5.11.1990. [DOI] [PubMed] [Google Scholar]
- Thuriaux P, Nurse P, Carter B. Mutants altered in the control co-ordinating cell division and cell growth in the fission yeast Schizosaccharomyces pombe. Mol Gen Genet. 1978;161:215–220. doi: 10.1007/BF00274190. [DOI] [PubMed] [Google Scholar]
- Tournier S, Gachet Y, Hyams JS. Identification and preliminary characterization of p31, a new PSTAIRE-related protein in fission yeast. Yeast. 1997;13:727–734. doi: 10.1002/(SICI)1097-0061(19970630)13:8<727::AID-YEA134>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Wright A, Maundrell K, Heyer WD, Beach D, Nurse P. Vectors for the construction of gene banks and the integration of cloned genes in Schizosaccharomyces pombe and Saccharomyces cerevisiae. Plasmid. 1986;15:156–158. doi: 10.1016/0147-619x(86)90051-x. [DOI] [PubMed] [Google Scholar]
- Wu L, Kazuhiro S, Alugue R, Russell P. Spatial organization of the Nim1-Wee1-Cdc2 mitotic control network in Schizosaccharomyces pombe. Mol Biol Cell. 1996;7:1749–1758. doi: 10.1091/mbc.7.11.1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L, Russell P. Nim1 kinase promotes mitosis by inactivating Wee1 tyrosine kinase. Nature. 1993;363:738–741. doi: 10.1038/363738a0. [DOI] [PubMed] [Google Scholar]
- Wu L, Russell P. Roles of wee1 and nim1 protein kinases in regulating the switch from mitotic division to sexual development in Schizosaccharomyces pombe. Mol Cell Biol. 1997;17:10–17. doi: 10.1128/mcb.17.1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita M, Yoshikuni M, Hirai T, Fukada S, Nagahama Y. A monoclonal antibody against the PSTAIRE sequence of p34cdc2, catalytic subunit of maturation-promoting factor and key regulator of the cell cycle. Dev Growth & Differ. 1991;33:617–624. doi: 10.1111/j.1440-169X.1991.00617.x. [DOI] [PubMed] [Google Scholar]
- Young PG, Fantes PA. Changed division response mutants function as allosuppressors. In: Skehan P, Friedman SJ, editors. Growth, Cancer, and the Cell Cycle. Clifton, NJ: Humana Press; 1984. pp. 221–228. [Google Scholar]
- Young PG, Fantes PA. Schizosaccharomyces pombe mutants affected in their division response to starvation. J Cell Sci. 1987;88:295–304. doi: 10.1242/jcs.88.3.295. [DOI] [PubMed] [Google Scholar]