Abstract
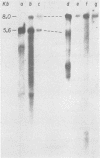
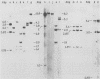
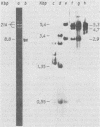
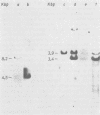
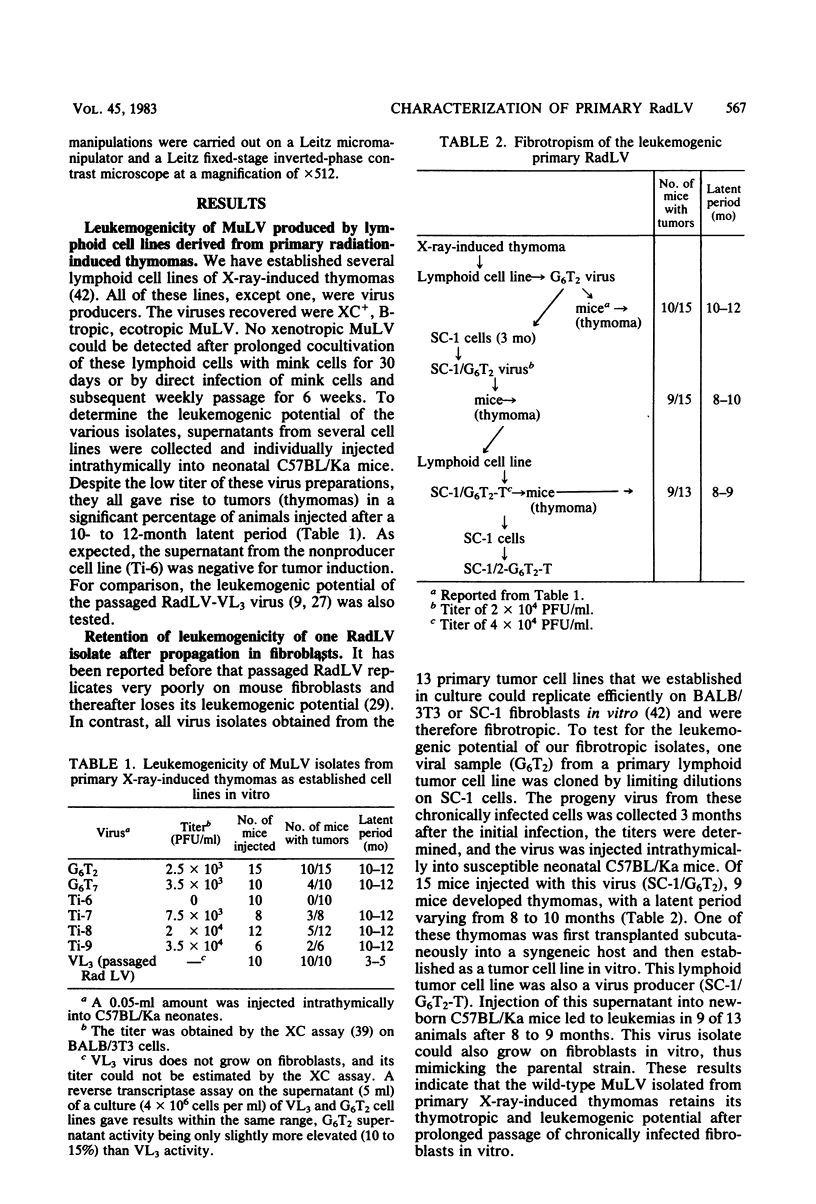
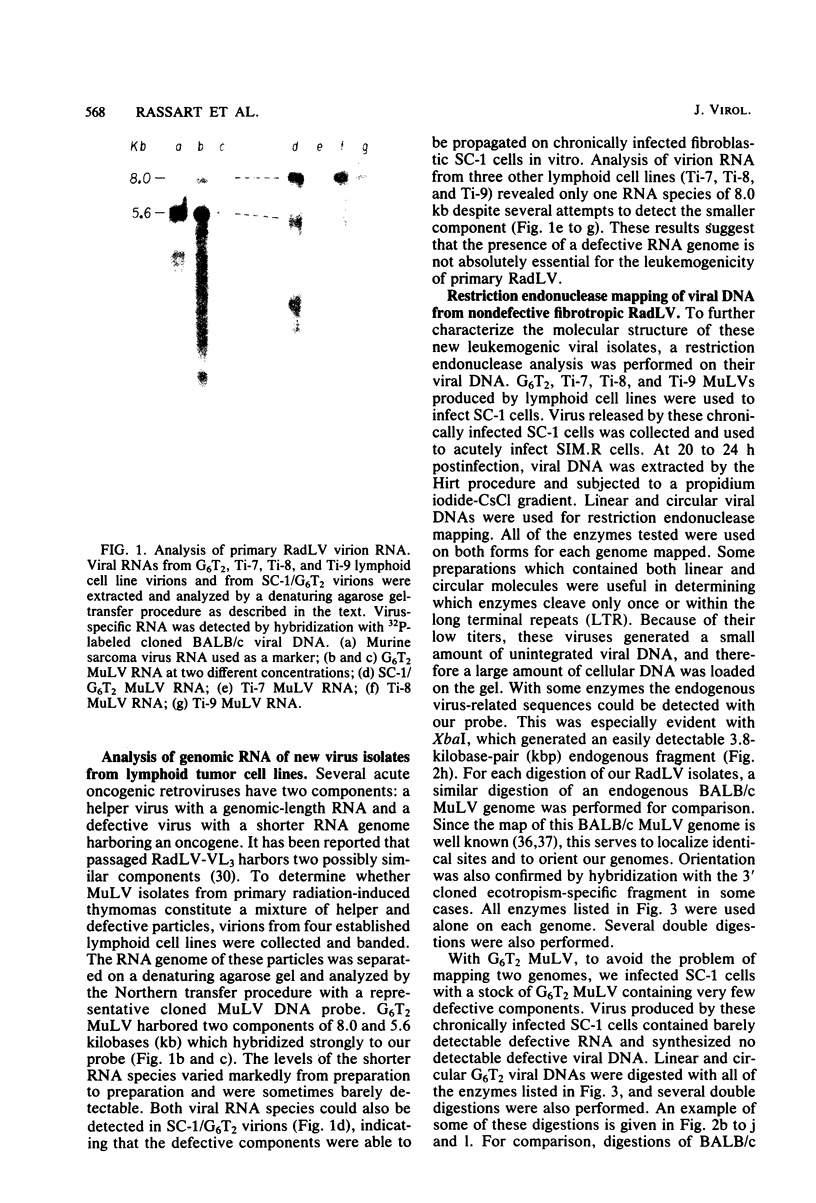
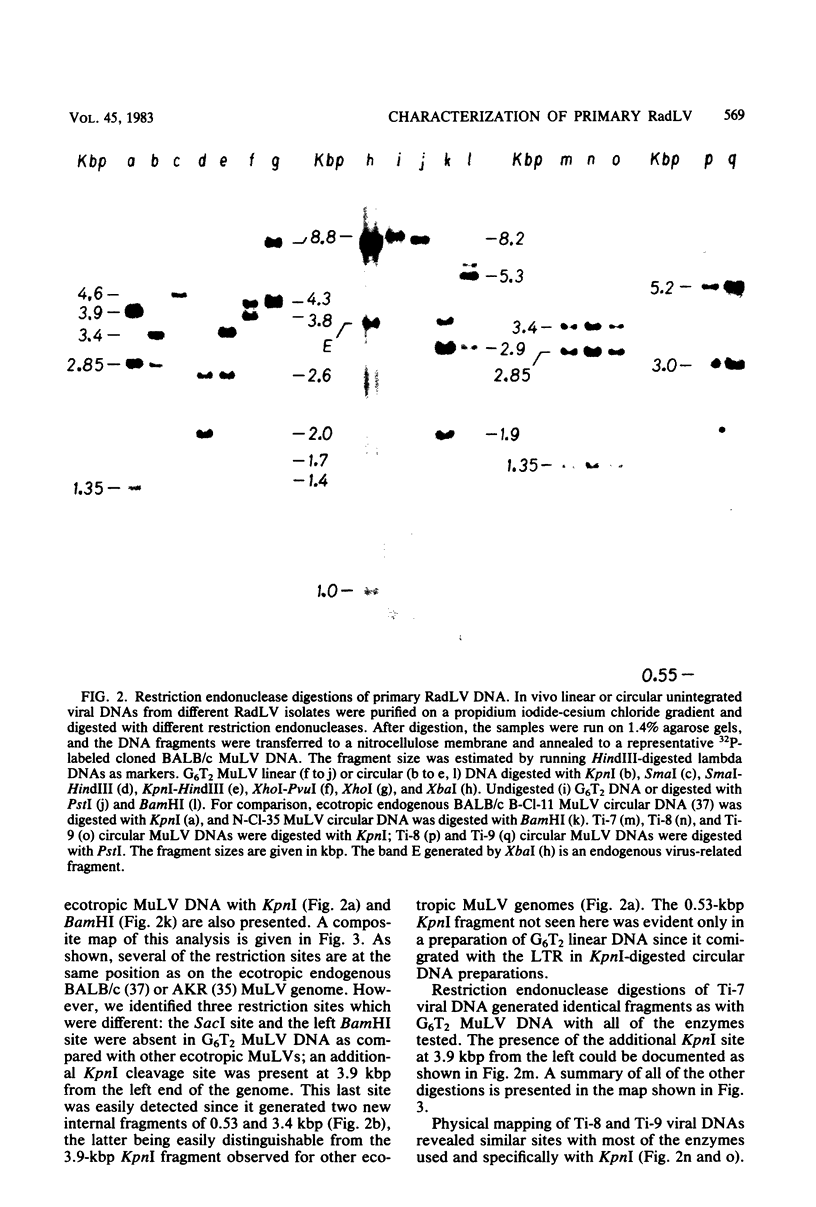
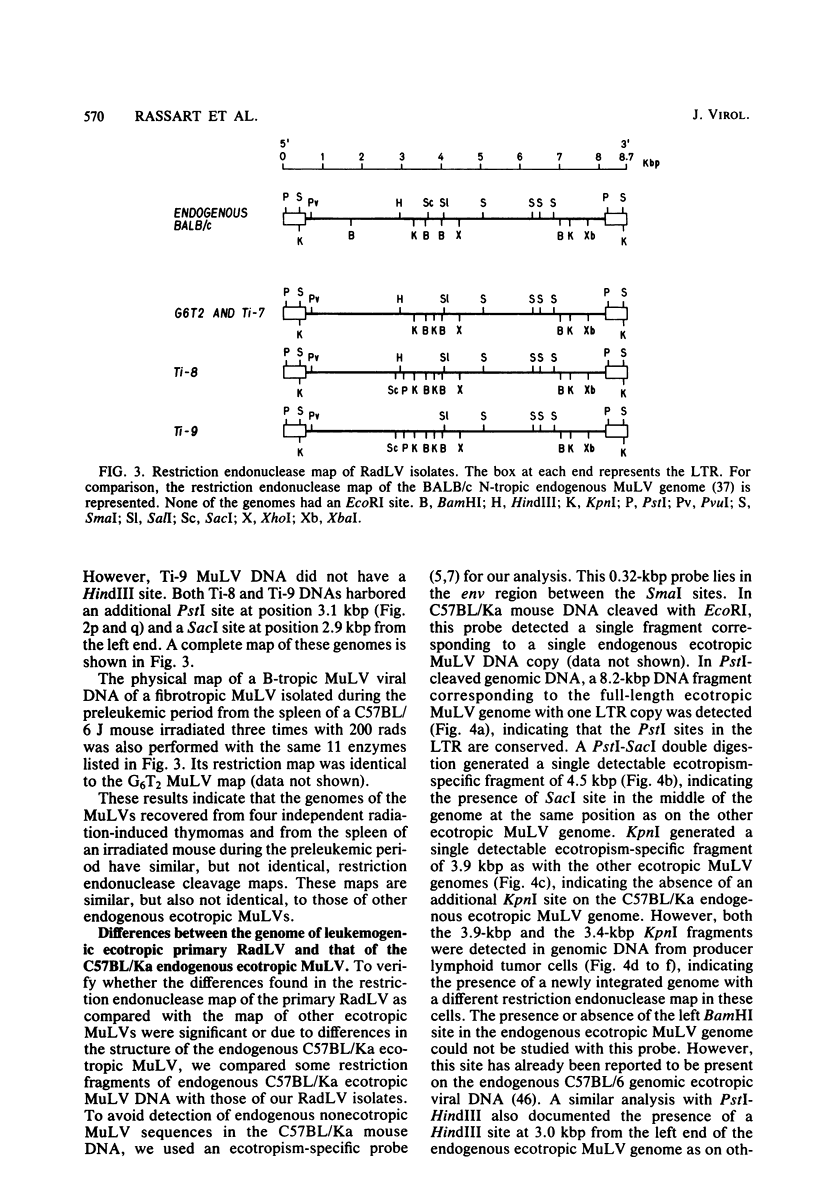
We previously reported the establishment of several lymphoid cell lines from X-ray-induced thymomas of C57BL/Ka mice, and all, except one, produce retroviruses (P. Sankar-Mistry and P. Jolicoeur, J. Virol.35:270-275, 1980). Biological characterization of five of these new primary radiation leukemia viruses (RadLVs) indicated that they had a B-tropic, fibrotropic, and ecotropic host range and were leukemogenic when reinjected into C57BL/Ka newborn mice. The leukemogenic potential of one isolate (G6T2) was further assessed and shown to be retained after prolonged passaging on fibroblasts in vitro. Restriction endonuclease analysis of the DNA of four of our new RadLV isolates (G6T2, Ti-7, Ti-8, and Ti-9) revealed that G6T2 and Ti-7 murine leukemia virus (MuLV) genomes had identical restriction maps, whereas Ti-8 and Ti-9 genomes were different from each other and from the G6T2 and Ti-7 genomes. The physical maps of these genomes were similar to that of known ecotropic MuLV genomes (including the C57BL/Ka endogenous ecotropic MuLV) within their long terminal repeats, env, the right portion of pol, and the left portion of gag. However, a region covering the end of gag and the beginning of pol was different and showed several similarities with xenotropic MuLV genomes of BALB/c, AKR, and C58 mice previously mapped. Our results suggest that these primary RadLV genomes are recombinants between the parental ecotropic MuLV genome and a nonecotropic (xenotropic) sequence. This nonecotropic gag-pol region might be important in conferring the leukemogenic potential to these isolates. Therefore, these RadLVs appear to form a new class of leukemogenic recombinant MuLVs recovered from leukemic tissues of mice. They appear to be distinct from the recombinant AKR mink cell focus-inducing MuLVs which have a dual-tropic host range and harbor xenotropic env sequences. To further study the leukemogenic potential of these RadLVs, the genome of one of them (G6T2) was cloned in Charon 21A as an infectious molecule.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bailey J. M., Davidson N. Methylmercury as a reversible denaturing agent for agarose gel electrophoresis. Anal Biochem. 1976 Jan;70(1):75–85. doi: 10.1016/s0003-2697(76)80049-8. [DOI] [PubMed] [Google Scholar]
- Benade L. E., Ihle J. N., Declève A. Serological characterization of B-tropic viruses of C57BL mice: possible origin by recombination of endogenous N-tropic and xenotropic viruses. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4553–4557. doi: 10.1073/pnas.75.9.4553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besmer P., Fan H., Paskind M., Baltimore D. Isolation and characterization of a mouse cell line containing a defective Moloney murine leukemia virus genome. J Virol. 1979 Mar;29(3):1023–1034. doi: 10.1128/jvi.29.3.1023-1034.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blattner F. R., Williams B. G., Blechl A. E., Denniston-Thompson K., Faber H. E., Furlong L., Grunwald D. J., Kiefer D. O., Moore D. D., Schumm J. W. Charon phages: safer derivatives of bacteriophage lambda for DNA cloning. Science. 1977 Apr 8;196(4286):161–169. doi: 10.1126/science.847462. [DOI] [PubMed] [Google Scholar]
- Chattopadhyay S. K., Lander M. R., Gupta S., Rands E., Lowy D. R. Origin of mink cytopathic focus-forming (MCF) viruses:comparison with ecotropic and xenotropic murine leukemia virus genomes. Virology. 1981 Sep;113(2):465–483. doi: 10.1016/0042-6822(81)90175-6. [DOI] [PubMed] [Google Scholar]
- Chattopadhyay S. K., Lander M. R., Rands E., Lowy D. R. Structure of endogenous murine leukemia virus DNA in mouse genomes. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5774–5778. doi: 10.1073/pnas.77.10.5774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Declève A., Lieberman M., Ihle J. N., Kaplan H. S. Biological and serological characterization of radiation leukemia virus. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4675–4679. doi: 10.1073/pnas.73.12.4675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Declève A., Lieberman M., Ihle J. N., Rosenthal P. N., Lung M. L., Kaplan H. S. Physicochemical, biological and serological properties of a leukemogenic virus isolated from cultured RadLV-induced lymphomas of C57BL/Ka mice. Virology. 1978 Oct 1;90(1):23–35. doi: 10.1016/0042-6822(78)90329-x. [DOI] [PubMed] [Google Scholar]
- Declève A., Sato C., Lieberman M., Kaplan H. S. Selective thymic localization of murine leukemia virus-related antigens in C57BL-Ka mice after inoculation with radiation virus. Proc Natl Acad Sci U S A. 1974 Aug;71(8):3124–3128. doi: 10.1073/pnas.71.8.3124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautsch J. W., Elder J. H., Jensen F. C., Lerner R. A. In vitro construction of a B-tropic virus by recombination: B-tropism is a cryptic phenotype of xenotropic murine retroviruses. Proc Natl Acad Sci U S A. 1980 May;77(5):2989–2993. doi: 10.1073/pnas.77.5.2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas M., Patch V. Cell-surface antigens associated with dualtropic and thymotropic murine leukemia viruses inducing thymic and nonthymic lymphomas. J Exp Med. 1980 Jun 1;151(6):1321–1333. doi: 10.1084/jem.151.6.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas M. Transient virus expression during murine leukemia induction by X-irradiation. J Natl Cancer Inst. 1977 Feb;58(2):251–257. doi: 10.1093/jnci/58.2.251. [DOI] [PubMed] [Google Scholar]
- Haran-Ghera N. Leukemogenic activity of centrifugates from irradiated mouse thymus and bone marrow. Int J Cancer. 1966 Jan;1(1):81–87. doi: 10.1002/ijc.2910010111. [DOI] [PubMed] [Google Scholar]
- Haran-Ghera N., Peled A. Induction of leukemia in mice by irradiation and radiation leukemia virus variants. Adv Cancer Res. 1979;30:45–87. doi: 10.1016/s0065-230x(08)60894-5. [DOI] [PubMed] [Google Scholar]
- Hopkins N., Jolicoeur P. Variants of N-tropic leukemia virus derived from BALB/c mice. J Virol. 1975 Oct;16(4):991–999. doi: 10.1128/jvi.16.4.991-999.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihle J. N., Joseph D. R., Pazmino N. H. Radiation leukemia in C57BL/6 mice. II. Lack of ecotropic virus expression in the majority of lymphomas. J Exp Med. 1976 Dec 1;144(6):1406–1423. doi: 10.1084/jem.144.6.1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihle J. N., McEwan R., Bengali K. Radiation leukemia in C57BL/6 mice. I. Lack of serological evidence for the role of endogenous ecotropic viruses in pathogenesis. J Exp Med. 1976 Dec 1;144(6):1391–1405. doi: 10.1084/jem.144.6.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolicoeur P., Rassart E. Effect of Fv-1 gene product on synthesis of linear and supercoiled viral DNA in cells infected with murine leukemia virus. J Virol. 1980 Jan;33(1):183–195. doi: 10.1128/jvi.33.1.183-195.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolicoeur P., Rosenberg N., Cotellessa A., Baltimore D. Leukemogenicity of clonal isolates of murine leukemia viruses. J Natl Cancer Inst. 1978 Jun;60(6):1473–1476. doi: 10.1093/jnci/60.6.1473. [DOI] [PubMed] [Google Scholar]
- KAPLAN H. S. THE ROLE OF RADIATION ON EXPERIMENTAL LEUKEMOGENESIS. Natl Cancer Inst Monogr. 1964 May;14:207–220. [PubMed] [Google Scholar]
- Kaplan H. S. On the natural history of the murine leukemias: presidential address. Cancer Res. 1967 Aug;27(8):1325–1340. [PubMed] [Google Scholar]
- Kopchick J. J., Ju G., Skalka A. M., Stacey D. W. Biological activity of cloned retroviral DNA in microinjected cells. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4383–4387. doi: 10.1073/pnas.78.7.4383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LATARJET R., DUPLAN J. F. Experiment and discussion on leukaemogenesis by cell-free extracts of radiation-induced leukaemia in mice. Int J Radiat Biol Relat Stud Phys Chem Med. 1962 Aug;5:339–344. doi: 10.1080/09553006214550911. [DOI] [PubMed] [Google Scholar]
- LIEBERMAN M., KAPLAN H. S. Leukemogenic activity of filtrates from radiation-induced lymphoid tumors of mice. Science. 1959 Aug 14;130(3372):387–388. doi: 10.1126/science.130.3372.387. [DOI] [PubMed] [Google Scholar]
- Lieberman M., Declève A., Ricciardi-Castagnoli P., Boniver J., Finn O. J., Kaplan H. S. Establishment, characterization and virus expression of cell lines derived from radiation- and virus-induced lymphomas of C57BL/Ka mice. Int J Cancer. 1979 Aug;24(2):168–177. doi: 10.1002/ijc.2910240208. [DOI] [PubMed] [Google Scholar]
- Lieberman M., Niwa O., Declève A., Kaplan H. S. Continuous propagation of radiation leukemia virus on a C57BL mouse-embryo fibroblast line, with attenuation of leukemogenic activity. Proc Natl Acad Sci U S A. 1973 Apr;70(4):1250–1253. doi: 10.1073/pnas.70.4.1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manteuil-Brutlag S., Liu S. L., Kaplan H. S. Radiation leukemia virus contains two distinct viral RNAs. Cell. 1980 Mar;19(3):643–652. doi: 10.1016/s0092-8674(80)80041-9. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer A., Dorsch-Häsler K. Endogenous MuLV infection does not contribute to onset of radiation- or carcinogen-induced murine thymoma. Nature. 1982 Jan 21;295(5846):253–255. [PubMed] [Google Scholar]
- Nowinski R. C., Hays E. F., Doyle T., Linkhart S., Medeiros E., Pickering R. Oncornaviruses produced by murine leukemia cells in culture. Virology. 1977 Sep;81(2):363–370. doi: 10.1016/0042-6822(77)90152-0. [DOI] [PubMed] [Google Scholar]
- Peters R. L., Sass B., Stephenson J. R., Al-Ghazzouli I. K., Hino S., Donahoe R. M., Kende M., Aaronson S. A., KElloff G. J. Immunoprevention of x-ray-induced leukemias in the C57BL mouse. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1697–1701. doi: 10.1073/pnas.74.4.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rands E., Lowy D. R., Lander M. R., Chattopadhyay S. K. Restriction endonuclease mapping of ecotropic murine leukemia viral DNAs: size and sequence heterogeneity of the long terminal repeat. Virology. 1981 Jan 30;108(2):445–452. doi: 10.1016/0042-6822(81)90451-7. [DOI] [PubMed] [Google Scholar]
- Rassart E., DesGroseillers L., Jolicoeur P. Molecular cloning of B- and N-tropic endogenous BALB/c murine leukemia virus circular DNA intermediates: isolation and characterization of infectious recombinant clones. J Virol. 1981 Jul;39(1):162–171. doi: 10.1128/jvi.39.1.162-171.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rassart E., Jolicoeur P. Restriction endonuclease mapping of unintegrated viral DNA of B- and N-tropic BALB/c murine leukemia virus. J Virol. 1980 Sep;35(3):812–823. doi: 10.1128/jvi.35.3.812-823.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rein A. L., Gerwin B. I., Bassin R. H., Schwarm L., Schidlovsky G. A replication-defective variant of Moloney murine leukemia virus. I. Biological characterization. J Virol. 1978 Jan;25(1):146–156. doi: 10.1128/jvi.25.1.146-156.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rein A., Athan E., Benjers B. M., Bassin R. H., Gerwin B. I., Slocum D. R. Isolation of a replication-defective murine leukaemia virus from cultured AKR leukaemia cells. Nature. 1979 Dec 13;282(5740):753–754. doi: 10.1038/282753a0. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Rowe W. P., Pugh W. E., Hartley J. W. Plaque assay techniques for murine leukemia viruses. Virology. 1970 Dec;42(4):1136–1139. doi: 10.1016/0042-6822(70)90362-4. [DOI] [PubMed] [Google Scholar]
- Sankar-Mistry P., Jolicoeur P. Frequent isolation of ecotropic murine leukemia virus after x-ray irradiation of C57BL/6 mice and establishment of producer lymphoid cell lines from radiation-induced lymphomas. J Virol. 1980 Jul;35(1):270–275. doi: 10.1128/jvi.35.1.270-275.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields A., Rosenberg N., Baltimore D. Virus production by Abelson murine leukemia virus-transformed lymphoid cells. J Virol. 1979 Aug;31(2):557–567. doi: 10.1128/jvi.31.2.557-567.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields A., Witte W. N., Rothenberg E., Baltimore D. High frequency of aberrant expression of Moloney murine leukemia virus in clonal infections. Cell. 1978 Jul;14(3):601–609. doi: 10.1016/0092-8674(78)90245-3. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Steffen D. L., Bird S., Weinberg R. A. Evidence for the Asiatic origin of endogenous AKR-type murine leukemia proviruses. J Virol. 1980 Sep;35(3):824–835. doi: 10.1128/jvi.35.3.824-835.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockert E., Old L. J. Preleukemic expression of TL antigens in x-irradiated C57BL/6 mice. J Exp Med. 1977 Jul 1;146(1):271–276. doi: 10.1084/jem.146.1.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]