Abstract
Background
Myoclonus dystonia syndrome (MDS) is an autosomal dominant movement disorder caused by mutations in the epsilon‐sarcoglycan gene (SGCE) on chromosome 7q21.
Methods
We have screened for SGCE mutations in index cases from 76 French patients with myoclonic syndromes, including myoclonus dystonia (M‐D), essential myoclonus (E‐M), primary myoclonic dystonia, generalised dystonia, dystonia with tremor, and benign hereditary chorea. All coding exons of the SGCE gene were analysed. The DYT1 mutation was also tested.
Results
Sixteen index cases had SGCE mutations while one case with primary myoclonic dystonia carried the DYT1 mutation. Thirteen different mutations were found: three nonsense mutations, three missense mutations, three splice site mutations, three deletions, and one insertion. Eleven of the SGCE index cases had M‐D and five E‐M. No SGCE mutations were detected in patients with other phenotypes. The total number of mutation carriers in the families was 38, six of whom were asymptomatic. Penetrance was complete in paternal transmissions and null in maternal transmissions. MDS patients with SGCE mutation had a significantly earlier onset than the non‐carriers. None of the patients had severe psychiatric disorders.
Conclusion
This large cohort of index patients shows that SGCE mutations are primarily found in patients with M‐D and to a lesser extent E‐M, but are present in only 30% of these patients combined (M‐D and E‐M).
Keywords: epsilon sarcoglycan gene, essential myoclonus, mutation, myoclonus dystonia, phenotype
Myoclonus dystonia syndrome (MDS), an autosomal dominant disorder, is characterised by myoclonic jerks mainly involving the arms and axial muscles in combination with dystonia. It often develops during childhood or early adolescence. Myoclonus is the predominant feature, with “lightning jerks” that are often responsive to alcohol. Dystonia classically appears later in the course of the disease and is often more focal and less spectacular than the myoclonus.1
In 1999, the MDS gene locus was linked to a 28 cM region of chromosome 7q21–q31,2 and in 2001, the gene was identified as the ε‐sarcoglycan gene (SGCE).3 Epsilon‐sarcoglycan (SGCE) is a transmembrane glycoprotein homologous to α‐sarcoglycan. Sarcoglycan proteins (α, β, γ, and δ) are components of the dystrophin‐glycoprotein complex, which links the muscle cytoskeleton to the extracellular matrix,4 and are involved in autosomal recessive muscular dystrophies.5,6 The SGCE protein is expressed in many human tissues: muscle, lung, liver, kidney, spleen, testis, and brain.7 The SGCE gene is composed of 12 exons that span 71 kb. Exon 10 is alternatively spliced and is missing in the majority of transcripts. Recently, a new exon, 11b, was identified in a transcript from mouse brain.8 Maternal imprinting of the SGCE gene has been demonstrated in mice9 and later in humans,10,11 and is responsible for the reduced penetrance observed in individuals who inherited the mutated allele from their mother. Since 2001, 24 mutations have been reported (table 1). In this study, we have evaluated the frequency of SGCE mutations and the corresponding phenotype in a large cohort of French patients with myoclonic syndromes, but also with clinically related disorders such as dystonia with tremor, generalised dystonia, or benign hereditary chorea (BHC).
Table 1 Characteristics of the published patients with SGCE mutations.
| Nucleotide or amino acid change | Localis‐ ation in gene | Affected (n) | Origin | Age at onset (year)* | Myoclonus (distribution) | Dystonia (distribution) | Alcohol sensitivity | References |
|---|---|---|---|---|---|---|---|---|
| Truncating mutations | ||||||||
| p. 97R>X | Exon 3 | 3 | German | 3.5 | NK | H, N | NK | Zimprich et al3 |
| p. 97R>X | Exon 3 | 1 | NK | 0.3 | A, L, N | N | Yes | Valente et al12 |
| p. 97R>X | Exon 3 | 1 | NK | 3 | A, N | A, N | No | Valente et al12 |
| p.102R>X | Exon 3 | 7 | German | 4 | NK | H, N | NK | Zimprich et al3 |
| p.102R>X | Exon 3 | 8 | German | 8 | NK | N | NK | Zimprich et al3 |
| p.102R>X | Exon 3 | 5 | German | 5–18 | A, N, T | H, N | Yes | Asmus et al13 |
| p.102R>X | Exon 3 | 3 | German | 2–6 | A, L, N, T | H, L | Yes | Asmus et al13 |
| p.102R>X | Exon 3 | 3 | French | 0.5–6 | A, F, N, T | A, H, N | Yes | Asmus et al13 |
| p.102R>X | Exon 3 | 13 | Canadian | Childhood | A, F, N | N | Yes | Han et al14 |
| p.102R>X | Exon 3 | 2 | Canadian | 1–14 | A, N | None | NK | Han et al14 |
| p.102R>X | Exon 3 | 1 | German | 7 | A | H | Yes | Hedrich et al15 |
| p.102R>X | Exon 3 | 1 | German | 13 | A, N | Segmental | NK | Hedrich et al15 |
| p.286Q>X | Exon 7 | 3 | French | Childhood | A, N, T | A, L, T | NK | Asmus et al13 |
| p.372R>X | Exon 9 | 1 | NK | 3 | A, L, N, T | A, L, N | Yes | Valente et al12 |
| p.372R>X | Exon 9 | 1 | NK | 11 | A, L, N | A, L | Yes | Valente et al12 |
| Missense mutations | ||||||||
| p.60H>P | Exon 2 | 1 | German | 7 | A, F, T | N | Yes | Hedrich et al15 |
| p.60H>P | Exon 2 | 5 | Serbian | 7–15 | A, H, L | T, H, L; none for 2 | Yes | Schule et al16 |
| p.196L>R | Exon 5 | 2 | German/ | 2–10 | A, F, L, V | A, H | Yes | Klein et al17 |
| English/Welsh | ||||||||
| Splicing mutations | ||||||||
| c.233−1G>A | Intron 2 | 3 | French | 3–10 | A, N | G | Yes | Asmus et al13 |
| c.391−3T>C | Intron 3 | 1 | NK | 30 | A, N | None | NK | Valente et al12 |
| c.463+6T>C | Intron 4 | 1 | United Kingdom | 3 | A, N, T | A, L | Yes | Asmus et al13 |
| c.662+5G>A | Intron 5 | 3 | German | 1.5–12 | N, A or G | H, N | Yes | Asmus et al13 |
| c.825+1G>A | Intron 6 | 4 | German | 4 | NK | N | Yes | Zimprich et al3 |
| Deletions | ||||||||
| c.164delG | Exon 2 | 5 | Welsh/Czech | <6 | A, F, N | N | Yes | Hedrich et al15 |
| c.276delG | Exon 3 | 1 | German | 4 | A, L, N | None | Yes | Asmus et al13 |
| c.391_405del | Exon 4 | 4 | German | 7 | NK | N | NK | Zimprich et al3 |
| c.402–405del | Exon 4 | 6 | French | 2–30 | NK | A, H, L | Yes | Marechal et al18 |
| c.483delA | Exon 5 | 11 | German | 4–12 | NK | H, N | Yes | Zimprich et al3 |
| c.564_576del | Exon 5 | 1 | NK | 2 | A, N | A, N | Partial | Valente et al12 |
| c.734_737delAATT | Exon 6 | 2 | French | 8–20 | A, T | N | Yes | Asmus et al13 |
| c.832_836delAAAAC | Exon 7 | 6 | Canadian | Childhood | A, F, L, N | A | Yes | Han et al14 |
| c.835_839delACAAA | Exon 7 | 8 | German/ | 2–16 | A, F, H, L, T, V | A, L, N , T | NK | Klein et al17 |
| English/Welsh | ||||||||
| c.966delT | Exon 7 | 2 | German | 4–9.5 | NK | NK | NK | Muller et al11 |
| c.966delT | Exon 7 | 6 | Serbian | 4–15 | A, H, L | T, L; none for 2 | NK | Schule et al16 |
| c.974delC | Exon 7 | 9 | Danish | 1–4 | L, N, T, V | A, H, L | Yes | Hjermind et al19 |
| Insertions | ||||||||
| c.625insG | Exon 5 | 1 | German | 2 | NK | NK | NK | Muller et al11 |
| c.885insT | Exon 7 | 4 | German | 15–17 | A, F, H, N | N, T | NK | Foncke et al20 |
A, arm; F, face; G, generalised; H, hand; L, leg; N, neck; NK, not known; S, shoulders; T, trunk; V, voice.
*Range of age at onset. When only one value is given, it is the mean age at onset in the family.
Methods
Families and patients
We included 76 index patients with myoclonus‐dystonia (M‐D; n = 49), essential myoclonus (E‐M; n = 5), and primary myoclonic dystonia (n = 13), as well as dystonia associated with tremor (n = 5), generalised dystonia (n = 3), and BHC (n = 1) to broaden the phenotypic spectrum. All patients were French and of Caucasian origin.
M‐D was defined by the presence of lightning jerks (myoclonus) and dystonia in patients.1 E‐M was defined by isolated myoclonus.21,22 Primary myoclonic dystonia was defined by primary dystonia with superimposed jerky movements. These patients had predominant dystonia, and their jerks were not lightning jerks but the slower jerks commonly seen in primary dystonia. Dystonia and tremor was defined by the association of a postural, localised, and irregular tremor (dystonic tremor) superimposed on more sustained dystonic spasms.23 Idiopathic generalised dystonia was characterised by involuntary sustained muscle contractions, causing twisting and repetitive movements or abnormal postures, involving at least one leg and the trunk and any other segment.24 BHC was defined by the presence of early onset chorea with or without other movement disorders in patients with a positive family history but without the CAG repeat expansion responsible for Huntington's disease.25 In all cases, history and investigations suggested a primary cause of the disease. All patients were interviewed and examined by a neurologist expert in the field of movement disorders. All index cases were tested for the GAG deletion in the DYT1 gene (c.946delGAG mutation). When possible, relatives were also investigated blind to the genetic results. After written informed consent, a standardised questionnaire, based on an interview and a clinical examination, was filled out for each individual (index case or relative) and a blood sample was obtained for DNA extraction.
PCR amplification and sequence analysis of the SGCE gene
Genomic DNA was extracted from peripheral leucocytes by standard methods (phenol/chloroform). The 12 exons and the exon‐intron junctions were amplified by the polymerase chain reaction (PCR). In addition, the 11b exon recently identified in a transcript from mouse brain was also amplified by PCR. PCR primers for amplification of the SGCE gene and the amplification conditions are shown in table 2. The amplified fragments were sequenced on a ABI 3730 automated sequencer using the BigDye Version 3.1 cycle sequencing kit (Applied Biosystems, Foster City, CA). Ninety unrelated control subjects were screened for the three new missense and three splice site mutations to exclude possible polymorphisms.
Table 2 Primer sequences and PCR conditions of SGCE exons.
| Exons | Primers | Annealing temperature (C°) | Amplimer size (bp) | |
|---|---|---|---|---|
| 1 | 1F | TGCTGAACTGGCCAAGCTGG | 64 | 301 |
| 1R | AGAGAGGCTGGTGCCCAAAG | |||
| 2 | 2F | GGCGTATCTCATTATTTGTC | 55 | 434 |
| 2R | AGGTAGATCACTTGTCAGAG | |||
| 3 | 3F | CATGTGTGAAAATAACTGTC | 53 | 311 |
| 3R | GGTAACTTTAGTTTCAACAC | |||
| 4 | 4F | ATGAAAATGGAAAGAATGAC | 55 | 290 |
| 4R | AGTTATATTAGGTATGTGGC | |||
| 5 | 5F | CCAGGATTATGACAGAACTC | 55 | 393 |
| 5R | GCAATAGGCCATCTTCCATC | |||
| 6 | 6F | AGGGATGAGTCTAGTTAATC | 57 | 343 |
| 6R | CAAACGTTAACTCCAGCCAC | |||
| 7 | 7F | GAATGCTTTAGTGTATCCAG | 53 | 348 |
| 7R | GTTGTTATCTTAGCAGGATC | |||
| 8 | 8F | GCATATAGTCTTAATGTTCC | 53 | 171 |
| 8R | CACATGTATGGAGCATGATG | |||
| 9 | 9F | AATTGATGACCCATCAGGCT | 55 | 341 |
| 9R | CACAACAACAGAAAGCTCTG | |||
| 10 | 10F | CAGTTGCATTTGGCAGACC | 52 | 574 |
| 10R | TTCTGCATAGCCATTCCATC | |||
| 11 | 11F | TCATTCTAGTATGTCTGCTC | 53 | 220 |
| 11R | TTTGGTGAAGATAAAGCTTC | |||
| 11b | 11bF | GGCATTGTGGTAGGGAAAC | 58 | 304 |
| 11bR | GCTTACAAAGTAGCACCAAC | |||
| 12 | 12F | GTATCCATGCCCTGACTAAC | 55 | 158 |
| 12R | AGCTCATGCATTATTGGAAG |
F, forward primer; R, reverse primer.
RT‐PCR analysis of the c.232 +2T>C mutation
Total RNA was extracted from immortalised lymphocytes of a patient in family 1 with the c.232 +2T>C splice site mutation using the RNeasy Mini kit (Qiagen, Valencia, CA). RNA was reverse transcribed using oligo(dT) primer (ImProm‐II Reverse Transcriptase; Invitrogen, Carlsbad, CA), and the full length SGCE cDNA was amplified by PCR using the forward primer 5'GAATCGAGGACGGACGGC3' in the promoter region and reverse 12R primer shown in the table 2. The PCR products corresponding to the expected 1437 bp cDNA were extracted from 1.5% agarose gel with a commercial kit (Qiaquick PCR purification kit; Qiagen) and sequenced with both forward and reverse primers.
Statistical analysis
Data are expressed as mean±SD (min−max) or % (n). To increase the power of the comparison between SGCE mutation carriers and non‐carriers, we added four previously published French families with SGCE mutations recruited with the same inclusion criteria as the patients reported in the present study.13 Age of onset was compared using Student's t test. Alcohol responsiveness and distribution of the symptoms were compared using the χ2 test or the Fisher exact test when appropriate. Sensitivity and specificity, that is the probability of a correct diagnosis based on phenotype given the diagnosis based on genotype, were also computed. Only index cases were used for these analyses. All statistical tests were two tailed. Statistical significance was defined as p<0.05. Statistical analyses were performed with the use of the SAS statistical package, version 8.2 (SAS Institute, Cary, NC).
Results
Patients with mutations in the SGCE gene
In the 76 index patients tested, we found 13 different mutations in the SGCE gene in 16 patients. Eleven index cases had M‐D and five E‐M, two of whom had at least one relative with myoclonus dystonia (M‐D). The total number of mutation carriers in the families was 38 (16 index cases and 22 relatives), six of whom were asymptomatic (table 3).
Table 3 Clinical features of carriers of SGCE mutations.
| Family no./ sex | Nucleotide change | Amino acid change | Exon | Familial trans‐ mission* | Diagnosis | Age of onset | Age at examination | First symptom | Dystonia (location)† | Myoclonus (location)† | Sensitivity to alcohol¶ |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Splicing mutations | |||||||||||
| 1/M‡ | c.232 +2T>C | Splicing | 2 | NK | E‐M | 23 | 64 | Myoclonus | – | NK | NK |
| 1/F | c.232 +2T>C | Splicing | 2 | F | E‐M | 4 | 30 | Myoclonus | – | N, T | NK |
| 1/F | c.232 +2T>C | Splicing | 2 | F | E‐M | 24 | 25 | Myoclonus | – | N, A, T | NK |
| 1/M | c.232 +2T>C | Splicing | 2 | F | E‐M | NK | 34 | Myoclonus | – | F, N, A | NK |
| 1/M | c.232 +2T>C | Splicing | 2 | F | E‐M | 4 | 9 | Myoclonus | – | NK | NK |
| 372/M | c.233 −1G>T | Splicing | 3 | NK | Unaffected | – | 37 | – | – | – | – |
| 372/F‡ | c.233 −1G>T | Splicing | 3 | F | M‐D | 4 | 13 | Myoclonus | A, T | N, A | NK |
| 372/F | c.233 −1G>T | Splicing | 3 | F | E‐M | 4 | 6 | Myoclonus | – | L | NK |
| 462/M‡ | c.232 +1G>A | Splicing | 2 | F | M‐D | 6 | 12 | M‐D | A | N, A | NK |
| Nonsense mutations | |||||||||||
| 302/M | c.1114 C>T | R372X | 9 | NK | E‐M | Adolescence | 73 | Myoclonus | – | F, A, T | No |
| 302/M‡ | c.1114 C>T | R372X | 9 | F | E‐M | Childhood | 43 | Myoclonus | – | NK | NK |
| 302/F | c.1114 C>T | R372X | 9 | F | M‐D | Childhood | 44 | Myoclonus | A | A | NK |
| 414/M | c.300 G>A | W100X | 3 | NK | Unaffected | – | 47 | – | – | – | – |
| 414/M | c.300 G>A | W100X | 3 | F | E‐M | 2 | 14 | Myoclonus | – | N, A | NK |
| 414/F‡ | c.300 G>A | W100X | 3 | F | M‐D | 2 | 4 | Myoclonus | L | A, L | NK |
| 565/M‡ | c.300 G>A | W100X | 3 | NK | E‐M | 3 | 20 | Myoclonus | – | N, A, L, T | Yes (0.5) |
| 598/M | c.208 G>T | E70X | 2 | NK | M‐D | 5 | 49 | NK | N, A, T | N, A | Yes (0.8) |
| 598/F | c.208 G>T | E70X | 2 | F | M‐D | 12 | 28 | Myoclonus | A | N | NK |
| 598/F‡ | c.208 G>T | E70X | 2 | F | E‐M | 10 | 20 | Myoclonus | – | N, A | Yes (1) |
| 664/F‡ | c.300 G>A | W100X | 3 | NK | E‐M | 3 | 20 | Myoclonus | – | N, A, L, T | Yes (1) |
| Missense mutations | |||||||||||
| 127/M‡ | c.812 G>A | C271Y | 6 | NK | M‐D | 1.5 | 9 | Dystonia | F, A, L, T | A, L | NK |
| 429/F | c.344 A>G | Y115C | 3 | NK | E‐M | NK | 68 | Myoclonus | – | F | NK |
| 429/M | c.344 A>G | Y115C | 3 | F | M‐D | Adolescence | 43 | Dystonia | A | A | No |
| 429/F‡ | c.344 A>G | Y115C | 3 | F | M‐D | 5 | 39 | Dystonia | N, A, T | N, A | No |
| 429/F | c.344 A>G | Y115C | 3 | M | Unaffected | – | 39 | – | – | – | – |
| 429/F | c.344 A>G | Y115C | 3 | M | Unaffected | – | 32 | – | – | – | – |
| 429/M | c.344 A>G | Y115C | 3 | M | Unaffected | – | 30 | – | – | – | – |
| 429/F | c.344 A>G | Y115C | 3 | F | M‐D | 12 | 19 | Dystonia | N, A | N, A | NK |
| 429/F | c.344 A>G | Y115C | 3 | F | E‐M | 1 | 5 | Myoclonus | – | N, F , A | NK |
| 632/F‡ | c.274 T>C | M92T | 3 | F | M‐D | 1.75 | 9 | Subacute ataxia | L | A, L | NK |
| Insertion | |||||||||||
| 294/F‡ | c.745_746insTGTA | S249fsX250 | 6 | F | M‐D | Before 6 | 46 | Myoclonus | L | N, A | Yes |
| Deletions | |||||||||||
| 6/F‡ | c.832_836delAAAAC | K278fsX295 | 7 | F | M‐D | 2 | 10 | Dystonia | N, L | N, A | NK |
| 6/F | c.832_836delAAAAC | K278fsX295 | 7 | F | M‐D | 7 | 7 | M‐D | A | A | NK |
| 138/M | c.444_447delTAAT | N149fsX149 | 4 | NK | Unaffected | – | 53 | – | – | – | – |
| 138/F‡ | c.444_447delTAAT | N149fsX149 | 4 | NK | M‐D | 18 | 23 | Myoclonus | N, T | N | Yes (1) |
| 138/F | c.444_447delTAAT | N149fsX149 | 4 | NK | E‐M | 6 | 27 | Myoclonus | – | F, N, T | NK |
| 486/F‡ | c.444_447delTAAT | N149fsX149 | 4 | NK | M‐D | 3 | 20 | Dystonia | N, A | N, A, L | Yes (0.7) |
| 562/F‡ | c.221delA | Y74fsX85 | 2 | NK | M‐D | 3 | 29 | Myoclonus | A | N, A, L | NK |
E‐M, essential myoclonus; F, female; M, male; M‐D, myoclonus‐dystonia; NK, not known.
*F, father; M, mother; †A, arm; F, face; G, generalised; L, leg; N, neck; NK, not known; T, trunk; V, voice; ‡index cases; ¶percentage of decrease in symptoms after alcohol intake is shown in parentheses.
Seventeen of the 32 affected patients had M‐D and 15 had E‐M. Their mean age at onset was 7.2 (SD 6.7) years (range: 1–24), whereas the mean age at examination of the asymptomatic carriers was 40.2 (SD 8.8) (range: 30–53). In most patients (69%), myoclonus was the first symptom. Seven of the 10 patients tested for a response to alcohol experienced a mean decrease in their symptoms of 79% (SD 23%). None of the patients reported alcohol addiction. No severe psychiatric disorders were detected: a careful interview of the patients and their families uncovered no past medical histories, no complaints of marked depression, or no patent obsessive or compulsive symptoms.
In 12 families (75%), at least one other member of the family had a myoclonic syndrome corresponding to M‐D (10 families) or E‐M (two families). There was no known family history for the remaining four. Information on the transmitting parent was available for 24 mutation carriers. All carriers who received the mutated allele from their father were affected (complete penetrance), whereas all carriers who received the mutated allele from their mother were asymptomatic (null penetrance).
Comparison of patients with and without SGCE mutations
The features of the 60 index patients without mutations in the SGCE gene are given in table 4. A family history of M‐D or dystonia was found in 38% (n = 23) of the patients (family history unknown for four patients). The mother transmitted the disease in 11 cases, the father in the other 12. Interestingly, one patient with primary myoclonic dystonia that responded extremely well to alcohol carried the DYT1 mutation. This patient developed writer's cramp at age 18 and myoclonus 4 or 5 years later. Both upper and lower limbs were affected. His mother, of Ashkenazi Jewish origin, suffered from writer's cramp without myoclonus.
Table 4 Clinical features of non‐carriers of SGCE mutations.
| Diagnosis | No. of cases | Mean age at onset | Myoclonus (distribution) | Dystonia (distribution) | Sensitivity to alcohol | Family history |
|---|---|---|---|---|---|---|
| Myoclonus dystonia | 35 (M:15/F:20) | 13.7±15.5 | UB: 20 | UB: 19 | Yes: 5 | AD(P): 3 |
| UB+LB: 14 | UB+LB: 15 | No: 10 | AD(M): 4 | |||
| LB: 1 | UK: 1 (618) | NK: 20 | AD(NK): 1 | |||
| None: 24 | ||||||
| NK: 2 | ||||||
| Essential myoclonus | 3 (M:2/F:1) | 26.0±17.3 | UB+LB: 2 | None | Yes: 1 | AD(P): 1 |
| UB: 1 | No: 2 | None: 2 | ||||
| Primary myoclonic | 13 (M:6/F:7) | 18.7±15.8 | UB+LB: 3 | UB+LB: 8 | Yes: 3 | AD(P):2 |
| dystonia | UB: 10 | UB: 5 | No: 7 | AD(M):4 | ||
| NK: 3 | ASP: 1 | |||||
| None: 6 | ||||||
| Dystonia with tremor | 5 (M:3/F:2) | 16.1±13.6 | None | UB+LB: 2 | Yes: 1 | AD(P):1 |
| UB: 3 | No: 2 | AD(M):1 | ||||
| NK: 2 | ASP: 1 | |||||
| None: 1 | ||||||
| Generalised dystonia | 3 (F:3) | 4, 14 | None | UB+LB | No: 1 | AD(M): 1 |
| NK: 2 | ASP: 1 | |||||
| BHC | 1 (M) | Childhood | UB | UB+LB | NK | AD(P) |
AD(M), autosomal dominant with maternal inheritance; AD(P), autosomal dominant with paternal inheritance; AD(UK), autosomal dominant with transmitting parent unknown; ASP, two or more affected sibs; BHC, benign hereditary chorea; F, female; LB, lower body; M, male; NK, not known; UB, upper body.
Index patients without SGCE mutations were compared to those with SGCE mutations, including four previously published families recruited using the same inclusion criteria.13 The four previously published families comprised 16 carriers (nine with M‐D, two with E‐M, and five asymptomatic subjects) who were added to our 38 carriers. M‐D patients with SGCE mutations were significantly younger at onset than non‐carriers (8.2 (SD 7.6) (0.5–38) years v 15.6 (SD 15.0) (0.25–64) years, respectively; p<0.003) and their symptoms were more frequently sensitive to alcohol (82% v 31%, respectively; p<0.0007). However, information on alcohol responsiveness was available for only 17 carriers and 32 non‐carriers, but 40% of the patients where this information was not available were under the age of 18. Since SGCE mutations were only found in patients with M‐D or E‐M, the analyses were also performed after excluding all other phenotypes and the results were similar. When patients with M‐D or E‐M phenotypes with or without SGCE mutations were compared, the location of the myoclonus was similar in both groups, but the location of dystonia differed (p<0.001): 73% of mutation carriers had dystonia in upper limbs only, 12% in lower limbs only, and 15% in both upper and lower limbs, whereas 47% of non‐carriers had dystonia in upper limbs only, 0% in lower limbs only, and 48% in both upper and lower limbs (5% had no dystonia).
Which patients should be tested for SGCE mutations to optimise screening
All patients with SGCE mutations had an M‐D or E‐M phenotype, indicating a sensitivity of 100%. However, 63% of the patients without SGCE mutations also had an M‐D or E‐M phenotype, indicating a specificity of only 37%. Conversely, 30% of M‐D or E‐M patients had SGCE mutations, whereas no mutations were found in patients with other phenotypes. Taking into account the age at onset and genetic parameters improves specificity. If patients with mother to child transmission of the disease were excluded, the specificity of the mutation screen increased to 43%. In addition, if only patients with onset before the age of 25 were tested, the specificity increased to 55%. In both cases, sensitivity was still 100%.
Molecular genetics
Mutation analysis
Eleven of the 13 different SGCE mutations identified were novel (table 3). The two previously reported mutations were a splice site mutation in intron 2 (c.233 –1G>A, French family from a different region)13 and a deletion in exon 7 (c.832_836delAAAAC, Canadian family).14 The types of mutation found in the 16 index carriers were five nonsense mutations, three missense mutations, three splice site mutations, four deletions, and one insertion. These mutations are all likely to be causative. All the deletions and the insertion caused a frameshift in the coding region that introduced a premature stop codon resulting in a truncated protein, as for the five nonsense mutations. The three putative splice site mutations affected major bases of the consensus splice site sequence. One (c.233 –1G>A) was previously reported, the other (c.232 +2T>C) has been validated by RT‐PCR (see below). The two new putative splice mutations were not present on 180 chromosomes from Caucasian controls. The SGCE missense mutations were all non‐conservative amino acid changes affecting conserved amino acids and were also absent from the control chromosomes.
In addition, three variants were identified in the alternative exon 10 in our patients. The A to C substitution at nucleotide 40 of exon 10 had already been reported in a genomic database (www.ensembl.org), the other two were G to A substitutions at nucleotides 41 and 43 of exon 10. These variants were also found in 156 unrelated controls, indicating that they are most likely to be polymorphisms.
Identification of alternative exon 2 splicing in SGCE transcripts
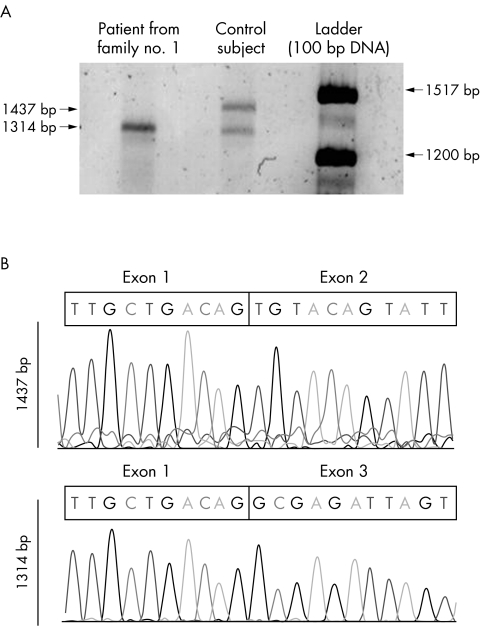
Lymphocytes were available from only one patient with the c.232 +2T>C splice site mutation (fig 1). RT‐PCR amplification of mRNA extracted from the cells produced a transcript that was smaller (1314 bp) than the expected size (1437 bp). Sequence analysis showed that this fragment lacked exon 2, resulting in the loss of 41 amino acids (p.37–77) without interrupting the reading frame. In the lymphocytes of a control subject, both the 1437 bp transcript and the 1314 bp transcript were detected, suggesting that exon 2 can be alternatively spliced, at least in lymphoblasts.
Figure 1 Alternative splicing of exon 2 in SGCE transcripts. (A) RT‐PCR amplification of SGCE produced evidence of two SGCE transcripts in the lymphocytes of a control subject, a 1437 bp sequence and a 1314 bp sequence, visualised after agarose gel electrophoresis. Only the smaller fragment was amplified in a patient with the c.232 +2T>C splice site mutation. (B) Sequencing of the two transcripts showed that only the 1437 bp fragment contains exon 2.
Discussion
In this study, we screened the SGCE gene in 76 index patients with myoclonic syndromes or phenotypically related disorders, the largest series analysed so far. Mutations in the SGCE gene were found only in patients with typical M‐D or E‐M phenotypes. Although most were familial cases, four patients with apparently sporadic M‐D also had SGCE mutations. This can be explained by maternal imprinting or because a family history was not available. This suggests that sporadic cases should be screened for SGCE mutations when the phenotype is consistent with M‐D.
In both the familial and sporadic patients, the initial symptom of the disease was either dystonia or myoclonus that could affect any part of the body. Unlike the patients of Valente et al,12 some of our patients had lower limb involvement. The syndrome developed during childhood or early adolescence and started before the age of 25 in all patients. This is in agreement with previous reports (table 1). There was no information about sensitivity to alcohol for 28 cases, but 40% of these were under the age of 18. When asked, however, several patients reported no response to alcohol. Unlike previous reports,18,26 no major psychiatric disturbances were found in our patients by careful clinical examination. Although we did not used standardised algorithms for the diagnosis of mental disorders or obsessive compulsive rating scales, the patients did not complain of alcohol addiction, severe depression, or major obsessive or compulsive disorders.
Molecular analysis detected 13 different SGCE mutations distributed along the gene between exons 2 and 9, which corresponds to the sarcoglycan domain (tables 1 and 3). However, no mutations were found in exon 8. This might be explained by alternative splicing of exon 8, as in mouse brain, where exon 11b is expressed.8 However, our systematic screening of exon 11b, the first to be performed, did not detect mutations in this sequence either. All of the missense mutations identified affected amino acids that are conserved in other species, suggesting that they alter an important function. The W100X nonsense mutation in exon 3 was the most frequent mutation in our patients (3/16). Except for the R102X nonsense mutation found in nine families (six German, two Canadian, and one French), the majority of mutations are different in each family. All types of mutations have been found (tables 1 and 3): nonsense mutations (20%), missense mutations (14%), splice site mutations (20%), deletions (37%), and insertions (9%). The great majority (86%) give rise to aberrant or truncated proteins. There were no obvious differences, however, in the phenotypes (clinical features or age of onset) of patients with different mutations.
The phenotypes of 38 of the 60 patients without SGCE mutations were very similar to those of patients with mutations (that is, M‐D and E‐M). However, there were significant group differences. Mutation carriers had significantly earlier onset, more frequent responses to alcohol, and lower limb dystonia. The frequency of SGCE mutations increased when patients with onset after the age of 25 or who inherited the disease from their mother were excluded. The clinical pattern of myoclonus and dystonia does not predict the genetic status of the patients,12,27 but more detailed analyses, including electrophysiological investigations, could increase specificity. This should be confirmed in future studies.
One of our patients of Ashkenazi origin with primary myoclonic dystonia, whose mother had writer's cramp, had a DYT1 mutation. This patient had symptoms that responded to alcohol as do M‐D patients. Since this patient was first examined at the age of 69, the age at onset given (18) might not be accurate. Although a myoclonic syndrome resembling M‐D is relatively rare in patients suffering from early onset primary dystonia, it has been previously reported.28,29,30 We tested one patient with BHC and found no mutation in this patient. A total of four patients with BHC have been tested negative for mutations in the SGCE gene.12 The number of tested patients with primary myoclonic dystonia, dystonia associated with tremor, generalised dystonia, and BHC is relatively small, so that only a frequent involvement of the SGCE gene in these diseases can be excluded.
In conclusion, this large cohort of patients has enabled us to show that SGCE mutations are exclusively found in patients with typical myoclonus‐dystonia or E‐M, but only in a subset (30%), indicating further genetic heterogeneity in this disease. Excluding patients with onset after the age of 25 and those who inherited the disease from their mothers would increase the specificity of the analysis. Furthermore, our data suggest that testing for SGCE mutations in other related phenotypes is likely to be negative. In SGCE negative families, especially when several members are affected, other as yet unidentified genes may be involved.
Acknowledgements
The authors are grateful to the patients who participated in this study. The authors would like to thanks Jean‐Pierre Azulay (Marseille), Philippe Castelnau (Paris), Victor Chan (Valence), Perrine Charles (Paris), David Devos (Lille), Daniel Fontan (Bordeaux), Cyril Goizet (Bordeaux), Philippe Kassiotis (Vannes), Pierre Krystkowiak (Lille), Isabelle Le Ber (Paris), James Lespinasse (Chambéry), Alexandre Mendes (Porto, Portugal), Jean‐Philippe Neau (Poitiers), Karine Nguyen (Marseille), Véronique Paquis (Nice), Danielle Ranoux (Paris), Jean‐Sébastien Vidal (Paris), Marie‐Laure Welter (Paris) for clinical investigation, Michèle Viemont for molecular analysis of the DYT1 gene, Thomas Gasser and Friedrich Asmus for their contribution, and Merle Ruberg for reviewing the manuscript.
Electronic‐database information
The Ensembl web site can be found at www.ensembl.org. Mutations are registered in the Human Gene Mutation Database at http://www.hgmd.cf.ac.uk
Abbreviations
BHC - benign hereditary chorea
E‐M - essential myoclonus
M‐D - myoclonus dystonia
MDS - myoclonus dystonia syndrome
PCR - polymerase chain reaction
Footnotes
This work was supported by INSERM, Réseau National Dystonies and GIS‐Maladies Rares, and the patients' associations AMADYS and Ligue Française Contre la Dystonie
Competing interests: none declared
The Ensembl web site can be found at www.ensembl.org. Mutations are registered in the Human Gene Mutation Database at http://www.hgmd.cf.ac.uk
References
- 1.Asmus F, Gasser T. Inherited myoclonus‐dystonia. Adv Neurol 200494113–119. [PubMed] [Google Scholar]
- 2.Nygaard T G, Raymond D, Chen C, Nishino I, Greene P E, Jennings D, Heiman G A, Klein C, Saunders‐Pullman R J, Kramer P, Ozelius L J, Bressman S B. Localization of a gene for myoclonus‐dystonia to chromosome 7q21–q31. Ann Neurol 199946(5)794–798. [DOI] [PubMed] [Google Scholar]
- 3.Zimprich A, Grabowski M, Asmus F, Naumann M, Berg D, Bertram M, Scheidtmann K, Kern P, Winkelmann J, Muller‐Myhsok B, Riedel L, Bauer M, Muller T, Castro M, Meitinger T, Strom T M, Gasser T. Mutations in the gene encoding epsilon‐sarcoglycan cause myoclonus‐dystonia syndrome. Nat Genet 200129(1)66–69. [DOI] [PubMed] [Google Scholar]
- 4.Yoshida M, Ozawa E. Glycoprotein complex anchoring dystrophin to sarcolemma. J Biochem (Tokyo) 1990108(5)748–752. [DOI] [PubMed] [Google Scholar]
- 5.Bonnemann C G, Modi R, Noguchi S, Mizuno Y, Yoshida M, Gussoni E, McNally E M, Duggan D J, Angelini C, Hoffman E P. Beta‐sarcoglycan (A3b) mutations cause autosomal recessive muscular dystrophy with loss of the sarcoglycan complex. Nat Genet 199511(3)266–273. [DOI] [PubMed] [Google Scholar]
- 6.Vainzof M, Passos‐Bueno M R, Canovas M, Moreira E S, Pavanello R C, Marie S K, Anderson L V, Bonnemann C G, McNally E M, Nigro V, Kunkel L M, Zatz M. The sarcoglycan complex in the six autosomal recessive limb‐girdle muscular dystrophies. Hum Mol Genet 19965(12)1963–1969. [DOI] [PubMed] [Google Scholar]
- 7.McNally E M, Ly C T, Kunkel L M. Human epsilon‐sarcoglycan is highly related to alpha‐sarcoglycan (adhalin), the limb girdle muscular dystrophy 2D gene. FEBS Lett 1998422(1)27–32. [DOI] [PubMed] [Google Scholar]
- 8.Nishiyama A, Endo T, Takeda S, Imamura M. Identification and characterization of ε‐sarcoglycans in the central nervous system. Mol Brain Res 2004125(1–2)1–12. [DOI] [PubMed] [Google Scholar]
- 9.Piras G, El Kharroubi A, Kozlov S, Escalante‐Alcalde D, Hernandez L, Copeland N G, Gilbert D J, Jenkins N A, Stewart C L. Zac1 (Lot1), a potential tumor suppressor gene, and the gene for epsilon‐sarcoglycan are maternally imprinted genes: identification by a subtractive screen of novel uniparental fibroblast lines. Mol Cell Biol 200020(9)3308–3315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grabowski M, Zimprich A, Lorenz‐Depiereux B, Kalscheuer V, Asmus F, Gasser T, Meitinger T, Strom T M. The epsilon‐sarcoglycan gene (SGCE), mutated in myoclonus‐dystonia syndrome, is maternally imprinted. Eur J Hum Genet 200311(2)138–144. [DOI] [PubMed] [Google Scholar]
- 11.Muller B, Hedrich K, Kock N, Dragasevic N, Svetel M, Garrels J, Landt O, Nitschke M, Pramstaller P P, Reik W, Schwinger E, Sperner J, Ozelius L, Kostic V, Klein C. Evidence that paternal expression of the epsilon‐sarcoglycan gene accounts for reduced penetrance in myoclonus‐dystonia. Am J Hum Genet 200271(6)1303–1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Valente E M, Edwards M J, Mir P, DiGiorgio A, Salvi S, Davis M, Russo N, Bozi M, Kim H T, Pennisi G, Quinn N, Dallapiccola B, Bhatia K P. The epsilon‐sarcoglycan gene in myoclonic syndromes. Neurology 200564(4)737–739. [DOI] [PubMed] [Google Scholar]
- 13.Asmus F, Zimprich A, Tézenas du Montcel S, Kabus C, Deuschl G, Kupsch A, Ziemann U, Castro M, Kuhn A A, Strom T M, Vidailhet M, Bhatia K P, Durr A, Wood N W, Brice A, Gasser T. Myoclonus‐dystonia syndrome: epsilon‐sarcoglycan mutations and phenotype. Ann Neurol 200252(4)489–492. [DOI] [PubMed] [Google Scholar]
- 14.Han F, Lang A E, Racacho L, Bulman D E, Grimes D A. Mutations in the epsilon‐sarcoglycan gene found to be uncommon in seven myoclonus‐dystonia families. Neurology 200361(2)244–246. [DOI] [PubMed] [Google Scholar]
- 15.Hedrich K, Meyer E ‐ M, Schule B, Kock N, de Carvalho Aguiar P, Wiegers K, Koelman J H, Garrels J, Durr R, Liu L, Schwinger E, Ozelius L J, Landwehrmeyer B, Stoessl A J, Tijssen M A, Klein C. Myoclonus‐dystonia: detection of novel, recurrent, and de novo SGCE mutations. Neurology 200462(7)1229–1231. [DOI] [PubMed] [Google Scholar]
- 16.Schule B, Kock N, Svetel M, Dragasevic N, Hedrich K, De Carvalho Aguiar P, Liu L, Kabakci K, Garrels J, Meyer E M, Berisavac I, Schwinger E, Kramer P L, Ozelius L J, Klein C, Kostic V. Genetic heterogeneity in ten families with myoclonus‐dystonia. J Neurol Neurosurg Psychiatry 200475(8)1181–1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Klein C, Liu L, Doheny D, Kock N, Muller B, de Carvalho Aguiar P, Leung J, de Leon D, Bressman S B, Silverman J, Smith C, Danisi F, Morrison C, Walker R H, Velickovic M, Schwinger E, Kramer P L, Breakefield X O, Brin M F, Ozelius L J. Epsilon‐sarcoglycan mutations found in combination with other dystonia gene mutations. Ann Neurol 200252(5)675–679. [DOI] [PubMed] [Google Scholar]
- 18.Marechal L, Raux G, Dumanchin C, Lefebvre G, Deslandre E, Girard C, Campion D, Parain D, Frebourg T, Hannequin D. Severe myoclonus‐dystonia syndrome associated with a novel epsilon‐sarcoglycan gene truncating mutation. Am J Med Genet B Neuropsychiatr Genet 2003119(1)114–117. [DOI] [PubMed] [Google Scholar]
- 19.Hjermind L E, Werdelin L M, Eiberg H, Krag‐Olsen B, Dupont E, Sorensen S A. A novel mutation in the epsilon‐sarcoglycan gene causing myoclonus‐dystonia syndrome. Neurology 200360(9)1536–1539. [DOI] [PubMed] [Google Scholar]
- 20.Foncke E M, Klein C, Koelman J H, Kramer P L, Schilling K, Muller B, Garrels J, de Carvalho Aguiar P, Liu L, de Froe A, Speelman J D, Ozelius L J, Tijssen M A. Hereditary myoclonus‐dystonia associated with epilepsy. Neurology 200360(12)1988–1990. [DOI] [PubMed] [Google Scholar]
- 21.Caviness J N, Brown P. Myoclonus: current concepts and recent advances. Lancet Neurol 20043(10)598–607. [DOI] [PubMed] [Google Scholar]
- 22.Quinn N P. Essential myoclonus and myoclonic dystonia. Mov Disord 199611(2)119–124. [DOI] [PubMed] [Google Scholar]
- 23.Jedynak C P, Bonnet A M, Agid Y. Tremor and idiopathic dystonia. Mov Disord 19916(3)230–236. [DOI] [PubMed] [Google Scholar]
- 24.Fahn S, Bressman S B, Marsden C D. Classification of dystonia. Adv Neurol 1998781–10. [PubMed] [Google Scholar]
- 25.Breedveld G J, Percy A K, MacDonald M E, de Vries B B, Yapijakis C, Dure L S, Ippel E F, Sandkuijl L A, Heutink P, Arts W F. Clinical and genetic heterogeneity in benign hereditary chorea. Neurology 200259(4)579–584. [DOI] [PubMed] [Google Scholar]
- 26.Saunders‐Pullman R, Shriberg J, Heiman G, Raymond D, Wendt K, Kramer P, Schilling K, Kurlan R, Klein C, Ozelius L J, Risch N J, Bressman S B. Myoclonus dystonia: possible association with obsessive‐compulsive disorder and alcohol dependence. Neurology 200258(2)242–245. [DOI] [PubMed] [Google Scholar]
- 27.Doheny D, Danisi F, Smith C, Morrison C, Velickovic M, De Leon D, Bressman S B, Leung J, Ozelius L, Klein C, Breakefield X O, Brin M F, Silverman J M. Clinical findings of a myoclonus‐dystonia family with two distinct mutations. Neurology 200259(8)1244–1246. [DOI] [PubMed] [Google Scholar]
- 28.Chinnery P F, Reading P J, McCarthy E L, Curtis A, Burn D J. Late‐onset axial jerky dystonia due to the DYT1 deletion. Mov Disord 200217(1)196–198. [DOI] [PubMed] [Google Scholar]
- 29.Gatto E M, Pardal M M F, Micheli F E. Unusual phenotypic expression of the DYT1 mutation. Parkinsonism Relat Disord 20039(5)277–279. [DOI] [PubMed] [Google Scholar]
- 30.Grundmann K, Laubis‐Herrmann U, Bauer I, Dressler D, Vollmer‐Haase J, Bauer P, Stuhrmann M, Schulte T, Schols L, Topka H, Riess O. Frequency and phenotypic variability of the GAG deletion of the DYT1 gene in an unselected group of patients with dystonia. Arch Neurol 200360(9)1266–1270. [DOI] [PubMed] [Google Scholar]